High-performance phosphate supported on HZSM-5 catalyst for dehydration of glycerol to acrolein☆
Feng Zhang,Xin Ren,He Huang,Jun Huang,Medak Sudhakar,Licheng Liu*
Qingdao Institute of Bioenergy and Bioprocess Technology,Chinese Academy of Sciences,Qingdao 266101,China
1.Introduction
Glycerol is the by-product in renewable biodiesel production.Generally,10 wt%of glycerol can be obtained along with the production of biodiesel and it is considered to be converted into raw material for value-added chemicals[1,2].Dehydration of glycerol to acrolein is a significant choice,which could replace the current gas phase oxidation of propylene.Meanwhile,acrolein is mostly applied as a versatile intermediate for the chemical industry and a feedstock for glutaraldehyde,acrylic acid and its esters,methionine,polyurethanes and polyester resins[3,4].
The catalytic dehydration of glycerol to acrolein has been widely investigated in the gas phase over solid acid catalysts such as heteropoly acids[5,6],metal oxides[7,8],functionalized mesoporous silica[9,10]and supported zeolites[11,12].Among various types of solid-acid catalysts,zeolites have been widely used in glycerol dehydration because of their well-defined porous structure,shape selectivity,and high hydrothermal stability[13-15].Maet al.[16]reported that phosphotungstic acid supported on mesoporous silica(HPW/MCM-41)was prepared by using wet impregnation method and results exhibited good catalytic activity with 63.8%acrolein selectivity and 51.3%acrolein yield even after a 5 h reaction in the dehydration of glycerol to acrolein.Zhanget al.[17]designed typical hierarchical HZSM-5 catalysts with diverse mesoporosity and similar microporosity/acidity,which exhibited good catalytic activity with above 80%acrolein selectivity and high acrolein yield even after 10 h in the dehydration of glycerol to acrolein.Yueet al.[18]demonstrated a nano-copper over ZSM-5(Cu/ZSM-5)catalyst prepared byin situimpregnated reduction was used for the catalytic dehydration of glycerol under solvent-free conditions.The conversion of glycerol and selectivity of acrolein were 92.1 wt%and 80.4 wt%,respectively.However,these porous catalysts are often severely constrained by the rapid catalyst deactivation due to coking formation.
A few studies have focused on the effect of coke formation on catalyst performance.Erfleet al.[19]analyzed the used vanadium based catalysts by FTIR spectroscopy and concluded that coking formed on Brønsted sites and this conclusion was con firmed by Suprunet al.[20].Although high density of Brønsted acid sites contributes to produce acrolein[21,22],it also accelerates the deactivation caused by severe coking which covers the active sites or blocks the microchannels of the catalysts.In order to decelerate deactivation and promote the glycerol dehydration,a feasible pathway has been proposed by taking advantage of doping with heteroatoms such as Zr[23],Fe[24],and Ti[25]that allows modifying either acid-base or even redox properties.For example,Liuet al.[26]investigated HPW supported on Cs+modified SBA-15(HPW/Cs-SBA)in gas-phase dehydration of glycerol and they found that the 50HPW/Cs-SBA with the largest amount of moderate acid sites showed 86%of acrolein yield and gave 85% of acrolein yield at TOS=20 h under N2/O2atmosphere.Huanget al.[27]presented HP-ZSM-5 with large intracrystal mesopores as a long-life catalyst for dehydration of glycerol to acrolein.Compared with commercial ZSM-5 catalyst,HP-ZSM-5 catalyst exhibited remarkable stability in the glycerol dehydration reaction.A nearly complete glycerol conversion over hierarchical ZSM-5 zeolite was achieved in nearly 50 h and the selectivity of acrolein remained 82%under N2/O2atmosphere.Ceciliaet al.[28]synthesized a sort of WO3supported on Zr doped mesoporous SBA-15 catalyst for the one-potconversion of glycerolto acrolein,which displayed the high glycerol conversion and acrolein yield values(97%and 41%after 2 h,and 90%and 38%after 8 h of TOS,respectively)under N2atmosphere.
Zeolites have shown excellent catalytic performance in vapor phase glycerol dehydration to acrolein,because of their tunable Brønsted and Lewis acidity and shape selectivity associated with well-defined pore size.It is well known that the acidity of the active phase is a key parameter which affects the catalytic performance,being relevant to the type of acid sites,since several authors have proved that Brønsted acid sites contribute to higher value of acrolein selectivity than Lewis acid sites[29,30].In this sense,phosphoric acid has been widely used in the literature to increase the total acidity of catalysts by providing new Brønsted acid sites[31,32].The previous studies of glycerol dehydration about metal phosphates supported on colloidal silica were patented in 2008[33].The yield of acrolein was more than 75%over the silica supported phosphate catalysts.On the other hand,ZSM-5 with satisfactory conversion and acrolein selectivity is the most studied molecular sieve in this reaction[34].However,the main disadvantage of zeolites is the rapid deactivation caused by severe coking which covers the active sites or blocks the microchannels of the catalysts.The microporous structure in traditional zeolites significantly limits their catalytic applications due to the mass transfer limitations.Therefore,we have focused on mesoporous molecular sieves,which have been widely studied in the glycerol dehydration.Decolattiet al.[35]investigated the dehydration of glycerol to acrolein by using treated HZSM-5 zeolite in order to develop mesoporosity by desilication and the alkaline treatment increased the mesoporous surface area.The catalytic results indicated that modified catalysts showed an improved activity in glycerol dehydration.Zhouet al.[36]used micro-and mesoporous ZSM-5 as the catalyst for this reaction and obtained a 72%acrolein yield.
To the bestofourknowledge,no scientific papers have been published doping different types of metal hydrophosphates supported on treated HZSM-5 zeolites as catalysts for the dehydration of glycerol to acrolein.The combination of phosphates and HZSM-5 is expected to make the acidity more tunable and improve catalytic performance and stability.Herein,we have tested four kinds of metal hydrophosphates overalkaline treatment and ammonium exchange HZSM-5 supporters.We focused on the effect of pore enlargement and acidic site modulation on the yield of acrolein,distribution of by-products and capacity of coking resistance.
2.Experimental
2.1.Catalyst preparation
2.1.1.Preparation of mesoporous HZSM-5
The HZSM-5(SiO2/Al2O3=300)was purchased from the Nankai University Catalyst Co.Ltd.Normally,HZSM-5 was treated with 0.2 mol·L-1sodium hydroxide aqueous solution at 60 °C for 6 h in an oil bath.Then,the resulting white solid was washed with deionized water and centrifuged for 4 times to ensure pH=7.After the solid powder was dried overnight at 70°C,the product was immersed in 1.0 mol·L-1ammonium nitrate solution and kept stirring for 6 h at 60°C in a water bath so that Na+could be exchanged by NH4+.The ion-exchange was repeated for3 times.At last,the product was calcined at 600°C in air for 6 h in a muffle furnace.
2.1.2.Preparation of phosphate/HZSM-5
Four kinds of transitional metal hydrophosphates were introduced(Sn,Cr,Mn,Mo).The molar ratio of PO42-/HZSM-5 was set to 15 mol%.Among all the active ingredients,precursors of transition metal cations were derived from SnCl4,Cr(NO3)3,Mn(NO3)2and (NH4)6Mo7O24·2H2O,respectively.The HPO42-and H2PO42-were offered by diammonium hydrogen phosphate((NH4)2HPO4)and ammonium dihydrogen phosphate(NH4H2PO4).
In the synthesis process,the Treated HZSM-5 and deionized water with a mass ratio of 1:3 were mixed fully with ultrasound and vigorous stirring.The suspension was poured into diammonium hydrogen phosphate solution and ultrasound treated for 30 min,then the obtained mixture was poured into transition metal corresponding to nitrate or acetate aqueous solution.The solid product was kept stirring for 24 h at 40°C and then evaporated.Finally,the sample was calcined at 350°C in air for 6 h in a muffle furnace.
