Effect of thermodynamic parameters on prediction of phase behavior and process design of extractive distillation☆
Hui Jia ,Huixin Wang ,Kang Ma ,Mengxiao Yu ,Zhaoyou Zhu ,Yinglong Wang ,*
1 College of Chemical Engineering,Qingdao University of Science and Technology,Qingdao 266042,China
2 National Registration Center for Chemicals,SINOPEC Research Institute of Safety Engineering,Qingdao 266071,China
1.Introduction
Distillation is a common method for separating binary and multicomponent liquid mixtures.A conventional distillation process is used to separate general mixtures.Azeotrope-forming mixtures can be separated by special techniques such as pressure-swing distillation[1-4],azeotropic distillation[5-8],extractive distillation[9-15],adsorption[16-18],and pervaporation[19-22].Distillation can be effective,only if the compositions of the vapor and liquid phases that are in phase equilibrium with each other,are clearly different[23].Thus,a feasible way to achieve efficient separation is to increase the relative volatilities of the components,which can be realized by adding a third solvent to the binary system.The third component is commonly known as a solvent in extractive distillation,and an entrainer in azeotropic distillation.Extractive distillation[24,25]is widely used for separating azeotropes and other mixtures with a relative volatility of key compositions below 1.1.
Extractive distillation is one of the most efficient processes to separate azeotropic mixtures.Several researchers[26-29]have compared extractive distillation with other techniques,such as heteroazeotropic distillation,dividing-wall distillation,and pressure-swing distillation.Chenet al.[26]compared extractive and heterogeneous azeotropic distillations for the separation of the propylene-glycol methyl-ether/water system and found that extractive distillation was more effective,could significantly lower the stream cost by 39.7%,and could reduce the total annual cost(TAC)by 32.7%.Daiet al.[27]made a comparison between the extractive dividing-wall column and conventional extractive distillation,for TAC and dynamic performance.The results showed that although the extractive dividing-wall column technique could save 44.0%energy and 35.8%TAC,it was difficult to control.Luoet al.[28]investigated dynamic controls of extractive and pressure-swing distillation processes for separating a mixture of isopropyl alcohol/diisopropyl ether.Both processes could be used for the separation of the mixture,from the stand point of dynamic control stability.The above studies demonstrate the high efficiency of extractive distillation.
The accurate prediction of vapor-liquid equilibrium(VLE)plays a key role in distillation processes,and can identify the phase behavior.Based on the idea of maximum likelihood estimation[30],the thermodynamic model parameters can be fitted to the experimental dataviadata regression.VLE data can be regressed to obtain exact binary interaction parameters(BIPs)by the most frequently used thermodynamic models such as NRTL,UNIQUAC,and Wilson,ensuring process accuracy.In recent years,several authors have studied data regression through different thermodynamic models,to simulate and calculate binary mixtures.Nicolae and Oprea[31]applied regression analysis to VLE data of the dipropylene glycol/aromatic hydrocarbon binary system,using NRTL and UNIQUAC thermodynamic models,to get the BIPs,and calculated theT-x-ydiagrams from the resulting parameters.Liet al.[32]reported the isobaric VLE data for the binary cinnamaldehyde/benzaldehyde system at 10,20,and 30 kPa.The data were then regressed with Wilson and NRTL thermodynamic models,and compared to the group contribution model(UNIFAC).Regression results from the three models were in good agreement with the experimental data.
Most binary mixtures,consisting of water and alcohol,form azeotropes.Alcohols containing four or more carbons form a heterogeneous mixture with water.N-propanol is one of the simplest alcohols,and can form a minimum azeotrope with water.Since VLE data on thenpropanol/water binary system have been reported,the thermodynamic properties and phase behavior of the mixture can be easily studied[33-36].Gabaldónet al.[34]explored the isobaric VLE of then-propanol/water system at 30,60,100 kPa,and studied the effect of pressure on the behavior of the azeotrope.The BIPs were obtained by experimental data regression using different thermodynamic models,and mean absolute deviations were calculated.The effect of salt on the phase behavior of propanol-water has been studied by some researchers.Iliutaet al.[35]studied the behavior of then-propanol/water system after adding calcium chloride,and the VLE data were regressed using the NRTL,UNIQUAC,and Wilson models.Carlson[37]measured the liquid-liquid equilibrium(LLE)data for the ternary system 1-propanol(2-propanol)/water/potassium fluoride at different temperatures,and the results showed that potassium fluoride has a greater salting-out effect on 1-propanol than on 2-propanol.All the above studies have made contributions to determine the accurate BIPs.
Several researchers have reported that extractive distillation is an efficient method to separaten-propanol/water.Anet al.[38]separated the azeotrope ofn-propanol/water usingN-methyl-2-pyrrolidone(NMP)as solvent.Pla-Francoet al.[36]selected ethylene glycol as the solvent to separaten-propanol/water.Although the solvent and distillation process were not the same,NRTL was selected as the thermodynamic model for both extractive distillation processes.It should be noted that regressed NRTL model parameters were used by Pla-Franco,while Aspen Plus built-in NRTL model parameters were used by An.The phase behavior using regressed BIPs was significantly different than that using built-in parameters from the simulation software.The simulation results,with built-in BIPs,were inconsistent with the experimental results.
This paper explores the effect of thermodynamic parameters on the phase behavior ofn-propanol and water,and the extractive distillation process of separatingn-propanol and water using NMP and ethylene glycol as solvents.All the separation processes reported in this paper were simulated by Aspen Plus V8.4.Experimental data from published work was regressed to obtain the BIPs.The detailed separation process was based on optimally regressed parameters,to obtain accurate separation results.
2.Thermodynamic Model
2.1.Phase behavior
N-propanol is a common solvent,used in the study of VLE or LLE.Experimental results indicate that the binary mixture ofn-propanol/water is completely miscible.The mixture is a homogeneous azeotrope,according to Azeotropic Data[39].In an earlier study,NRTL was chosen as the thermodynamic method to separate the system,based on previous experience.The simulation results with the built-in BIPs showed that the mixture was partially miscible(see Table 1).A“Decanter Model”in Aspen Plus was employed to perform a simple split simulation test,at a temperature of 298.15 K,under atmospheric pressure,using the NRTL model.The simulation results showed that the mixture was homogeneous when then-propanol mole-concentration was less than 39.86%,and became heterogeneous when then-propanol mole-concentration was above 39.86%.In addition to the NRTL model,the UNIQUAC model built-in Aspen was also used to study then-propanol/water system,and the simulation results showed similar heterogeneous characteristics.

