Effect of the chelating agents on bio-sorption of hexavalent chromium using Agave sisalana fibers
H.Bendjeffal*,A.Djebli,H.Mamine ,T.Metidji,M.Dahak ,N.Rebbani,Y.Bouhedja
1 Higher School of Technological Education,ENSET-Skikda,21000,Algeria
2 Laboratory of Water Treatment and Valorization of Industrial Wastes,Badji-Mokhtar University,P.O.Box 12,Annaba 23000,Algeria
3 Centre de Recherche Scientifique,et Technique en Analyses Physico-Chimiques,BP 384,Siège ex-Pasna Zone Industrielle,Bou-Ismail CP 42004,Tipaza,Algeria
1.Introduction
The elimination of chemical hazards(radioactive elements,textile dyes,heavy metals,and their derivatives)from aquatic environment became an essential environmental process to protect our life.The large diffusion of these pollutants is due essentially to the great development of the main human industrial activities[1-3].Many industrial processes(manufacture of steel,leather,textiles…)throw liquid and solid wastes contaminated by a lot of heavy metals,such as mercury,chromium,and cadmium[4-5].These metals became one of the mosttoxically compounds due to their detrimental effect on the environment and ecosystems,causing damage to the gills of aquatic organisms and disrupting their spawning sites and refuges.The toxicity of these compounds is enhanced through bioaccumulation and they can be transported and released elsewhere,in water sources,sediments,and foods[6-8].
The hexavalent chromium is one of the most toxic metals;people can be exposed to this element by breathing,eating,drinking or by direct contact with chrome compounds[9].This element is hazardous to health,especially for those working in steel and textile industries.Smokers also have a greater risk of exposure to chromium.It is known that chromium(VI)has various health consequences.In leather factories,it can cause allergic reactions,such as rashes[10]and inhaling it can cause nasal irritation and nosebleeds.Chromium(VI)may have other consequences(rashes,stomach upset and ulcers,respiratory problems,immune system weakness liver and kidney damage,alteration of genetic material,lung cancer,death)[9-10].Considering the hexavalent chromium carcinogenic nature,the contamination level limit for Cr(VI)in domestic water supplies is 0.05 mg·L-1.Its concentration in industrial wastewaters ranges from 0.5 to 270 mg·L-1.The tolerance limit for Cr(VI)for discharge into inland surface waters is 0.1 mg·L-1and in potable water is 0.05 mg·L-1[11,12].
Several practical physicochemical techniques have been developed to remove hexavalent chromium from wastewater,including solid phase extraction[13],adsorption[5,14],photocatalyzed reduction[15],chemical precipitation[16],ion exchange[17],reverse osmosis[18],and electrokinetic technique[19].However,many of these procedures are too costly,especially when used for treating large waste streams.However,the bio-sorption using natural adsorbents such as activated carbon,natural clays,marine algae,and plant fibers is among the most practical,green,and economical techniques used for the removal of chromium and their derivatives from aqua mediums[5].
In the past 20 years,natural fibers such as Jute,Hemp,Kapok,andAgave sisalanabecame among the most and low-cost bio-adsorbents,due to their interesting properties,such as surface morphology,good surface area,wide-range porosity,high gas permeability,good diameters,and smallinter- fibrous pore size[20].However,few studies related to the removal of heavy metals using these materials make reference to the bio-sorption of Cr(IV)usingA.sisalanafibers(Fig.1.).
Recently,great efforts have been devoted to improving the adsorptive properties of these fibers by essential physicochemical modifications of their surface.E.Padminiet al.[21]treated the sisal using sodium carbonate for the removal of nickel from aqueous solutions;whereas,T.Hajeethet al.[22]modified the surface of sisal fibers using acrylic acid to remove Cr(VI)from aqueous solution.The main objective of the present study is the removal of chromium(IV)from aqueous solution using sisal fibers as a bioadsorbent.Hence,in order to obtain a suitable removal of this toxic metal,we treated the natural fibers with various chelating agents such as urea(UR),thiocarbamide(TC),diphenyl carbazide(DCZ),and ethylenediaminetetraacetic acid(EDTA);the high chelating properties of these ligands,can improve the adsorptive capacity of the treated sisal fibers for the removal of this toxic element from aqueous medium.