2.2.Catalyst characterization
X-ray diffraction(XRD)patterns of the catalysts were recorded on an X-ray diffractometer(Bruker AXS D8 Focus)equipped with a Cu Kαsource(λ=1.5406 nm)operated at 40 kV and 40 mA.The SEM images were observed by using a scanning electron microscope(SEM JSM-6700f)with the acceleration voltage of 10 kV.Temperature-programmed desorption of ammonia(NH3-TPD)and temperature-programmed desorption of carbon dioxide(CO2-TPD)of the catalysts were performed using a Micromeritics Autochem 2920 chemisorption analyzer.For the NH3-TPD characterization,a typical procedure was performed as follows.Firstly,0.1 g of sample was loaded in a quartz U-tube and degassed at 150°C for 2 h.After the adsorption of ammonia at 100 °C for 2 h,the sample was then heated to 800 °C at a rate of 10 °C·min-1.For the CO2-TPD analysis,0.1 g of as-prepared catalyst was loaded in a quartz U-tube and degassed at 150°C for 2 h,then the adsorption of ammonia at 100°C for 2 h,the sample was then heated to 800 °C at a rate of 10 °C·min-1.NH3and CO2were detected by using thermal conductivity detector(TCD)signals(Thermo TPD/R/O 1100)after saturation with NH3/CO2at 100°C for 30 min and then purging with helium gas at for 1 h.
2.3.Catalytic test and products analysis
Glycerol dehydration was conducted in a vertical fixed-bed reactor under atmospheric pressure.A quartz tube reactor with an internal diameter of 10 mm was used and 0.5 g of catalyst(40-60 mesh)was loaded in the middle section of the reactor.Before reaction,the catalyst was pretreated at 300 °C for 1 h in N2at a flow rate of 25 ml·min-1.Aqueous glycerol solution(20wt%)was fed by using a syringe pump with 1.5 ml·h-1.The N2flow with 40 ml·min-1has passed the reactor as co-feeding gas.The reaction temperature was fixed at 300°C.The products were collected hourly with a cold trap and analyzed by GC(Shimadzu 2014,Japan)equipped with an auto-sampler,a capillary column(Phenomenex,ZB-WAX,60 m × 0.25 mm × 0.5 μm)and a flame ionization detector(FID).
The results of the GC analysis of the liquid-phases were used to evaluate conversion,selectivity,and carbon balance.The conversion of glycerol(Xgly)and product yield(Yi,mol%)were calculated based on molar concentrations of product and glycerol according to equations:
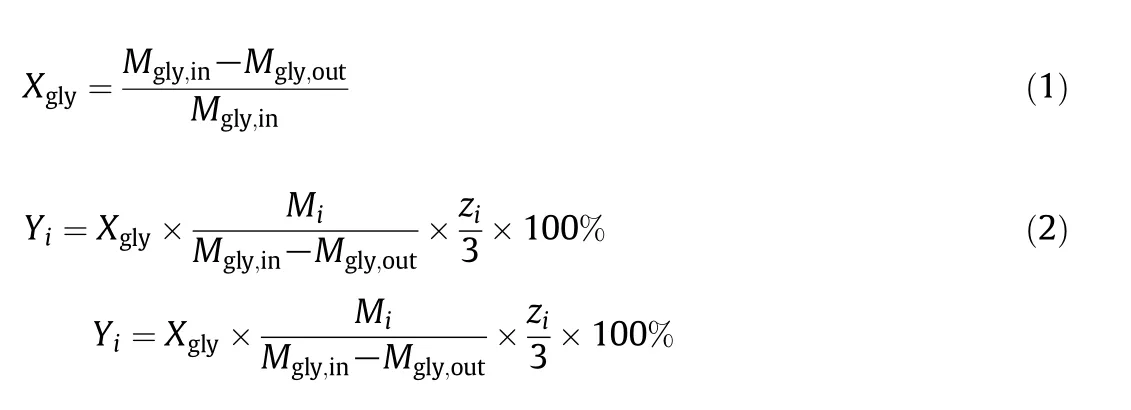
wherezrepresents the number of carbon atoms,for example,z=3 for acrolein andz=2 for acetaldehyde.Mirepresents the detected molar quantity of producti[37].
3.Results and Discussion
The XRD patterns of the catalysts are shown in Fig.1.All the catalysts are highly crystalline,as indicated by the strong intensity of the characteristic peaks in the XRD patterns,suggesting that the treatment of HZSM-5 did notobviously degrade the crystalline structure of the HZSM-5 zeolites.It is observed that the characteristic diffraction peaks that attributed to the HZSM-5 zeolite are preserved at 2θ =7°-9°and 2θ =23°-25°.In addition,the intensity of characteristic diffraction peaks is weaker than the treated HZSM-5 after being supported various metal hydrophosphates.It illustrates that metal hydrophosphate was successfully supported on the framework of treated HZSM-5 and slightly decreased the crystallinity of zeolite with the metal hydrophosphate loaded.However,there is no evident peak to be indicative of the presence of impurities.So it seems to be concluded that different metal hydrophosphates were highly dispersed on the catalyst surface.
Fig.2 shows the SEM images of all HZSM-5 and phosphates/HZSM-5 composite catalysts.The HZSM-5 shows clear cuboid shape with different side lengths in the range of 0.3-3 μm.The difference in morphology between the catalysts is not obvious,which homogeneously shows porous structure.Meanwhile,there is no visibly observed particles attached on the surface of supporters and it can be noted that the metal hydrophosphate particles highly dispersed on the framework and channel structure of the treated HZSM-5.The results of Energy Dispersive Spectra(EDS)of the above catalysts are present in Table 1.Due to the high Si/Al ratio(150),Al element is not detected beyond the low detection limit.The atomic percentages of other elements including O,Si,P and metal(Sn,Cr,Mn,Mo)are shown for all samples.However,it is found that the loadings of phosphates are all lower than the theoretical values(15 mol%)estimated from the P/Siratio.The unavoidable losthappened in the synthesis process.Considering the above XRD analysis,the targeted phosphate species were highly dispersed and not aggregated into large particles,so metal hydrophosphate crystals are not observed in the SEM pictures.
The N2adsorption and desorption isotherms were measured for studying the pore structure characteristics of HZSM-5 before and after alkali treatment,as well as the phosphate supported catalysts.As shown in Fig.3,the isotherm of Untreated HZSM-5 catalyst belongs to typicaltypeΙisotherm due to the microporous structure.The adsorption and desorption curves almost coincide with each other in the pressure range of 0.2-1.0,due to the full monolayer N2adsorption.Additionally,there is a hysteresis loop inP/P0=0.1-0.3 that relates to the super-micropores and small amount of narrow mesopores[37].In comparison,the Treated HZSM-5 catalyst shows a mixture of type Ι and type IV isotherms with enhanced N2adsorption quantity.The adsorption-desorption isotherm presents two hysteresis loops inP/P0=0.1-0.3 and 0.4-1.0.The low-pressure hysteresis loop is related to the supermicroporous and small mesopores in the HZSM-5 just like the Untreated HZSM-5.The high-pressure hysteresis loop is related to the artificial mesopores of HZSM-5[27].
For the Sn1/4H2PO4/HZSM-5 catalyst,the N2quantity adsorbed decreases obviously.This is also indicated from the surface area and textural properties(Table 2).The BET surface area of Untreated HZSM-5 is 469 m2·g-1,which is larger than Treated HZSM-5 considering the loss of part micropores(384 m2·g-1).But the pore volume of Treated HZSM-5 is much larger than that of Untreated HZSM-5(0.23versus0.14 cm3·g-1).The results came to a conclusion that alkaline treatment wasvery effective in expanding the pore volume ofHZSM-5.The surface area of Sn1/4H2PO4/HZSM-5 continues decreasing to 297 m2·g-1.The pore volume also decreases down to 0.16 cm3·g-1.It is supposed that the phosphate species occupy the channels or block them in the treated HZSM-5.The average pore size increases for the Treated HZSM-5 and Sn1/4H2PO4/HZSM-5 due to the introduction of mesopores by alkalinetreatment.The pore structure characteristics are important for mass transfer,coking resistance and product distribution.
The other phosphates/HZSM-5 catalysts were also measured by nitrogen adsorption-desorption(isotherms notshown)and the texturalproperties are summarized in Table 2.The similar results to Sn1/4H2PO4/HZSM-5 are observed with reduced surface area and pore volume.