Table 1 VLE data for n-propanol(1)-water(2)at 0.1 MPa predicted by built-in NRTL binary interaction parameters in Aspen Plus
Another phenomenon of interest is that the azeotropic composition ofn-propanol initially increases,and then decreases,with increasing pressure(see Fig.1),which is an unusual azeotropic behavior.Whether this is due to the special behavior ofn-propanol/water,or due to improper thermodynamic parameters,needs further study.
2.2.Thermodynamic consistency test
The isobaric and isothermal VLE data were obtainedviathe NIST data-bank in Aspen Plus V8.4,which contains almost all thenpropanol/water system-data published from 1910 to 2008.Although more than 20 sets of data have been published,the error in the experimental process is multifaceted,and cannot be completely avoided,requiring the user to judge whether the data is available.

Fig.1.Azeotropic composition and temperature predicted by built-in NRTL model under different pressure(1 atm=101325Pa).
Thermodynamic consistency tests were performed first to determine the VLE-data reliability.There are several methods to check the consistency of thermodynamics,such as the area test,point-to-point test,Herington test,Redlich-Kister test,and the Van Ness point-to-point test[40,41].However,the present state of knowledge indicates that no de finite test is available for thermodynamic consistency of VLE data.Five sets of experimental data[34,35,42],which were complete and passed the Herington test successfully,were selected as the typical data.The Herington test is an integral test based on the Gibbs-Duhem equation,

where γ is the activity coefficient,andxis the mole fraction of the liquidphase component.HEandVEare the excess enthalpy and volume,respectively,in the mixing process.The consistency of thermodynamic results was checked by the Herington semi-empirical analysis method.The value ofD-J,calculated by the following equations,must be less than 10:

TmaxandTminare the highest and lowest boiling points,respectively,in the binary systems(k).The test results for thermodynamic-consistency of the binary-system VLE,for five sets of typical data,are listed in Table 2 and Fig.2.

Table 2 The Herington test results for thermodynamic consistence of five sets of typical data
Many consistency test methods are provided in Aspen Plus,such as the area test,the Van Ness point-to-point test,and the in finite dilution test.Five sets of experimental data[34,35,42]were selected as the typical data.Four methods of consistency tests,the area test,the Van Ness point-to-point test,and the in finite dilution test,were used to check thermodynamic consistency of the typical data by Aspen Plus.The test results are shown in Table 3.
2.3.Data regression
To obtain precise separation results,the BIPs were regressed using experimental data from the NIST data bank in Aspen Plus V8.4.The accuracy of simulated results was estimated from the fitness of the thermodynamic property model parameters.Simulation and experimental results for then-propanol/water system were different,which indicated that the NRTL thermodynamic model,with builtin BIPs,cannot describe the phase behavior of then-propanol/water azeotropic system accurately.The built-in thermodynamic models such as NRTL,UNIQUAC,and Wilson can predict the liquid-phase nonideality,and have been tested for prediction results.With the built-in BIPs in Aspen Plus,however,the three models could not predict then-propanol/water system precisely.Hence,the BIPs,which were obtained using the“Data Regression”function in Aspen Plus with the experimental VLE data,were used to predict the most suitable thermodynamic property model.
The typical data were regressed using the NRTL,UNIQUAC,and Wilson models.The result-comparison was expressed by root mean square deviation(RMSD)between the experimental and calculated composition.RMSD is a frequently used measure that represents the sample standard-deviation of the differences between predicted and observed values.The formula is as follows:

(wherenis the total number of points,andMexpandMcalrepresent experimental and calculated values,respectively).
The RMSD was calculated to test the fitting-degree of the estimated and experimental data.Fig.3 shows the calculation results from the five sets of data,with the three thermodynamic models.The UNIQUAC model,and the third set of experimental data,had the smallest RMSD.To further verify the exactness of the UNIQUAC model,the third set of data were regressed by the UNIQUAC,NRTL,and Wilson models,and the azeotropic composition,temperature,and parameters were predicted for then-propanol-water system.The results are listed in Table 4.
Due to its superiority,UNIQUAC was selected as the thermodynamic model for the separation process,and the BIPs were obtained by regressing the third set of data.The optimal BIPs forn-propanol/water are shown in Table 5.Fig.4 demonstrates thatn-propanol/water formed a homogeneous azeotrope,indicating that the regressed thermodynamic parameters were feasible.
3.Process Design
3.1.Extractive distillation process with NMP as solvent
NMP was chosen as the solvent for the separation ofn-propanol/water,as discussed in a previous publication[38].The extractive distillation process included the preconcentration-distillation,extractive distillation,and solvent-recovery columns.The feed composition included 20 mol%n-propanol under the condition of saturated liquid for the preconcentration-distillation column,and the distillated composition feeding into the extractive-distillation column was 40 mol%n-propanol.The product purities were specified to 99.9 mol%.The operating pressure of the three columns was 0.1 MPa.The solvent flow-rate was approximately 330 kmol·h-1,and the deviation was adjusted according to the separation results.
For then-propanol/water system,the built-in BIPs predicted the phase behavior correctly,hence the VLE experimental data was regressed to obtain the accurate parameters.It was also necessary to investigate the accuracy of the built-in BIPs for both the NMP/water and NMP/n-propanol systems.For the case where the built-in parameters predicted the phase behavior correctly,they were used for the simulation.Many VLE data for NMP/water and NMP/n-propanol systems have been reported,but not every group of data reflects the phase behavior accurately.Due to the existence of experimental errors,some of the data cannot pass the thermodynamic consistency test.Experimental data reported in the literature[43-46],which passed the consistency test successfully,were selected as the typical data,and regressed to obtain two sets of BIPs for each system.Fig.5a shows the fitting results of VLE data,predicted by regressed parameters based on literature,and built-in parameters for the NMP/water system.Both sets of data passed the consistency test,but the fitting results were quite different.The prediction results from the built-in parameters were located between the two regression parameters.The average absolute deviation in vapor phase composition(AADy)was calculated between the VLE data predicted by the built-in,and the experimental data[44,45],to verify the accuracy of the built-in parameters.The formula is as follows:

Fig.2.Diagram of lnγ1 and lnγ2 to x1 for the five typical sets of data for n-propanol(x1)and water(x2)system.

The AADy results are presented in Table 6.Fig.5b shows the fitting results of VLE data predicted by built-in parameters,as reported in the literature[43,46]for the NMP/n-propanol system.The difference between the data predicted by the built-in parameters and experimental data was small,which implied that the built-in parameters were satisfactory.Hence,the built-in parameters were adopted in the simulation processes,except for the binaryn-propanol/water system.UNIQUAC was chosen as the optimal thermodynamic model.

Table 3 Thermodynamic consistence test results of five typical sets of data checked by different methods in Aspen Plus
The sequential iterative optimization procedure[47-49]was adopted to optimize the process based on minimum TAC,which is a common,objective function to estimate economics.The detailed calculation method for TAC has been described previously[50],the difference being that there were three columns in the current study.Both the processes with built-in and regressed BIPs were studied,and the TAC was calculated(see Table 7).A large deviation existed in the TAC with different BIPs.Detailed process-information,with built-in and regressed parameters,is shown in Fig.6.For the number of theoretical stages and feed locations in the distillation column,the calculation results using the built-in and the regression parameters were identical,but there were significant differences in the re flux ratio and diameter.The reboiler duties of the process,with the built-in and regressed BIPs,were 8775.9 and 10484.6 kW,and the condenser duties of the two processes were 8722.7 and 9680 kW,respectively.The energy consumption of the process using regression parameters,was 1.2 times that of the process with built-in parameters.The TAC calculated for the two processes was 2901988 and 3417849 USD·a-1,respectively.
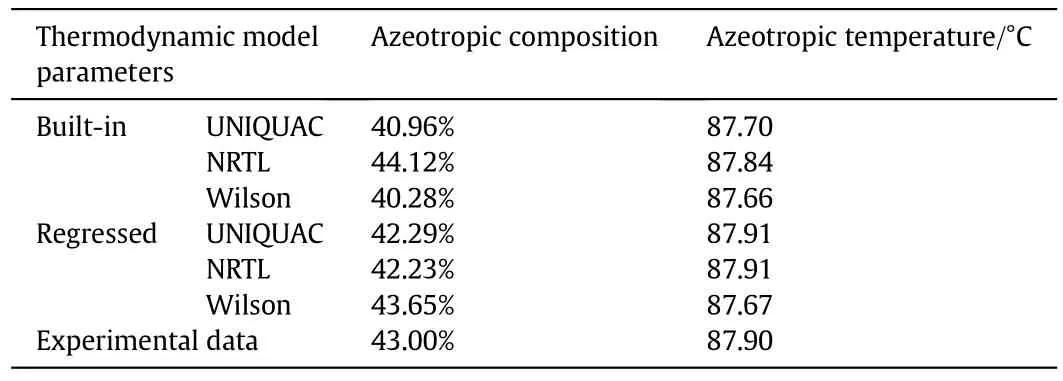
Table 4 The azeotropic composition and temperature predicted by UNIQUAC,NRTL and Wilson thermodynamic model and parameters for n-propanol-water system
3.2.Extractive distillation process with ethylene glycol as solvent