The bio-sorption mechanism was studied under the effects of some physicochemical factors such as the nature of the chelating agents(F@UR,F@TC,F@EDTA,and F@DCZ), fiber amount,contact time,solution pH,Cr(VI)initial concentration,and medium temperature.The bio-sorption thermodynamic parameters such as free energy(ΔG⊖),enthalpy(ΔH⊖),and entropy(ΔS⊖),have been determined at a different temperature.
2.Experimental
The usedA.sisalananatural fibers were perched from local farms.All solutions were prepared from analytical grade reagent(sodium dichromate,urea,thiocarbamide,ethylenediaminetetraacetic,and diphenyl carbazide).A stock standard solution of chromium(VI)was prepared by dissolving 500 mg of sodium dichromate Na2Cr2O7(Merck)in 1 L of double distilled water.The medium pH was adjusted using hydrochloric acid and sodium hydroxide solution(0.1 mol·L-1).
2.1.Preparation of adsorbents
The sisal natural fibers were chopped in small pieces of size approximately 10 mm and sieved using the appropriate mesh sieve.The collected fiber samples were cleaned at room temperature using nitric acid solution(0.1 mol·L-1),and with hydrogen peroxide(10%)for 1 h then,oven dried at 80°C for 24 h.The cleaned sisal fibers were treated using the various chelating agents,under stirring at room temperature for 48 h as shown in Fig.2.The treated fibers were then washed several times with double distilled water,centrifuged at 3000 r·min-1,and oven dried again for 24 h at 80 °C.The treated fibers have been characterized by infrared spectroscopy.
2.2.Batch adsorption studies

Fig.1.(a)Agave sisalana,(b)chopped sisal fibers,and(c)SEM image of their surface.

Fig.2.Bio-sorption mechanism of Cr(VI)on treated sisal fibers with chelating agents;(F@N)natural fibers,(F@DCZ) fibers charged with diphenyl carbazide,and F@DCZ@Cr(VI) fibers charged with chromium(VI).
The bio-sorption experiments were made in batch method under the effect of some physicochemical factors.All sorption experiments were realized in 100 ml flasks by mixing 50 ml of Cr(VI)aqueous solution with the corresponding amount of fibers(2-10 g·L-1),under stirring in a thermostatic bath.After the appropriate interaction time(0-120 min),the mixture was centrifuged at 3000 r·min-1.
The concentration of unadsorbed Cr(VI)was determined by UV-Vis spectrophotometry at λmax=540 nm using diphenyl carbazide as a complexing agent in an acidic medium(pH 2).Therefore,the adsorbed amount of chromium(VI)on used bio-adsorbents was calculated using the following equation[5]:

where,qeis the adsorbed amount(mg·g-1),C0andCeare respectively the initial and the equilibrium concentrations of Cr(VI)(mg·L-1),Vis the volume of the solution(L),andmis the amount of the used bioadsorbent(g)[5].
3.Results and Discussion
3.1.Characterization of the adsorbents
The naturalsisal fibers were constituted essentially of cellulose(74%),lignin(12%),hemicellulose(11%),and pectin(2%).The surface area of this adsorbentwas measured using the BET method.The obtained results show that the sisal fibers have a good specific surface area of 0.0301 m2·g-1.Therefore,the treatment of the used fibers by different chelating agents has been investigated using infrared spectroscopy(Shimadzu 8700 FTIR spectrometer)in the range 400 cm-1-4000 cm-1.Fig.3 shows typical FTIR spectra of the different fiber samples.FTIR spectrum of untreated fibers shows a large peak between 3000 cm-1and 3600 cm-1corresponding to the hydroxyl vibration(υ—OH).The peak at 1500-1750 cm-1was attributed to the stretching of carboxylic function(υ--C=O).However,spectra of treated fibers show the presence of new peaks due to the appearance of functional groups of the used chelator agent(--NH2,--C=O,--C=S,--C--NH…).In this case,the bands observed at 900-1125 cm-1were assigned to vibration bands of amino functions(υ--NH2and υ--C--NH--)and the ones observed at 1250-1500 cm-1can be assigned to the--C=S group.However,the F@UR spectra show a vibration band between 2000 and 2300 cm-1corresponding to the isocyanate function(υ--N=C=O)generated by the reaction between urea and the sisal surface.In addition,the frequency change observed for functional groups of the sisal fibers may be due to the interaction between the chelator agent and fiber surface.