The NH3-TPD experiments were conducted for investigating the acidity of different catalysts.A conventional NH3-TPD spectrum of HZSM-5 has two ammonia desorption peaks.The NH3-TPD profiles of the Treated HZSM-5 and different metal hydrophosphates/HZSM-5 catalyst are shown in Fig.4.According to Fig.4,the first peak for HZSM-5 is achieved at about180°C,which is associated with weak acid sites.The second peak is obtained at around 400°C,which is associated with strong acid sites.The peak at the low temperature(around 180°C)is assigned as that of ammonia adsorbed on non-acidic-OH groups or ammonium cations that formed by the reaction of ammonia and Brønsted acid sites,whereas the peak at the strong temperature(around 400°C)is assigned as the desorption peak of ammonia adsorbed on the Brønsted acid sites or Lewis acid sites[38].Thus,the acidic intensity at high temperature is weaker than low temperature for the Treated HZSM-5,suggesting the lack of strong acid concentration.With metal hydrophosphate loading,the specific peak areas of ammonia desorption increase and demonstrate that all the acidic intensity at about180°C is dramatically enhanced.However,element Mo merely contributes to improve the acidic intensity of HZSM-5 at around 400°C and then according to this order(Mn,Cr and Sn),the acidic intensity gradually transfers to high temperature(close to 500°C).It could be concluded that although crystals of these four atoms cannot be detected by XRD analysis,the best dispersion of them was observed.Hence,the dispersion of metal elements has a significant influence on the distribution of H+and P-OH and may further affect the catalyst activity in dehydration of glycerol.
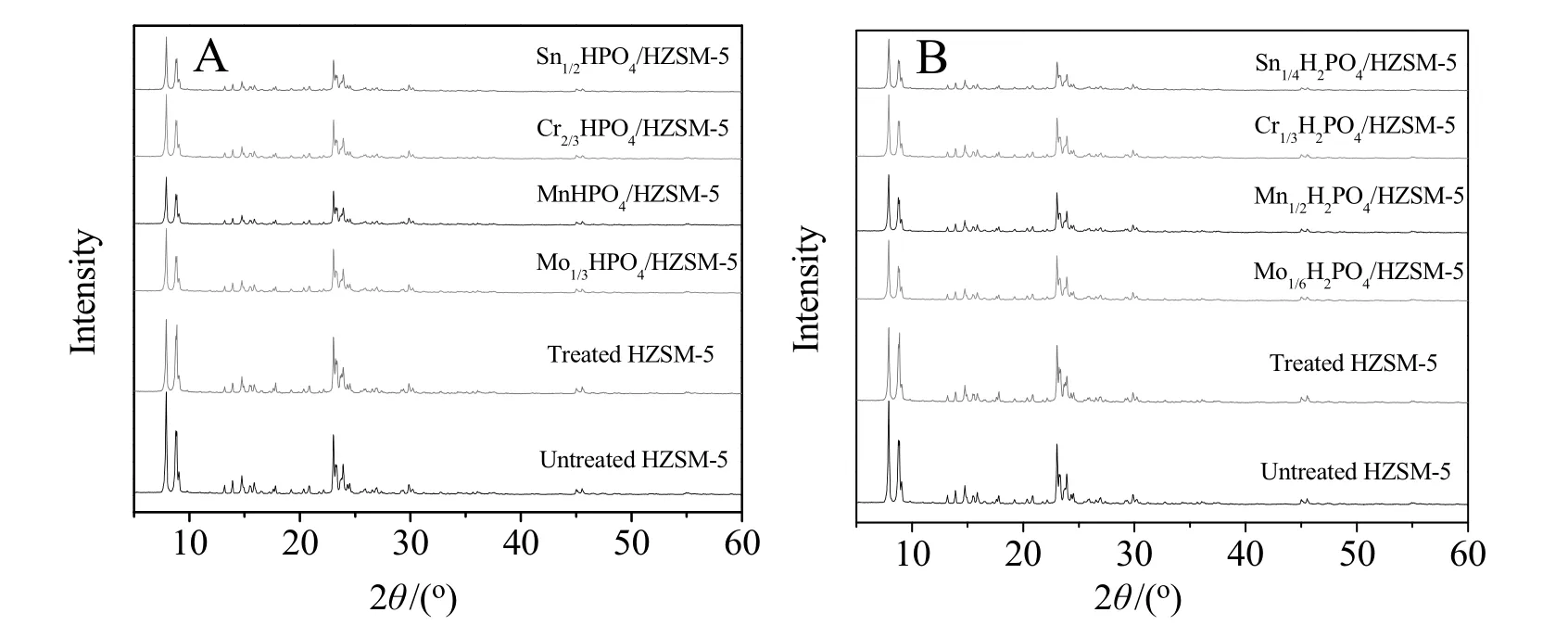
Fig.1.XRD patterns of as-prepared catalysts.
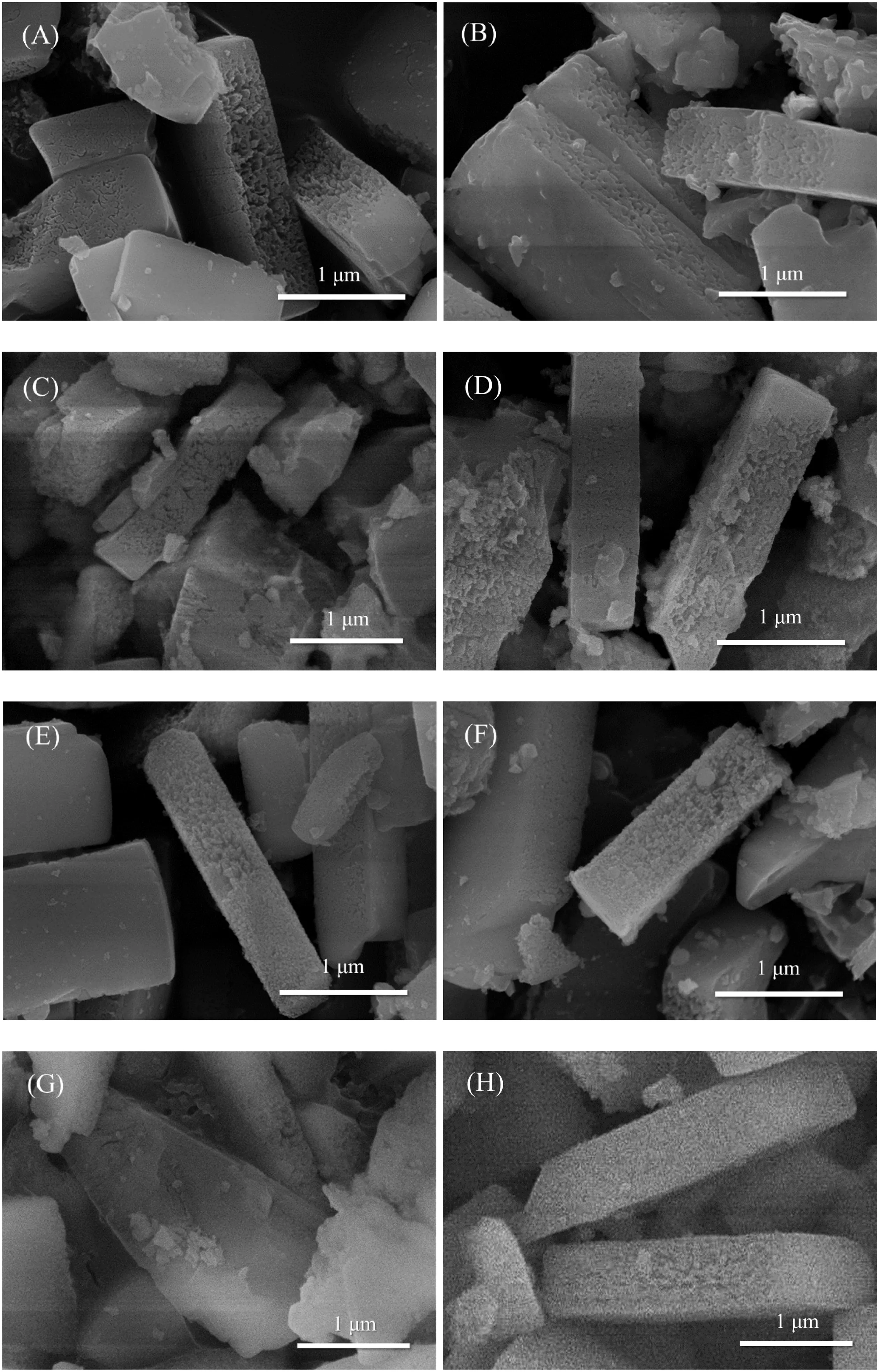
Fig.2.SEM images of different catalysts.(A)Untreated HZSM-5;(B)Treated HZSM-5;(C)Sn1/4H2PO4/HZSM-5;(D)Sn1/2HPO4/HZSM-5;(E)Cr1/3H2PO4/HZSM-5;(F)Cr2/3HPO4/HZSM-5;(G)Mn1/2H2PO4/HZSM-5;(H)MnHPO4/HZSM-5;(I)Mo1/6H2PO4/HZSM-5;(J)Mo1/3HPO4/HZSM-5.