Fig.3.The calculating results about the five sets of data.

Table 5 The regressed binary interaction parameters of n-propanol(i)and water(j)

Fig.4.The VLE data predicted by regressed UNIQUAC model and binary interaction parameters for n-propanol and water azeotrope(1 atm=0.1 MPa).
Pla-Francoet al.[36]applied regression analysis to the VLE data using the NRTL,UNIQUAC,and Wilson thermodynamic models to get the BIPs,and concluded that the NRTL thermodynamic model provided the best prediction forn-propanol/water.The data reported by Pla-Franco were regressed in this study,but the data predicted by the regressed parameters were quite different from the experimental data and could not be used.Through the regression of five typical sets of data,UNIQUAC was determined to be the appropriate thermodynamic model.To verify the reliability of the results mentioned above,a comparison was conducted between the UNIQUAC parameters regressed in this study,and the NRTL parameters regressed by Pla-Franco.Fig.7 shows the fitting of the two regression parameters to the experimental data at0.1 MPa.The azeotropic temperature calculated by the regressed NRTL was 361.34 K,which was higher than the experimental data.Theregressed UNIQUAC parameters were closer to the experimental data,which proved that it was the optimal thermodynamic model for the system.

Table 6 The results of AADy between the data predicted by built-in parameters and experimental data reported in different literatures

Table 7 The calculating results of TAC for different processes using UNIQUAC as the thermodynamic model

Fig.5.The fitting results of NMP-water with UNIQUAC thermodynamic model.Literature data 1 “■”;calculated data:“ ”predicted by regressed parameters based on literature data1,“---”predicted by regressed parameters based on literature data 2,“▬”predicted by built-in parameters.