Fig.3.The infrared spectrum of untreated and treated sisal fibers with various chelating agents,(a)F@N,(b)F@UR,(c)F@TC,(d)F@DCZ,and(e)F@EDTA.
The Scanning electron microscope is known to be the most important instrument in studying the surface morphology of adsorbent materials such as zeolites,clays,and fibers by direct two dimensional surface imaging.Fig.4 displays typical SEM images of the surface morphology of natural and treated sisal fibers.Fig.4(b)and(c)shows that the treatment with urea and thiocarbamide generates a small grain of the immobilized chelating agent on the surface of the treated fibers,with grain sizes in the range 1 μm-3 μm.However,the treatment with DCZ and EDTA generates a thin layer on all the surface of the treated fibers as shown in Fig.4(d)and(e).
3.2.Effect of the chelating agent on bio-sorption kinetic
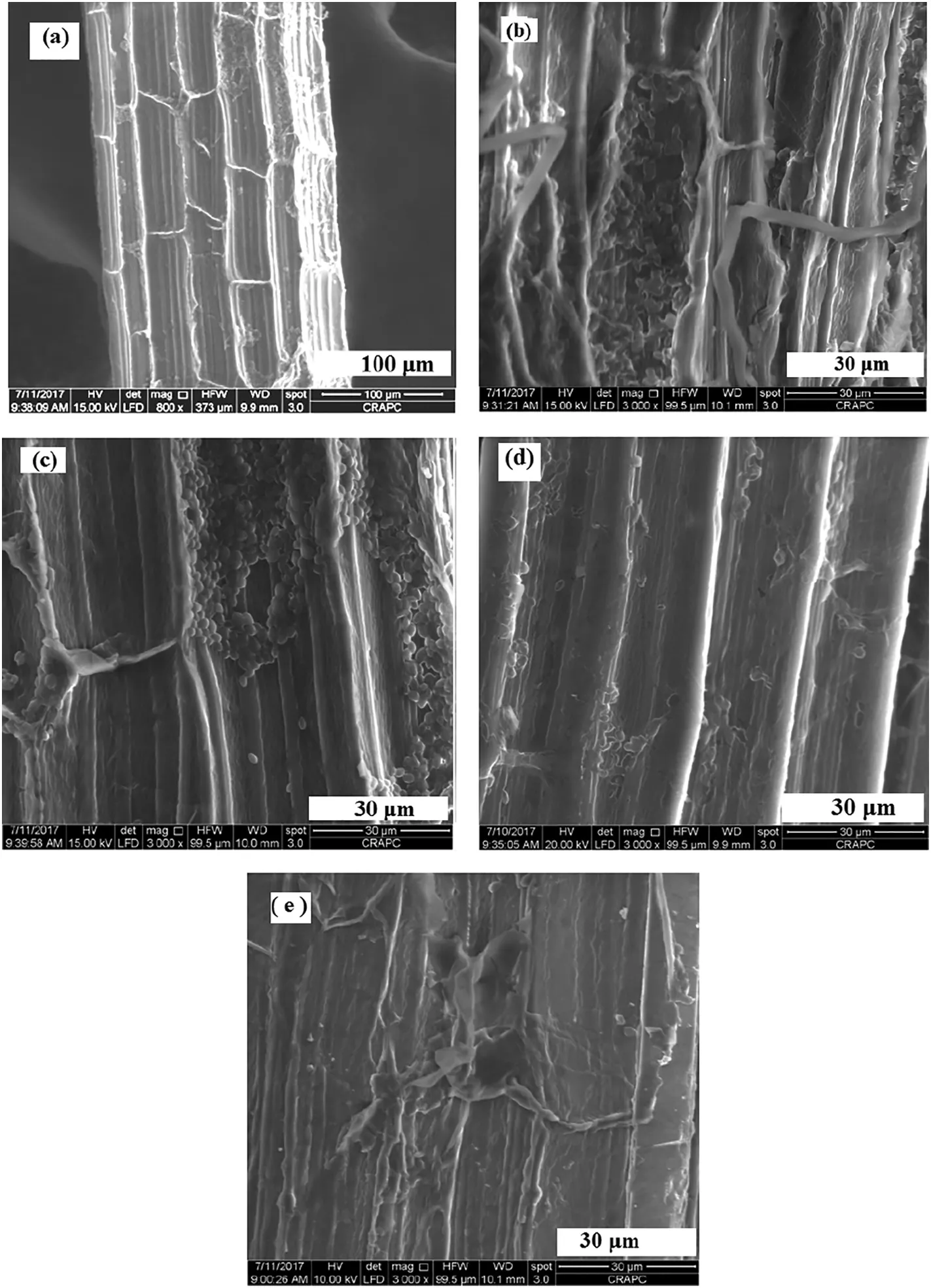
Fig.4.The Scanning electron microscope images of untreated and treated sisal fibers with various chelating agents,(a)F@N,(b)F@UR,(c)F@TC,(d)F@DCZ,and(e)F@EDTA.

Fig.5.Bio-sorption kinetics of Cr(VI)on sisal fibers treated with various chelating agent([Cr(VI)]0=200 mg·L-1,adsorbent amount=5 g·L-1,T=20 °C).
The bio-sorption kinetics was studied at various contact times ranging from 0 to 120 min,using a Cr(VI)aqueous solution of 200 mg·L-1and an amount of adsorbents(F@N,F@UR,F@TC,F@DCZ,and F@EDTA)of 5 g·L-1.The mixture was stirred in a thermostatic bath at 20 °C.The obtained results are shown in Fig.5.It can be seen that the Cr(VI)interacted rapidly with the bio-adsorbents and the maximum adsorption was observed after 50 min of contact.The equilibrium is reached in about 60 min of interaction.The untreated fibers(F@N)showed a modest removal capacity of the hexavalent chromium of 4.9 mg·g-1.However,the used chelating agents have a good effect on the adsorptive capacity of