The strong and weak acid concentrations were calculated by integration of peak area using 300°C as the dividing point.Considering the reaction was carried out at 300°C,the interaction of acid sites below and above 300°C may be important to the reaction.As shown in Table 3,the weak and strong acid amounts of the Treated HZSM-5 are much lower than the different metal hydrophosphates/HZSM-5 catalysts.It is noteworthy that metal hydrophosphate loaded on the supporter tremendously improves the total acid amounts and the main percentage of acid in total acid amounts is strong acid.The ratio of strong to weak acid concentration was calculated and listed in the last column.For the metal mono/di-hydrogen phosphates/HZSM-5 composite catalysts,the ratios can be divided into three levels:around 2 for all manganese and molybdenum phosphates,around 3 for both tin phosphates and chromium dihydrogen phosphate,and ultra-high 9.5 for chromiummonohydrogen phosphate.High proportion of strong acid is beneficial to the dehydration of glycerol to acrolein,this will be demonstrated next.
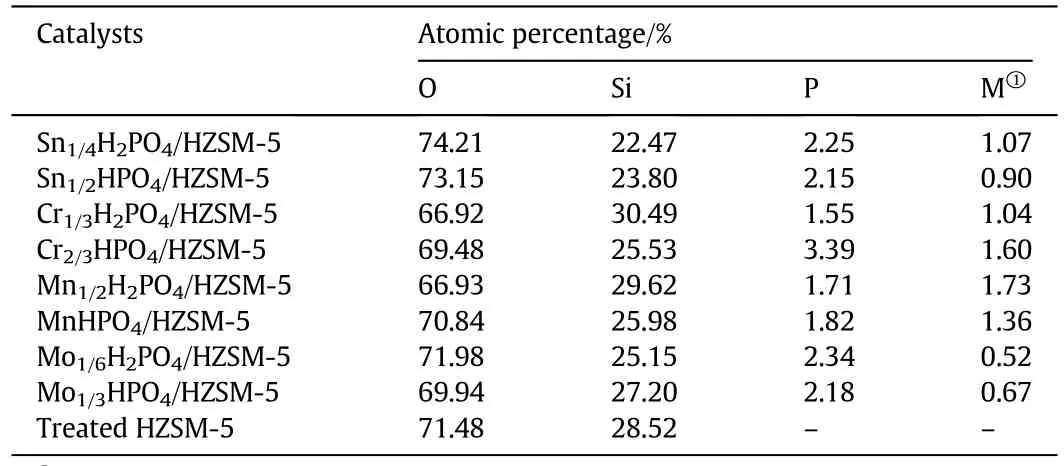
Table 1 EDS analysis of the catalysts
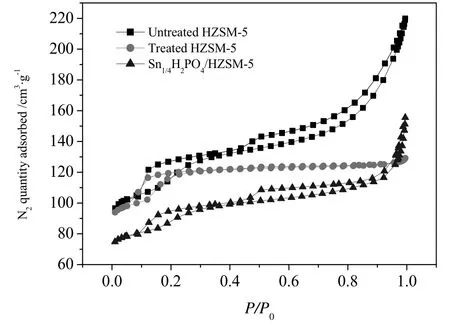
Fig.3.The N2 adsorption-desorption isotherms for the Untreated,Treated HZSM-5 and Sn1/4H2PO4/HZSM-5 catalysts.
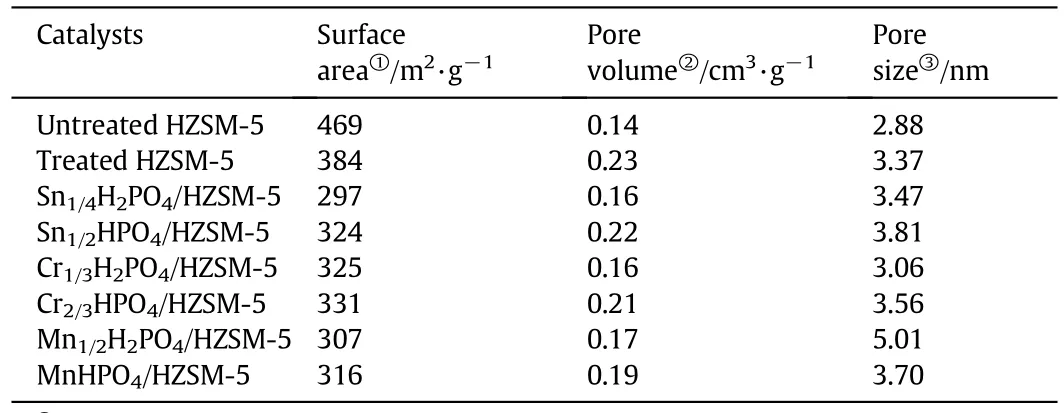
Table 2 Textural properties of the catalysts
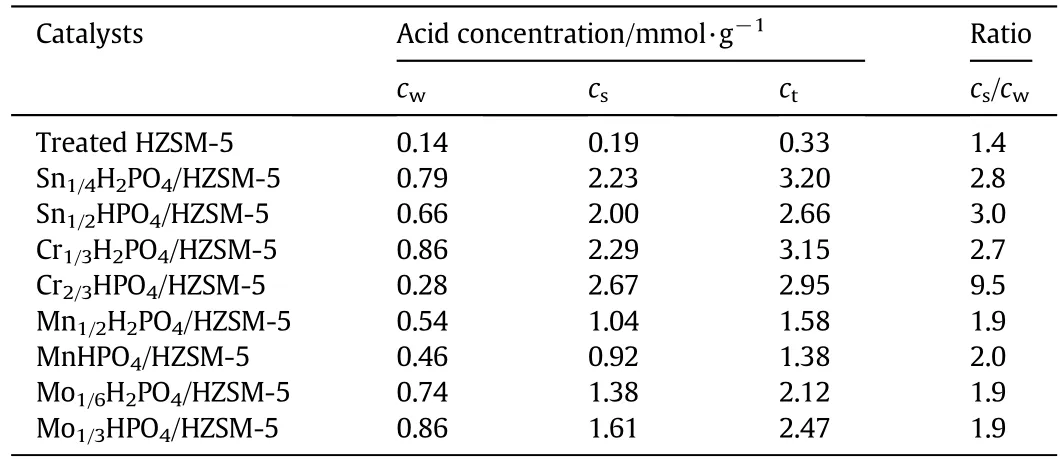
Table 3 Acidities of different composite catalysts
To further understand the effects of the different metal hydrophosphates on the acidity property of HZSM-5 zeolites,the pyridine-adsorbed FT-IR measurements were carried out and the results are presented in Fig.5.The three catalysts contain Brønsted and Lewis acid sites,which are indicated by the absorption peaks at about 1540 cm-1and 1450 cm-1,respectively.Additional absorption bands can also be observed at 1489 cm-1(co-adsorption onB/L),1616 and 1575 cm-1(strong and weakLacid sites)[39].It could be noticed that the peak assigned to Lewis acid sites at 1450 cm-1is actually composed of two overlapped bands for the metal hydrophosphates.By comparing with the peak of Treated HZSM-5,the overlapped peak at higher wavenumber can be assigned to Lewis acid sites on HZSM-5(labeled asL1),while the peak at lower wavenumber relates to the Lewis acid sites on phosphates(labeled asL2).
The peak areas assigned to both Brønsted and Lewis acid sites at 1540 and 1450 cm-1were integrated and summarized in Table 4.Because the sample amount for IR measurement is not fixed,it is hard to compare the acid amount directly.Therefore,the ratios ofL1/L2and(L1+L2)/Bare calculated to eliminate the effect of mass difference.The Sn1/4H2PO4/HZSM-5 shows a lowerL1/L2ratio(2.1)than Mn1/2H2PO4/HZSM-5(3.4),indicating that Sn1/4H2PO4contributes more Lewis acid than Mn1/2H2PO4.The(L1+L2)/Bratio shows that the Mn1/2H2PO4/HZSM-5 has higher Brønsted acid sites than Treated HZSM-5 and Sn1/4H2PO4/HZSM-5.
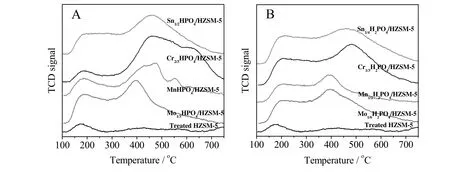
Fig.4.NH3-TPD profiles of different catalysts.