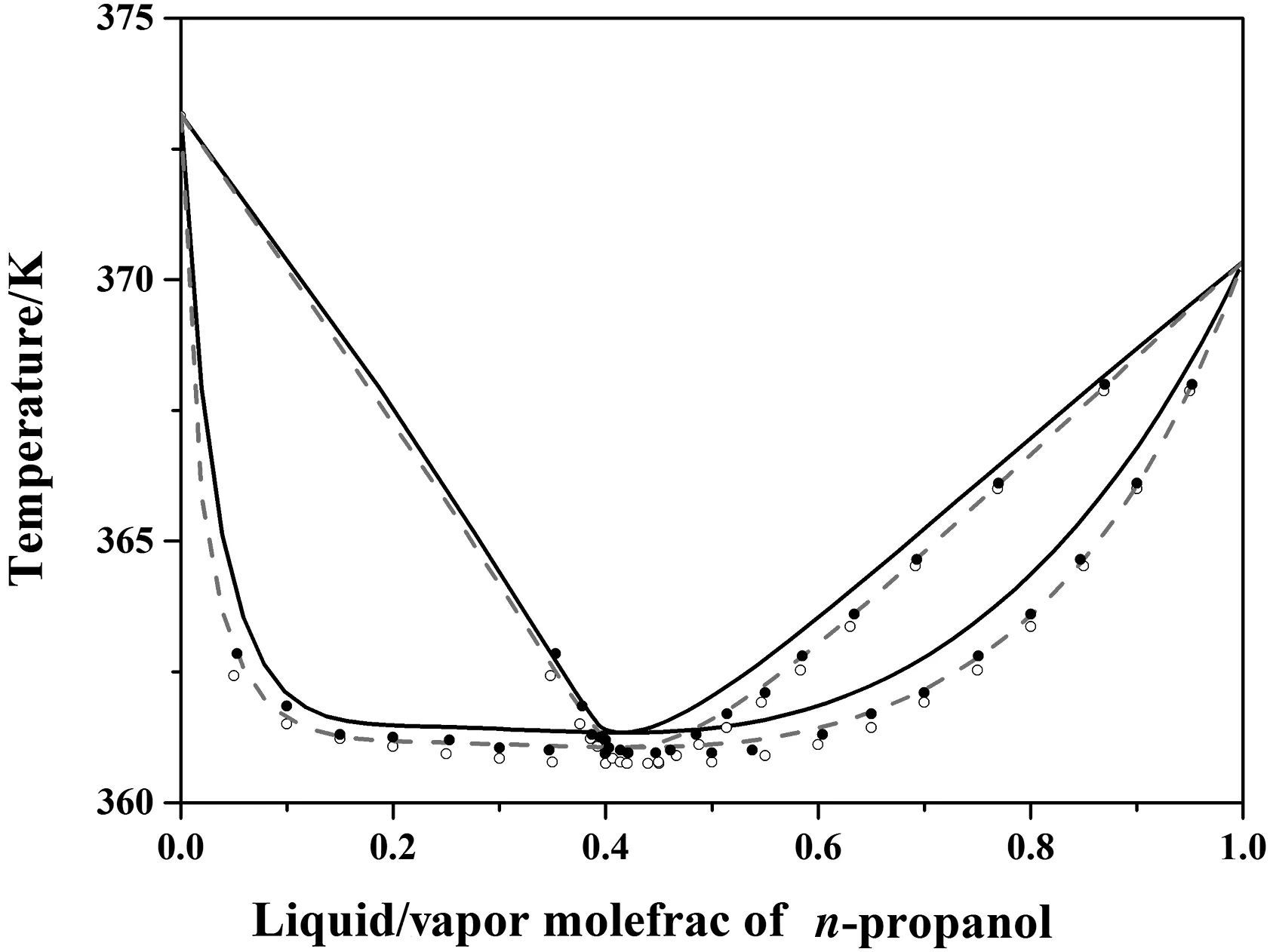
Fig.7.Experimental and calculated VLE diagram for the system of n-propanol and water at 101.32 kPa.“●”means experimental data from Maria C[35];“○”means experimental data from Kojima[42];“▬”means the data calculated by regressed NRTL parameters,“---”means the data calculated by regressed UNIQUAC parameters.
Pla-Francoet al.studied the separation process by the commercial software Aspen Hysys,while Aspen Plus was used in the current study.The feed flow rate was 1500 kg·h-1,and included 68.98 wt%npropanol and 31.02 wt%water,similar to the Pla-Francoet al.study.The initial feed temperature was 308.15 K,and the operating pressure was 101.3 kPa.The reboiler duty of the solvent recovery column was used to heat the initial feed.The solvent-to-feed ratio was approximately 5.5.The process with built-in BIPs was first simulated using Aspen Plus.Another process adopted regressed thermodynamic parameters for then-propanol/water system.The parameters of ethylene glycol/water and ethylene glycol/n-propanol were set as default.The fitting results are shown in Fig.8[36,51-53]and Table 6,and indicate that the VLE of ethylene glycol/water and ethylene glycol/n-propanol were predicted well by the built-in parameters.
TAC was calculated for the two processes.The sequential iterative optimization procedure was also used to optimize the processes.Table 6 gives the optimization results.The TAC for the process with regressed thermodynamic parameters was lower than that for the process with built-in parameters.Detailed information for two processes with different BIPs is given in Fig.9.There were significant deviations in the simulated results obtained from the regressed and the built-in BIPs.Both operating and capital costs for the process with regressed parameters were lower than those using the built-in parameters.The energy cost for the process using regressed parameters was only 90.8%of the cost using built-in parameters.
4.Guidelines
Engineering design requires equipment that considers the phase behavior of the system to be separated[54].The phase miscibility and azeotropy should always be checked before proceeding with a distillation design.To design a distillation scheme,the first step is to exactly identify the phase behavior of the mixture to be separated.Phase behavior is the mathematical relation between pressure,temperature,and composition,and includes the relationships between the pressure and composition(p-xy),temperature and composition(T-xy),and solubility and azeotropy.Experimental data,which have been published extensively,are the most important information to grasp the phase behavior of mixtures.However,due to experimental error,thermodynamic consistency tests need to be performed to ensure the VLE-data reliability.The VLE data that passes the thermodynamic consistency tests should be selected as the typical data,as a standard of comparison.If no VLE data passes the thermodynamic consistency tests,the users need to do experiments themselves to study the phase miscibility/splitting and azeotropic data,until reliable VLE data are available.The thermodynamic models provided by simulation software are used to predict the phase behavior of the separation system.If there are multiple models that can predict the phase behavior correctly,it is necessary to fit the experimental data to the thermodynamic models,and to calculate the RMSD and azeotropic data.The model with the minimum RMSD is optimal,and the built-in interaction parameters are satisfactory.If there are no models that can predict the phase behavior correctly,the experimental data need to be regressed by different thermodynamic models,and the RMSD between the experimental data and that predicted by regressed interaction parameters needs to be calculated.The model with the minimum RMSD is selected,and the regressed interaction parameters are used in subsequent process simulations.
5.Conclusions
Water andn-propanol formed a homogeneous azeotrope,but the heterogeneous behavior obtained from the built-in parameters was contrary to the experimental data.This demonstrated that the built-in thermodynamic parameters were unsuitable for simulation of then-propanol/water system separation.UNIQUAC was selected as the thermodynamic model,according to the calculation of the RMSD using published experimental data.