the treated fibers(F@N,F@UR,F@TU,F@DCZ,and F@EDTA)with an adsorption capacity up to 16.43 mg·g-1,17.12 mg·g-1,27.3 mg·g-1,and 36.2 mg·g-1respectively.Therefore,the used adsorbents show a different adsorption capacity in the removal of Cr(VI),this phenomenon can be explicated by the difference in the chelating properties between them,due essentially to the number of coordination atoms(N and/or O)in each ligand,for example urea and thiocarbamide contained two coordination sites.However,diphenyl carbazide has four sites and EDTA contained six coordination sites,as shown in Fig.6.
3.3.Optimization of bio-sorption factors
3.3.1.Effect of adsorbent dose

Fig.6.Electrostatic interactions between chromium(VI)anions and protonated amino groups of the used chelating agents(a)urea,(b)thiocarbamide,(c)ethylenediaminetetraacetic acid,and(d)the reaction between Cr(VI)and the pre-adsorbed diphenyl carbazide,on the surface of sisal fibers.
The effect of adsorbent dose on the removal of Cr(VI)from aqueous solution(200 mg·L-1)was carried out using an adsorbent dose in the range of 1-10 g·L-1while the other parameters such as contact time,medium pH,and temperature were kept constant.The mixture was stirred for 120 min at 20°C and then centrifuged for 10 min at 3000 r·min-1.The obtained results are shown in Fig.7;we notice an increase of the adsorbed quantity of Cr(VI)with an increase of fiber amount,caused by the availability of more active sorption sites.However,beyond a dose of 5 g·L-1,there is no considerable change in the adsorption amount.Such a phenomenon caused by a large amount of adsorbent may reduce the instauration of the adsorption sites and/or creates particle aggregation,leading to a reduction in the total surface area and an increase in diffusion path length both of which contribute to a decrease in the adsorbed amount per mass unit[22].