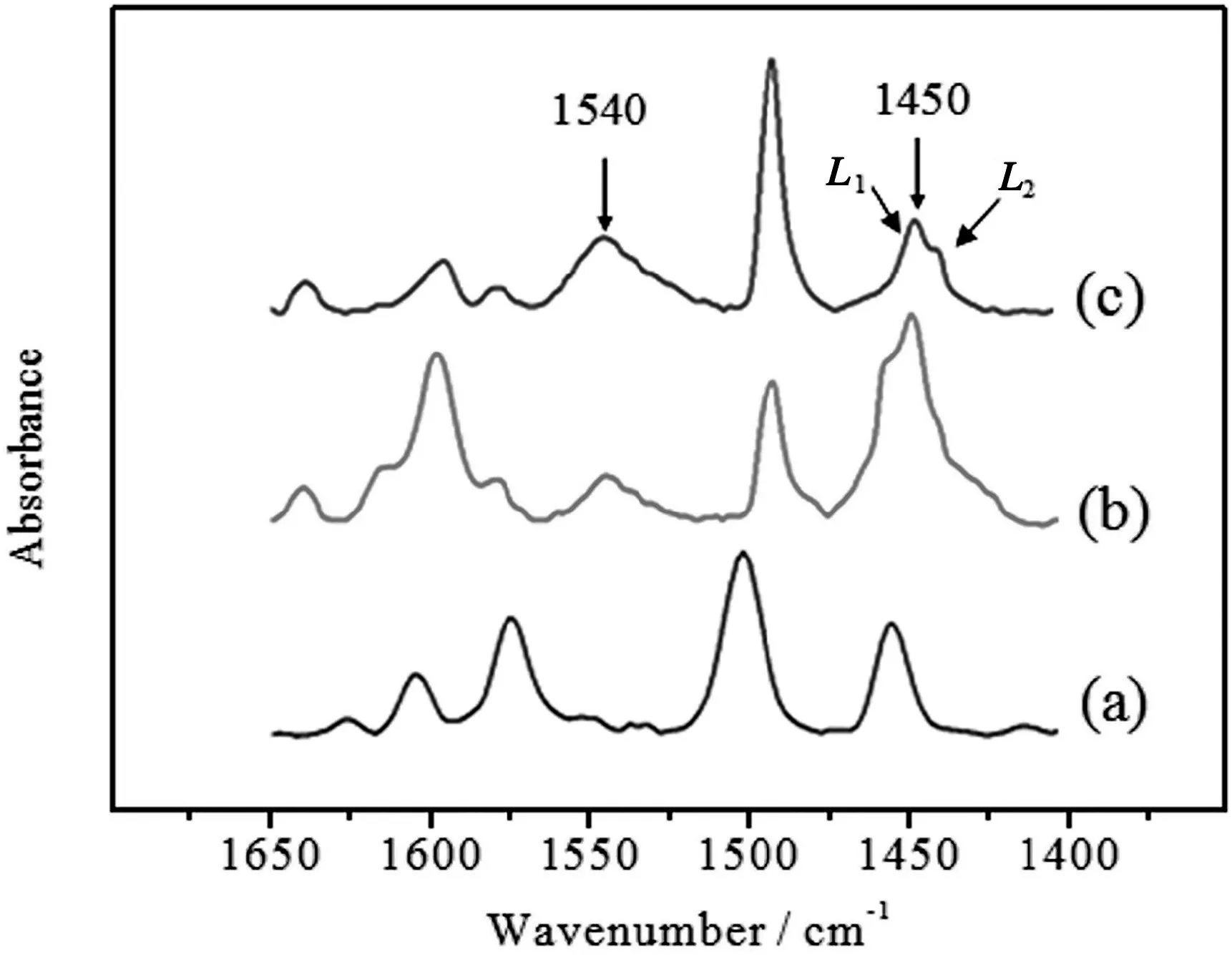
Fig.5.Pyridine-adsorption FT-IR spectra of different catalysts.(a)Treated HZSM-5;(b)Sn1/4H2PO4/HZSM-5;(b)Mn1/2H2PO4/HZSM-5.

Table 4 The acid type property derived from pyridine-adsorption FT-IR spectra of different catalysts

Fig.6.CO2-TPD profiles of different catalysts.

Table 5 Alkalinities of different phosphates/zeolite composite catalysts
Subsequently,we measured CO2-TPD profiles as shown in Fig.6.According to the patterns,low temperature peak atabout170°C is associated with weak alkaline sites and the high temperature peaks at around 400 °C and 550 °C are associated with strong alkaline sites.The weak peaks at the low temperature are assigned as CO2adsorbed on the supporters[40],whereas the strong peaks at the high temperature are assigned as the desorption peak of CO2adsorbed on the oxidative metal species with the dissociated-OH from the weak M-OH[41].Table 2 lists the alkalinity amounts of different catalysts obtained by CO2-TPD analysis.The low value of alkalinity below 2 mmol·g-1belongs to the alkaline sites on supporter and high amount of alkalinity above 12 mmol·g-1represents for oxidative metal species attached hydroxyl Lewis acid.By comparing the data of four samples in Table 5,the amount of strong alkalinity increases in the order of:Mo1/3HPO4/HZSM-5(12.3 mmol·g-1)< MnHPO4/HZSM-5(17.7 mmol·g-1)< Cr2/3HPO4/-HZSM-5(19.6 mmol·g-1) < Sn1/2HPO4/HZSM-5(27.1 mmol·g-1).According to this order,the amount of strong alkalinity and total alkalinity gradually increase,which is attributed to the local charge imbalance for different metals.It will show that the steadily catalytic performance in glycerol dehydration reaction may comply with this order.
The catalytic activity evaluation for glycerol dehydration was conducted in a fix-bed reactor for 30 h and the performance over the prepared catalysts is shown in Fig.7.The conversion of glycerol is 100%for all reactions.But some polymeric and pyrolysis products cannot be collected and analyzed,so as to make a poor carbon balance for some reactions.The yield curves of acrolein over the Untreated HZSM-5 and Treated HZSM-5 are corresponding to Fig.7(A)and(B).The Untreated HZSM-5 shows an initial acrolein yield of 50-60%in 2-5 h.It is a little higher than Yong Tae Kim's report on HZSM-5 with different SiO2/Al2O3ratios[34].Moreover,the present HZSM-5 exhibits extremely poor stability in the reaction with less than 25%of acrolein yield obtained after 30 h.After alkaline treatment,it shows enhanced initial acrolein yield,as well as the stability for the overnight reaction.The initial activity increases to 65-70%with acrolein yield above 50%after 30 h.Compared to the untreated one,pore enlargement is in favor of accelerating the mass transfer and avoiding rapid deactivation induced by coke deposition on the acid sites for the Treated HZSM-5 catalyst.
Doping different types of metal hydrophosphates on the structure of zeolites affected surface and textural chemistry as well as the catalytic activity for glycerol conversion and product distribution[42].Compared to pure HZSM-5 supporters,the catalytic activity ofSn1/4H2PO4/HZSM-5 and Sn1/2HPO4/HZSM-5 catalysts in Fig.7(C)shows outstanding performance with regard to their activity and stability,which respectively achieve acrolein yield up to 68%and 56%after the reaction for 30 h.Modification with metal hydrophosphate helps boost the acrolein selectivity as more Brønsted acidity is well dispersed on the catalyst surface at the cost of Lewis sites.The improved strong Brønsted acid sites were observed in the FTIR spectra of pyridine-adsorption.Catalytic difference divides for the Cr2/3HPO4/HZSM-5 and Cr1/3H2PO4/HZSM-5 catalysts in Fig.7(D).Compared to the Cr1/3H2PO4/HZSM-5 catalyst that exhibited above 64%of acrolein yield after 30 h,the Cr2/3HPO4/HZSM-5 catalyst shows poor catalytic activity with less than 36%of acrolein yield obtained after 30 h.The manganese hydrophosphates and molybdenum hydrophosphates on HZSM-5 in Fig.7(E)and(F)show very poor stability.Initially,all of them display good performance in the dehydration of glycerol to acrolein in the first 5 h,poor stability with less than 35%of acrolein yield emerges after 30 h.