Fig.9.The optimal process with built-in binary and regressed(the values in brackets)interaction parameters using ethylene glycol as solventa(1 atm=101325Pa).a:Composition data are mass fraction.
Extractive distillation processes,with built-in and regressed BIPs,were explored to separate the azeotrope by the Aspen Plus simulation software.Compared with the processes with built-in BIPs,the two processes with regressed parameters showed different optimal parameters,including the number of theoretical stages,locations of feed,re flux ratio,etc.The TAC,for the extractive distillation process with ethylene glycol as the solvent,using regressed parameters,was 90.2%of the process using built-in parameters.Conversely,the TAC for the process using regressed parameters was 1.2 times that of the process using built-in parameters,when NMP was selected as solvent.The differences in the simulation using different thermodynamic parameters show the importance of BIPs in the design and optimization of extractive distillation.
Accurate thermodynamic parameters are the basis for achieving simulation results as close as possible to actual industrial production.Simulation results from inaccurate parameters can mislead engineers into making wrong decisions.For some systems,such as NMP/water,the BIPs built into the simulation software can accurately predict the actual phase behavior,while for others,such asn-propanol/water,they cannot.For the systems that cannot be accurately predicted by the built-in parameters,it is necessary to regress the experimental VLE data,to obtain parameters that can ensure the accuracy of simulation results.
[1]A.M.Fulgueras,J.Poudel,D.S.Kim,J.Cho,Optimization study of pressure-swing distillation for the separation process of a maximum-boiling azeotropic system of water-ethylenediamine,Korean J.Chem.Eng.33(2016)46-56.
[2]Z.Zhu,D.Xu,X.Liu,Z.Zhang,Y.Wang,Separation of acetonitrile/methanol/benzene ternary azeotropeviatriple column pressure-swing distillation,Sep.Purif.Technol.169(2016)66-77.
[3]Y.Wang,Z.Zhang,D.Xu,W.Liu,Z.Zhu,Design and control of pressure-swing distillation for azeotropes with different types of boiling behavior at different pressures,J.Process Control42(2016)59-76.
[4]S.Liang,Y.Cao,X.Liu,X.Li,Y.Zhao,Y.Wang,Y.Wang,Insight into pressure-swing distillation from azeotropic phenomenon to dynamic control,Chem.Eng.Res.Des.117(2016)318-335.
[5]A.A.Kiss,J.David,P.Suszwalak,Enhanced bioethanol dehydration by extractive and azeotropic distillation in dividing-wall columns,Sep.Purif.Technol.86(2012)70-78.
[6]S.-J.Wang,D.S.Wong,Online switching of entrainers for acetic acid dehydration by heterogeneous azeotropic distillation,J.Process Control23(2013)78-88.
[7]I.L.Chien,K.-L.Zeng,H.-Y.Chao,J.H.Liu,Design and control of acetic acid dehydration systemviaheterogeneous azeotropic distillation,Chem.Eng.Sci.59(2004)4547-4567.
[8]X.Huang,W.Zhong,W.Du,F.Qian,Thermodynamic analysis and process simulation of an industrial acetic acid dehydration systemviaheterogeneous azeotropic distillation,Ind.Eng.Chem.Res.52(2013)2944-2957.
[9]H.Wang,Y.Li,W.Su,Y.Zhang,J.Guo,C.Li,Design and control of extractive distillation based on effective relative gain array,Chem.Eng.Technol.39(2016)2339-2347.
[10]I.Rodriguezdonis,V.Gerbaud,X.Joulia,Thermodynamic insights on the feasibility of homogeneous batch extractive distillation.4.azeotropic mixtures with intermediate boiling entrainer,Ind.Eng.Chem.Res.51(2012)6489-6501.
[11]Y.Wang,P.Cui,Y.Ma,Z.Zhang,Extractive distillation and pressure-swing distillation for THF/ethanol separation,J.Chem.Technol.Biotechnol.90(2015)1463-1472.
[12]X.You,I.Rodriguez-Donis,V.Gerbaud,Low pressure design for reducing energy cost of extractive distillation for separating diisopropyl ether and isopropyl alcohol,Chem.Eng.Res.Des.109(2016)540-552.