Fig.7.Effect of adsorbent dose on bio-sorption of Cr(VI)onto natural and treated sisal fibers([Cr(VI)]0=200 mg·L-1,contact time=120 min,T=20 °C).
3.3.2.Effect of medium pH
The medium pH has an essential effect on the adsorption phenomena of chromium.It can change in adsorbent surface charge as well as lead to the protonation of functional groups on the surface of the adsorbent.Effect of the pH on adsorption was realized by varying the solution pH in the range of 1-6,at room temperature,with an initial Cr(VI)concentration of 200 mg·L-1using 5 g·L-1as adsorbent dose.Fig.7 shows that the pH solution has a remarkable effect on the adsorption of Cr(VI).The optimal adsorption has been observed at low pHs(1-2)for all samples,with a chromium adsorption amount varied between 5 and 42.3 mg·g-1(Fig.8);this phenomenon can be explained by the straight interaction between fiber surface and various chromium anions such as Cr2O7-,,and HCrO4-.Hence,at low pHs the adsorbent surface acquired positive charge that is necessary for the adsorption process,this can be explained by the formation of quaternary ammonium cations(,>…)on the surface of the treated fibers resulting from the protonation of amino groups of the used chelating agents which assisted the electrostatic attraction toward the different chromium anions(,,and HCrO4-)as shown in Fig.6[5-23].On the other hand,the interaction between Cr(VI)and the pre-adsorbed diphenyl carbazide(DCZ)formed a reddish purple complex on the surface of the treated sisal fibers(Fig.2),the formation of this complex is favored in low pHs at room temperature,the mechanism of this reaction can be illustrated by Fig.6.
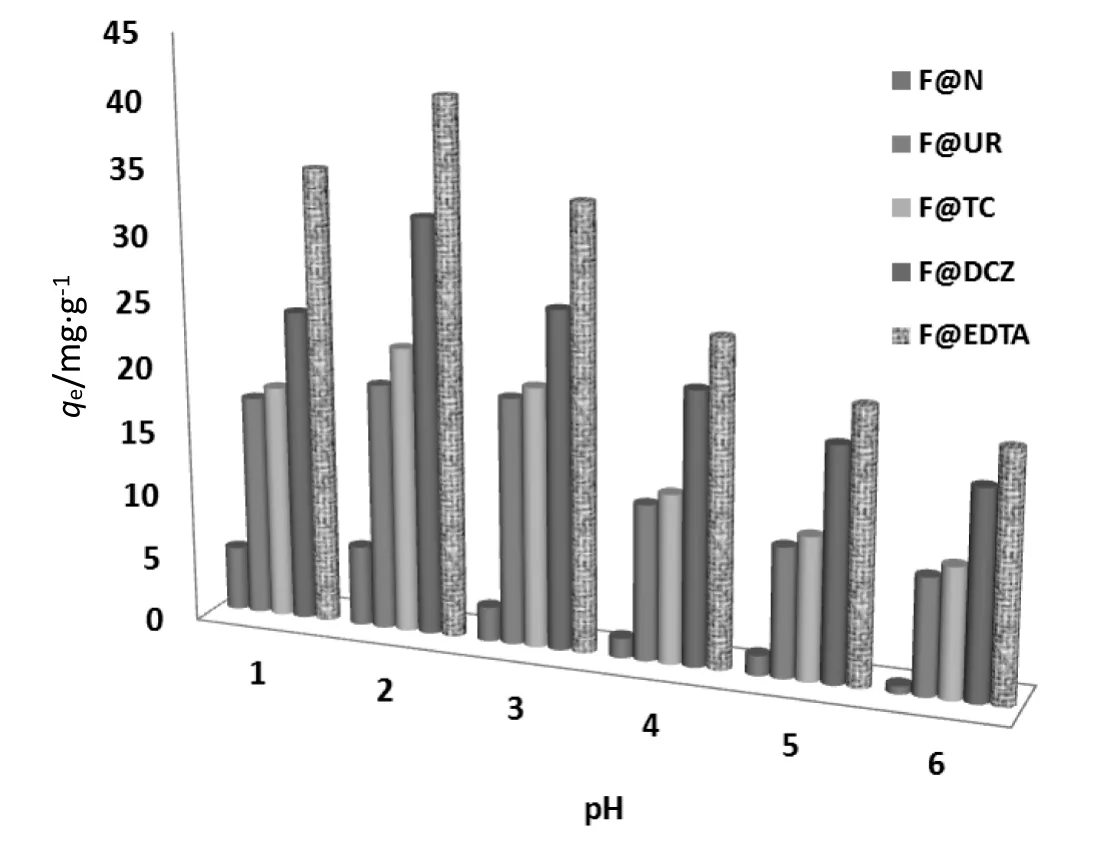
Fig.8.Effect of pH on bio-sorption of Cr(VI)onto natural and treated sisal fibers using various chelating agents([Cr(VI)]0=200 mg·L-1,contact time=120 min,T=20 °C,and adsorbent amount=5 g·L-1).