Considering the acidic properties obtained by NH3-TPD,the ratio of strong to weak acidity may play an important role in deciding the catalyst activity and stability.The initial acrolein yields on all composite catalysts may relate to the increase of total acid concentration,especially the strong acid sites.The Sn1/4H2PO4/HZSM-5,Sn1/2HPO4/HZSM-5 and Cr1/3H2PO4/HZSM-5 catalysts that exhibited relatively good stability can be attributed to their moderatecs/cwratio.The ratio is low(the manganese hydrophosphates and molybdenum hydrophosphates on HZSM-5)or too high(Cr2/3HPO4/HZSM-5),that both damage the stability.On the other hand,the acrolein yield for the four metal(in the order of Sn,Cr,Mn,Mo)monohydrogen phosphates on HZSM-5 catalysts shows a roughly positive relation with the alkalinity amount at reaction for5 h(70.4%,66.1%,69.9%,59.6%)and 30 h(54.1%,36.6%,35.8%,27.2%),respectively.So an appropriate proportion and distribution of acidity/basicity on the catalyst are crucial for the catalytic performance on glycerol dehydration to acrolein.

Fig.7.The yield of acrolein over different catalysts.(A)Untreated HZSM-5;(B)Treated HZSM-5.(C)Sn1/2HPO4/HZSM-5 and Sn1/4H2PO4/HZSM-5;(D)Cr2/3HPO4/HZSM-5 and Cr1/3H2PO4/HZSM-5;(E)MnHPO4/HZSM-5 and Mn1/2H2PO4/HZSM-5;(F)Mo1/3HPO4/HZSM-5 and Mo1/6H2PO4/HZSM-5.
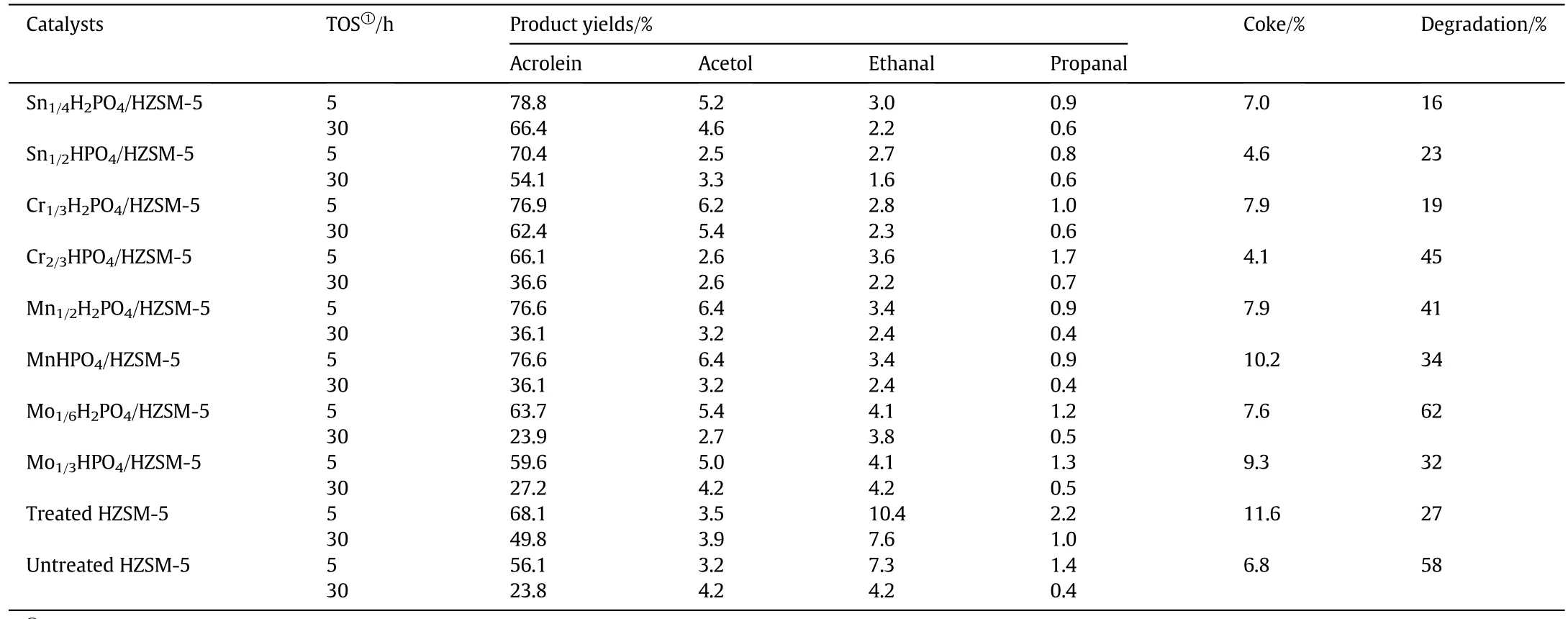
Table 6 The catalytic results on different zeolite catalysts
The primary byproducts summarized in Table 6 are hydroxyacetone,acetaldehyde and propanal.It was observed that hydroxyacetone is the maximum byproduct.Although the intensity of acidity was much enhanced,there is no obvious distinction in the yield of byproducts among the modified HZSM-5 and pure HZSM-5.Therefore,this modification method is practicable to be con firmed by the results.As faras the catalysts for glycerol dehydration are concerned,coking is the main reason for the catalyst deactivation and those coking deposits provoke a severe blockage of the porous network,drastically reducing catalytic activity.Coking quantitative analysis in Table 6 indicates that the Untreated HZSM-5 catalyst possessed the larger amount of coke(%)than the Treated HZSM-5.It is concluded that pore enlargement contributes to improve the resistant ability of coking formation.For investigating the stability of catalyst,the degradation level was defined as the percentage of difference for acrolein yield decrease in 5-30 h to the acrolein yield at 5 h.It is actually the digitization of Fig.7.The results are listed in the last column in Table 6.According to the carbon deposition and degradation level of different catalysts after 30 h,there is no obvious rule summarized among them.So the radar map was drawn to discuss their relations.
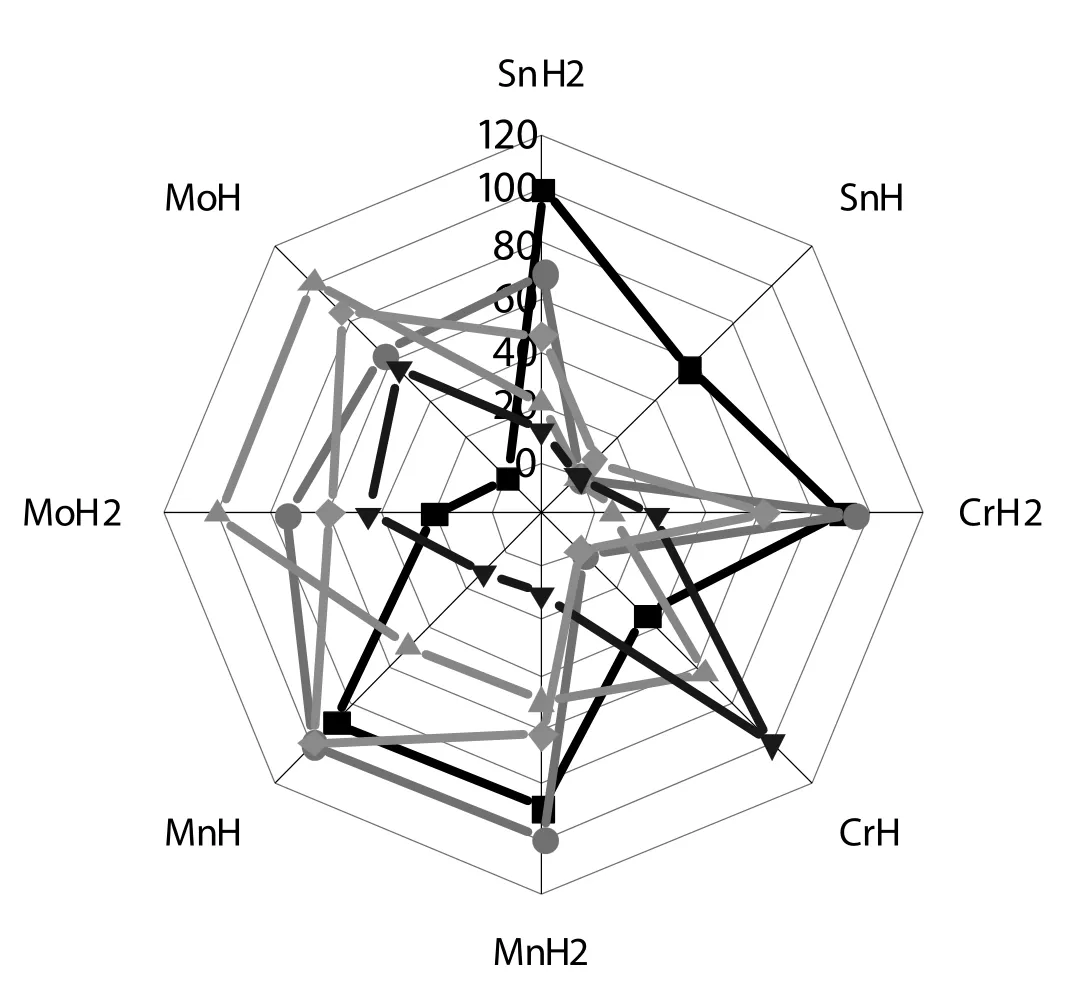
Fig.8.The radar map of product distribution for different composite catalyst.Acrolein;Acetol;Ethanal;Propanal;Coke().Designation:Sn H2=Sn1/4H2PO4/HZSM-5;SnH=Sn1/2HPO4/HZSM-5;CrH2=Cr1/3H2PO4/HZSM-5;CrH=Cr2/3HPO4/HZSM-5;MnH2=Mn1/2H2PO4/HZSM-5;MnH = MnHPO4/HZSM-5;MoH2 = Mo1/6H2PO4/HZSM-5;MoH =Mo1/3HPO4/HZSM-5.