[13]W.L.Luyben,Control comparison of conventional and thermally coupled ternary extractive distillation processes,Chem.Eng.Res.Des.106(2016)253-262.
[14]E.Lladosa,J.B.Montón,M.Burguet,Separation of di-n-propyl ether andn-propyl alcohol by extractive distillation and pressure-swing distillation:Computer simulation and economic optimization,Chem.Eng.Process.50(2011)1266-1274.
[15]Y.Wang,S.Liang,G.Bu,W.Liu,Z.Zhang,Z.Zhu,Effect of solvent flow rates on controllability of extractive distillation for separating binary azeotropic mixture,Ind.Eng.Chem.Res.54(2015)12908-12919.
[16]Y.Y.Loy,X.L.Lee,G.P.Rangaiah,Bioethanol recovery and purification using extractive dividing-wall column and pressure swing adsorption:An economic comparison after heat integration and optimization,Sep.Purif.Technol.149(2015)413-427.
[17]G.Lei,P.Mao,M.He,L.Wang,X.Liu,A.Zhang,Combination of column adsorption and supercritical fluid extraction for recovery of dissolved essential oil from distillation waste waterofYulania liliiflora,J.Chem.Technol.Biotechnol.91(2016)1896-1904.
[18]Y.Y.Loy,X.L.Lee,G.P.Rangaiah,Optimization and economic evaluation of bioethanol recovery and purification processes involving extractive distillation and pressure swing adsorption,Comput.Aided Chem.Eng.37(2015)413-418.
[19]D.Cai,S.Hu,Q.Miao,C.Chen,H.Chen,C.Zhang,P.Li,P.Qin,T.Tan,Two-stage pervaporation process for effective in situ removal acetone-butanol-ethanol from fermentation broth,Bioresour.Technol.224(2017)380-388.
[20]J.Fontalvo,J.T.F.Keurentjes,A hybrid distillation-pervaporation system in a single unit for breaking distillation boundaries in multicomponent mixtures,Chem.Eng.Res.Des.99(2015)158-164.
[21]Y.T.Ong,S.H.Tan,Pervaporation separation of a ternary azeotrope containing ethyl acetate,ethanol and water using a buckypaper supported ionic liquid membrane,Chem.Eng.Res.Des.109(2016)116-126.
[22]M.T.Del Pozo Gomez,J.-U.Repke,D.-y.Kim,D.R.Yang,G.N.Wozny,Reduction of energy consumption in the process industry using a heat-integrated hybrid distillation pervaporation process,Ind.Eng.Chem.Res.48(2009)4484-4494.
[23]W.L.Luyben,Distillation Design and Control Using Aspen Simulation,John Wiley&Sons,New Jersey,2013.
[24]Q.Wang,B.Yu,C.Xu,Design and control of distillation system for methylal/methanol separation.Part 1:Extractive distillation using DMF as an entrainer,Ind.Eng.Chem.Res.51(2012)1281-1292.
[25]J.D.Seader,E.J.Henley,Separation Process Principles,2nd edition John Wiley&Son,New Jersey,2011.
[26]Y.C.Chen,B.Y.Yu,C.C.Hsu,I.L.Chien,Comparison of heteroazeotropic and extractive distillation for the dehydration of propylene glycol methyl ether,Chem.Eng.Res.Des.111(2016)184-195.
[27]D.Xin,Q.Ye,J.Qin,Y.Hao,X.Suo,L.Rui,Energy-saving dividing-wall column design and control for benzene extraction distillationviamixed entrainer,Chem.Eng.Process.100(2015)49-64.
[28]H.Luo,L.Kai,W.Li,L.Ye,X.Ming,C.Xu,Comparison of pressure-swing distillation and extractive distillation methods for isopropyl alcohol/diisopropyl ether separation,Ind.Eng.Chem.Res.53(2014)15167-15182.
[29]Y.Wang,Z.Zhang,Y.Zhao,S.Liang,G.Bu,Control of extractive distillation and partially heat-integrated pressure-swing distillation for separating azeotropic mixture of ethanol and tetrahydrofuran,Ind.Eng.Chem.Res.54(2015)8533-8545.
[30]M.Teodorescu,K.Aim,I.Wichterle,Isothermal vapor-liquid equilibrium in the quaternary water+2-propanol+acetic acid+isopropyl acetate system with chemical reaction,J.Chem.Eng.Data46(2001)261-266.
[31]M.Nicolae,F.Oprea,Vapor-liquid equilibrium for the binary mixtures of dipropylene glycol with aromatic hydrocarbons:Experimental and regression,Fluid Phase Equilib.370(2014)34-42.