3.3.3.Effect of chromium initial concentration
To investigate the effect of the chromium initial concentration on the adsorptive capacity of the used adsorbents,the experiments were realized by varying Cr(VI)concentrations from 100 mg·L-1to 500 mg·L-1,while keeping the other parameters constant(pH 2,temperature 20°C,contact time 120 min,and adsorbent amount of 5 g·L-1).The results,shown in Fig.9,indicate that the Cr(VI)adsorbed amount initially increases by the increase of Cr(VI)initial concentration,and attained a maximum of 300 mg·L-1for all cases(F@UR,F@TC,F@DCZ,and F@EDTA)with a respective optimal adsorbed amount of chromium(VI),of 6.29 mg·g-1,24.3 mg·g-1,26.15 mg·g-1,42.3 mg·g-1,and 55.96 mg·g-1.However,at a concentration higher than 300 mg·L-1the Cr(VI)adsorbed amount of Cr(VI)was stabilized,this is due to the saturation in adsorptive sites and fiber surface area by the presence of height number of chromium molecules in the solutions[5,14,23].

Fig.9.Effect of initial concentration on bio-sorption of Cr(VI)onto natural and treated sisal fibers using chelating agents(pH 2,adsorbent amount=5 g·L-1,contact time=120 min,and T=20°C).
3.3.4.Effect of medium temperature
The effect of temperature on sorption of hexavalent chromium on untreated and treated sisal fibers was investigated at temperatures in the range of(20-50°C)using Cr(VI)solutions with the initial concentration of300 mg·L-1at pH 2,using 5 g·L-1of fiber amount.The obtained results indicate that the temperature has a negative effect on the adsorption of chromium by the different adsorbents as shown in Fig.10.This means that the interaction between chromium species(Cr2O7-2,CrO42-,and HCrO4-)and fiber surface is exothermic.The trends demonstrate a tendency of the adsorbate to escape from the adsorbent to the liquid phase with the increase in the temperature due to the excess energy promoting desorption of Cr(VI)species from the surface of adsorbents.The best result was obtained with the F@EDTA at 20°C giving a maximum adsorption amount of 56.96 mg·g-1.
3.4.Bio-sorption kinetic modeling
Several studies related to the sorption of Cr(VI)used kinetic models in order to highlight the indispensable factors of adsorption kinetics.The sorption of Cr(VI)on the natural and treated sisal fibers was modeled using the equation of the pseudo- first-order(Eq.(2))[24]and pseudo-second-order model(Eq.(3))[25-28].