Firstly,the radar map of product distribution for different composite catalysts is present in Fig.8.For directly observing,the yield and coking amount were normalized before plot.From Fig.8,the two tin hydrophosphates/HZSM-5 catalysts with high acrolein yield show relatively low by-product and coking level.The molybdenum hydrophosphates/HZSM-5 catalysts with lower acrolein yield usually show high by-product and coking level.In contrast,the manganese hydrophosphates/HZSM-5 catalysts with higheracrolein yield show relatively high by-product and coking level at the same time,except the propanal.While the two chromium hydrophosphates/HZSM-5 catalysts show distinct behavior with regard to the product and coke.In general,to suppress coking and by-product is necessary to enhance acrolein yield.The effect of acidity on the reaction pathways is complicated in the glycerol dehydration reaction reported here.
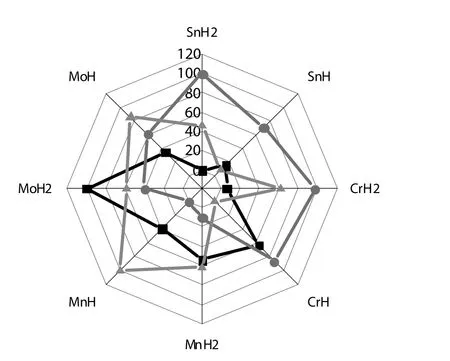
Fig.9.The radar map of degradation level,total acidityand cokefor different composite catalysts.Designation:SnH2=Sn1/4H2PO4/HZSM-5;SnH=Sn1/2HPO4/HZSM-5;CrH2=Cr1/3H2PO4/HZSM-5;CrH=Cr2/3HPO4/HZSM-5;MnH2=Mn1/2H2PO4/HZSM-5;MnH=MnHPO4/HZSM-5;Mo H2=Mo1/6H2PO4/HZSM-5;MoH=Mo1/3HPO4/HZSM-5.
For observing the relation between the stability/coking and acidity,another radar map is plotted in Fig.9.It can be seen that enhanced acidity is beneficial for reducing catalyst degradation and coking simultaneously,for tin and chromium based catalysts especially.The other catalysts show opposite conclusion that catalyst degradation and coking would be serious with low acidity.Therefore,the most important role of introducing phosphates is to create enough and favorable strong acid sites.Ganet al.reported[43]that the amorphous zirconium phosphate catalysts calcined at 400°C exhibited the largest fraction of medium strength acid sites and the best catalytic efficiency for the formation of acrolein with the yield of 81.5%.An appropriate amount of acid sites and acid strength were favorable for catalyst stability.This has the similar meaning to our conclusions.N.Pethan Rajan and his coworkers prepared a series of porous zirconium phosphate supported VPO catalysts for gas-phase dehydration of glycerol to acrolein[44].The 20 wt%VPO/ZrP showed the best activity towards glycerol conversion(100%)and acrolein selectivity(65%).It is a bit lower than our results.And they found that there is a significant decrease in the acidity and Brønsted acidic sites of the spent samples leading to a decrease in the conversion and selectivity of the catalyst.
4.Conclusions
In this paper,the alkaline treated HZSM-5 zeolites were successfully modified by different metal hydrophosphates.The resulting composite catalysts were applied to the catalytic dehydration of glycerol to acrolein in gas-phase.The influence of different metal hydrophosphates on the supporter and reaction performance was carefully investigated by various characterizations.From the results of XRD,SEM,EDS,NH3-TPD,CO2-TPD and Py-IR,metal hydrophosphate species had been well dispersed in the network of HZSM-5 zeolite,which affected surface and physico-chemical properties of the composite catalysts.It could be concluded that the total acid concentration,especially the strong acidity,was greatly improved by introducing metal hydrophosphate species.The catalytic activity and stability were obviously enhanced with appropriate acid strength and distribution.According to our investigation,Sn1/4H2PO4/HZSM-5 shows excellent performance in the catalytic activity and coking resistant ability,which results in a high acrolein yield of 83%initially and acrolein yield of 68%after 30 h.
[1]A.Behr,J.Eilting,K.Irawadi,J.Leschinski,F.Lindner,Improved utilisation of renewable resources:New important derivatives of glycerol,Green Chem.10(2008)13-30.
[2]B.Katryniok,S.Paul,M.Capron,F.Dumeignil,Towards the sustainable production of acrolein by glycerol dehydration,ChemSusChem2(2009)719-730.
[3]K.H.Bowmer,G.H.Smith,Herbicides for injection into flowing water:Acrolein and endothal-amine,Weed Res.24(1984)201-211.
[4]M.Pagliaro,R.Ciriminna,H.Kimura,M.Rossi,C.Della Pina,From glycerol to valueadded products,Angew.Chem.Int.Ed.46(2007)4434-4440.
[5]D.Stošić,S.Bennici,S.Sirotin,P.Stelmachowski,J.-L.Couturier,J.-L.Dubois,A.Travert,A.Auroux,Examination of acid-base properties of solid catalysts for gas phase dehydration of glycerol:FTIR and adsorption microcalorimetry studies,Catal.Today226(2014)167-175.
[6]M.H.Haider,N.F.Dummer,D.Zhang,P.Miedziak,T.E.Davies,S.H.Taylor,D.J.Willock,D.W.Knight,D.Chadwick,G.J.Hutchings,Rubidium-and caesium-doped silicotungstic acid catalysts supported on alumina for the catalytic dehydration of glycerol to acrolein,J.Catal.286(2012)206-213.
[7]W.Yan,G.J.Suppes,Low-pressure packed-bed gas-phase dehydration of glycerol to acrolein,Ind.Eng.Chem.Res.48(2009)3279-3283.
[8]M.Massa,A.Andersson,E.Finocchio,G.Busca,F.Lenrick,L.R.Wallenberg,Performance of ZrO2-supported Nb-and W-oxide in the gas-phase dehydration of glycerol to acrolein,J.Catal.297(2013)93-109.
[9]J.A.Cecilia,C.García-Sancho,J.M.Mérida-Robles,J.Santamaría-González,R.Moreno-Tost,P.Maireles-Torres,V and V-P containing Zr-SBA-15 catalysts for dehydration of glycerol to acrolein,Catal.Today254(2015)43-52.
[10]T.Ma,Z.Yun,W.Xu,L.Chen,L.Li,J.Ding,R.Shao,Pd-H3PW12O40/Zr-MCM-41:An efficient catalyst for the sustainable dehydration of glycerol to acrolein,Chem.Eng.J.294(2016)343-352.
[11]A.S.de Oliveira,S.J.S.Vasconcelos,J.R.de Sousa,F.F.de Sousa,J.M.Filho,A.C.Oliveira,Catalytic conversion of glycerol to acrolein over modified molecular sieves:Activity and deactivation studies,Chem.Eng.J.168(2011)765-774.
[12]L.G.Possato,R.N.Diniz,T.Garetto,S.H.Pulcinelli,C.V.Santilli,L.Martins,A comparative study of glycerol dehydration catalyzed by micro/mesoporous MFI zeolites,J.Catal.300(2013)102-112.