[32]H.Li,M.Han,X.Gao,X.Li,Isobaric vapor-liquid equilibrium for binary system of cinnamaldehyde+benzaldehyde at 10,20 and 30 kPa,Fluid Phase Equilib.364(2014)62-66.
[33]A.F.Cristino,S.Rosa,P.Morgado,A.Galindo,E.J.M.Filipe,A.M.F.Palavra,C.A.N.D.Castro,High-temperature vapour-liquid equilibrium for the(water+alcohol)systems and modelling with SAFT-VR:2.Water-1-propanol,J.Chem.Thermodyn.60(2013)15-18.
[34]C.Gabaldón,P.Marzal,J.B.Montón,M.A.Rodrigo,Isobaric vapor-liquid equilibria of the water+1-propanol system at 30,60,and 100 kPa,J.Chem.Eng.Data41(1996)1176-1180.
[35]M.C.Iliuta,F.C.Thyrion,O.M.Landauer,Effect of calcium chloride on the isobaric vapor-liquid equilibrium of 1-propanol+water,J.Chem.Eng.Data41(1996)402-408.
[36]J.Pla-Franco,E.Lladosa,S.Loras,J.B.Montón,Approach to the 1-propanol dehydration using an extractive distillation process with ethylene glycol,Chem.Eng.Process.91(2015)121-129.
[37]E.C.Carlson,Don't gamble with physical properties for simulations,Chem.Eng.Process.92(1996)35-46.
[38]Y.An,W.Li,Y.Li,S.Huang,J.Ma,C.Shen,C.Xu,Design/optimization of energysaving extractive distillation process by combining preconcentration column and extractive distillation column,Chem.Eng.Sci.135(2015)166-178.
[39]J.Gmehling,Azeotropic Data,Wiley-VCH,Wein-heim,Germany,2004.
[40]J.Wisniak,J.Ortega,L.Fernández,A fresh look at the thermodynamic consistency of vapour-liquid equilibria data,J.Chem.Thermodyn.105(2017)385-395.
[41]J.Gmehling,B.Kolbe,M.Kleiber,J.Rarey,Chemical Thermodynamics for Process Simulation,Wiley-VCH,Wein-heim,Germany,2012.
[42]J.N.Im,C.Gwak,Determination of vapor liquid equilibrium from boiling point curve,Korean Chem.Eng.Res.19(1981)681-687.
[43]P.Gierycz,M.Rogalski,S.Malanowski,Vapour—liquid equilibria in binary systems formed byN-Methylpyrrolidone with hydrocarbons and hydroxyl derivatives,Fluid Phase Equilib.22(1985)107-122.
[44]L.J.Ping,P.Yong,J.W.Mao,Vapor-liquid equilibria of acetic acid-water-N-methylpyrrolidone system at 26.67 kPa,J.Chem.Eng.Chin.Univ.25(2011)554-558.
[45]Z.Cui,Z.Li,Z.Gao,J.Li,Vapor-liquid equilibria ofN-methylpyrrolidone(1)-water(2)binary system by an ebulliometer,Chin.J.Chem.Eng.2(1994)119-124.
[46]L.Ping,Y.Peng,J.Mao,Vapor-liquid equilibria of acetic acid-water-N-methylpyrrolidone system at 26.67 kPa,Chin.J.Chem.Eng.25(2011)554-558.
[47]E.Hosgor,T.Kucuk,I.N.Oksal,D.B.Kaymak,Design and control of distillation processes for methanol-chloroform separation,Comput.Chem.Eng.67(2014)166-177.
[48]M.Piccolo,P.Douglas,P.Lee,Data reconciliation using AspenPlus,Dev.Chem.Eng.Miner.Process.4(1996)157-182.
[49]Y.-C.Wu,H.-Y.Lee,C.-H.Lee,H.-P.Huang,I.-L.Chien,Design and control of thermally-coupled reactive distillation system for esterification of an alcohol mixture containingn-amyl alcohol andn-hexanol,Ind.Eng.Chem.Res.52(2013)17184-17197.
[50]Y.Cao,M.Li,Y.Wang,T.Zhao,X.Li,Z.Zhu,Y.Wang,Effect of feed temperature on economics and controllability of pressure-swing distillation for separating binary azeotrope,Chem.Eng.Process.110(2016)160-171.
[51]N.Kamihama,H.Matsuda,K.Kurihara,K.Tochigi,S.Oba,Isobaric vapor-liquid equilibria for ethanol+water+ethylene glycol and its constituent three binary systems,J.Chem.Eng.Data57(2012)339-344.
[52]G.F.Qian,W.Liu,L.T.Wang,D.C.Wang,H.Song,(Vapour+liquid)equilibria in the ternary system(acetonitrile+n-propanol+ethylene glycol)and corresponding binary systems at 101.3 kPa,J.Chem.Thermodyn.67(2013)241-246.
[53]X.Xu,W.Liu,M.Li,Y.Ri,Y.Wang,Ternary liquid-liquid equilibrium of azeotropes(ester+alcohol)with different ionic liquids at T=298.15 K,J.Chem.Eng.Data62(2017)532-538.
[54]D.P.Tassios,Applied Chemical Engineering Thermodynamics,Springer,Berlin Heidelberg,1989.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