[13]A.Corma,From microporous to mesoporous molecular sieve materials and their use in catalysis,Chem.Rev.97(1997)2373-2419.
[14]D.Kubička,I.Kubičková,J.Čejka,Application of molecular sieves in transformations of biomass and biomass-derived feedstocks,Catal.Rev.Sci.Eng.55(2013)1-78.
[15]C.S.Carriço,F.T.Cruz,M.B.Santos,H.O.Pastore,H.M.C.Andrade,A.J.S.Mascarenhas,Efficiency of zeolite MCM-22 with different SiO2/Al2O3molar ratios in gas phase glycerol dehydration to acrolein,Microporous Mesoporous Mater.181(2013)74-82.
[16]T.Ma,J.Ding,R.Shao,W.Xu,Z.Yun,Dehydration of glycerol to acrolein over Wells-Dawson and Keggin type phosphotungstic acids supported on MCM-41 catalysts,Chem.Eng.J.316(2017)797-806.
[17]H.Zhang,Z.Hu,L.Huang,H.Zhang,K.Song,L.Wang,Z.Shi,J.Ma,Y.Zhuang,W.Shen,Y.Zhang,H.Xu,Y.Tang,Dehydration of glycerol to acrolein over hierarchical ZSM-5 zeolites:Effects of mesoporosity and acidity,ACS Catal.5(2015)2548-2558.
[18]C.-J.Yue,M.-M.Gan,L.-P.Gu,Y.-F.Zhuang,In situ synthesized nano-copper over ZSM-5 for the catalytic dehydration of glycerol under mild conditions,J.Taiwan Inst.Chem.Eng.45(2014)1443-1448.
[19]S.Er fle,U.Armbruster,U.Bentrup,A.Martin,A.Brückner,Impact of redox properties on dehydration of glycerol to acrolein over heteropolyacids assessed by operando-EPR spectroscopy,Appl.Catal.A Gen.391(2011)102-109.
[20]W.Suprun,M.Lutecki,T.Haber,H.Papp,Acidic catalysts for the dehydration of glycerol:Activity and deactivation,J.Mol.Catal.A Chem.309(2009)71-78.
[21]S.-H.Chai,H.-P.Wang,Y.Liang,B.-Q.Xu,Sustainable production of acrolein:investigation of solid acid-base catalysts for gas-phase dehydration of glycerol,Green Chem.9(2007)1130-1136.
[22]B.Katryniok,S.Paul,V.Bellière-Baca,P.Rey,F.Dumeignil,Glycerol dehydration to acrolein in the context of new uses of glycerol,Green Chem.12(2010)2079.
[23]D.J.Jones,J.Jimenez-Jimenez,A.Jimenez-Lopez,P.Maireles-Torres,P.Olivera-Pastor,E.Rodriguez-Castellon,J.Roziere,Surface characterisation of zirconium-doped mesoporous silica,Chem.Commun.(1997)431-432.
[24]A.Vinu,D.P.Sawant,K.Ariga,K.Z.Hossain,S.B.Halligudi,M.Hartmann,M.Nomura,Direct synthesis of well-ordered and unusually reactive FeSBA-15 mesoporous molecular sieves,Chem.Mater.17(2005)5339-5345.
[25]S.-Y.Chen,C.-Y.Tang,J.-F.Lee,L.-Y.Jang,T.Tatsumi,S.Cheng,Effect of calcination on the structure and catalytic activities of titanium incorporated SBA-15,J.Mater.Chem.21(2011)2255-2265.
[26]R.Liu,T.Wang,Y.Jin,Catalytic dehydration of glycerol to acrolein over HPW supported on Cs+modified SBA-15,Catal.Today233(2014)127-132.
[27]L.Huang,F.Qin,Z.Huang,Y.Zhuang,J.Ma,H.Xu,W.Shen,Hierarchical ZSM-5 zeolite synthesized by an ultrasound-assisted method as a long-life catalyst for dehydration of glycerol to acrolein,Ind.Eng.Chem.Res.55(2016)7318-7327.
[28]J.A.Cecilia,C.García-Sancho,J.M.Mérida-Robles,J.Santamaría González,R.Moreno-Tost,P.Maireles-Torres,WO3supported on Zr doped mesoporous SBA-15 silica for glycerol dehydration to acrolein,Appl.Catal.A Gen.516(2016)30-40.
[29]A.Alhanash,E.F.Kozhevnikova,I.V.Kozhevnikov,Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt,Appl.Catal.A Gen.378(2010)11-18.
[30]G.S.Foo,D.Wei,D.S.Sholl,C.Sievers,Role of Lewis and Brønsted acid sites in the dehydration of glycerol over niobia,ACS Catal.4(2014)3180-3192.
[31]J.Mohd Ekhsan,S.L.Lee,H.Nur,Niobium oxide and phosphoric acid impregnated silica-titania as oxidative-acidic bifunctional catalyst,Appl.Catal.A Gen.471(2014)142-148.
[32]Y.Y.Lee,K.A.Lee,N.C.Park,Y.C.Kim,The effect of PO4to Nb2O5catalyst on the dehydration of glycerol,Catal.Today232(2014)114-118.
[33]T.H.Peterson,A.H.Zacher,M.J.Gray,J.F.White,J.E.Holladay,T.A.Werpy,Chemical Production Process,System,and Catalyst Compositions,US,2009.
[34]Y.T.Kim,K.D.Jung,E.D.Park,Gas-phase dehydration of glycerol over ZSM-5 catalysts,Microporous Mesoporous Mater.131(2010)28-36.
[35]H.P.Decolatti,B.O.Dalla Costa,C.A.Querini,Dehydration of glycerol to acrolein using H-ZSM5 zeolite modified by alkali treatment with NaOH,Microporous Mesoporous Mater.204(2015)180-189.
[36]C.J.Zhou,C.J.Huang,W.G.Zhang,H.S.Zhai,C.J.Huang,H.L.Wu,Z.S.Chao,Synthesis of micro-and mesoporous ZSM-5 composites and their catalytic application in glycerol dehydration to acrolein,Stud.Surf.Sci.Catal.165(2007)527-530.
[37]Z.X.Yang,Y.D.Xia,R.Mokaya,Zeolite ZSM-5 with unique supermicropores synthesized using mesoporous carbon as a template,Adv.Mater.16(2004)727-732.
[38]K.Suzuki,Y.Aoyagi,N.Katada,M.Choi,R.Ryoo,M.Niwa,Acidity and catalytic activity of mesoporous ZSM-5 in comparison with zeolite ZSM-5,Al-MCM-41 and silica-alumina,Catal.Today132(2008)38-45.
[39]M.I.Zaki,M.A.Hasan,F.A.Al-Sagheer,L.Pasupulety,In situ FTIR spectra of pyridine adsorbed on SiO2-Al2O3,TiO2,ZrO2and CeO2:General considerations for the identification of acid sites on surfaces of finely divided metal oxides,Colloids Surf.A Physicochem.Eng.Asp.190(2001)261-274.
[40]O.Kikhtyanin,Y.Ganjkhanlou,D.Kubička,R.Bulánek,J.Čejka,Characterization of potassium-modified FAU zeolites and their performance in aldol condensation of furfural and acetone,Appl.Catal.A Gen.549(2018)8-18.
[41]S.Deng,H.Li,S.Li,Y.Zhang,Activity and characterization of modified Cr2O3/ZrO2nano-composite catalysts for oxidative dehydrogenation of ethane to ethylene with CO2,J.Mol.Catal.A Chem.268(2007)169-175.
[42]S.Thanasilp,J.W.Schwank,V.Meeyoo,S.Pengpanich,M.Hunsom,One-pot oxydehydration of glycerol to value-added compounds over metal-doped SiW/HZSM-5 catalysts:Effect of metal type and loading,Chem.Eng.J.275(2015)113-124.
[43]H.Gan,X.Zhao,B.Song,L.Guo,R.Zhang,C.Chen,J.Chen,W.Zhu,Z.Hou,Gas phase dehydration of glycerol to acrolein catalyzed by zirconium phosphate,Chin.J.Catal.35(2014)1148-1156.
[44]N.P.Rajan,G.S.Rao,V.Pavankumar,K.V.R.Chary,Vapour phase dehydration of glycerol over VPO catalyst supported on zirconium phosphate,Catal.Sci.Technol.4(2014)81-92.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