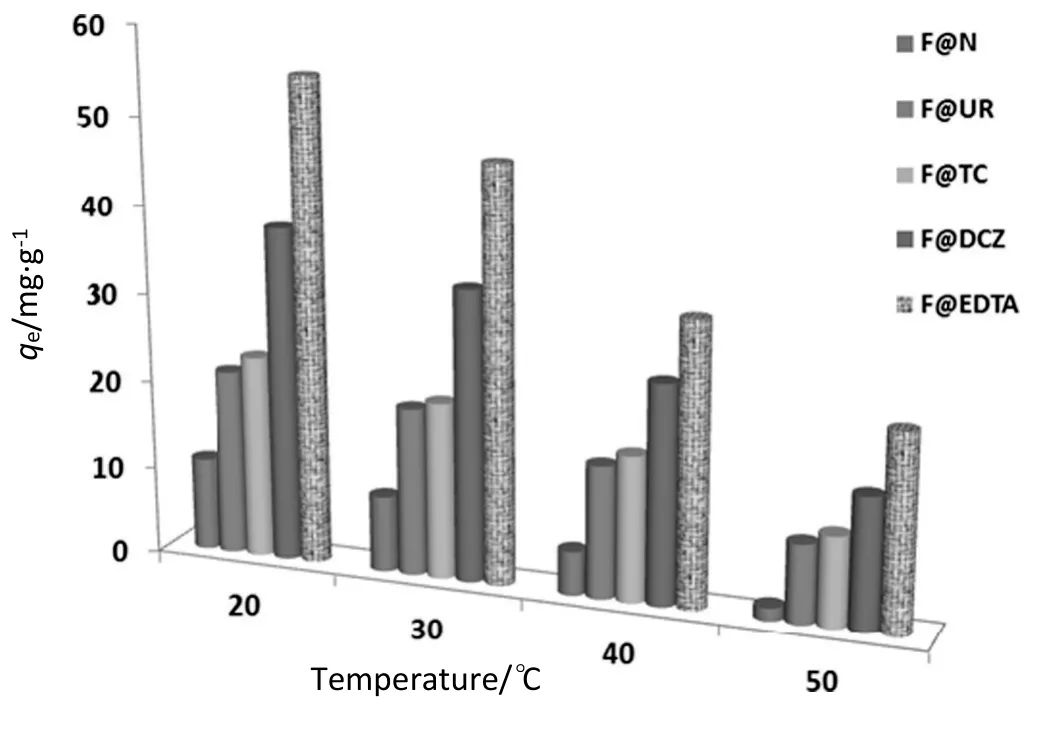
Fig.10.Effect of temperature on the bio-sorption of Cr(VI)onto natural and treated sisal fibers([Cr(VI)]=300 mg·L-1,adsorbent amount=5 g·L-1,pH 2,and contact time=120 min).

wherek1is the rate constant of the pseudo- first-order(min-1);k2is the pseudo-second-order adsorption rate constant(g·mg-1·min-1),tis the contact time,andqtandqeare adsorption amounts(mg·g-1),respectively,atttime and at equilibrium(min)[23-28].The results of the pseudo- first-order and pseudo-second-order models are represented by Fig.11 and gathered in Table 1.They show that the adsorption of hexavalent chromium using natural and treated sisal fibers follows the pseudo-second-order model with a correlation coefficient,R2up to(0.99).They also show that the theoretical values ofqetheoare better estimated by the pseudo-second-order model than by that the pseudo- first-order one which suggests that the bio-adsorption of chromium on the sisal fibers is dominated by a chemical process.

Fig.11.Plot of the pseudo- first-order(a)and pseudo-second-order,(b)of the bio-sorption of Cr(VI)onto natural and treated fibers([Cr(VI)]=300 mg·L-1,adsorbent amount=5 g·L-1,pH 2,contact time=120 min).

Table 1 Parameters of pseudo- first-order and pseudo-second-order models of Cr(VI)bio-sorption on natural and treated sisal fibers.
3.5.Adsorption isotherm
Adsorption isotherm study is indispensable to describe the adsorption mechanism.It also permits the determination of the maximum adsorbed amount on the used adsorbents and the identification of the adsorption type.According to the Gileset al.classification[29],the obtained results illustrate that the obtained isotherms are of type S as shown in Fig.12.
The adsorbed amount of Cr(VI)increases gradually with an increase of chromium initial concentration in the adsorbate solution(Fig.12).However,at low concentration,the ratio of the number of chromium ions to the number of available adsorption sites is small and consequently,adsorption is independent of initial concentration,but as the concentration of the Cr(VI)increases,the situation changes and the competition for adsorption sites becomes intense.Adsorption alters the distribution of a solute in the constituent phases and the interface between them[30].
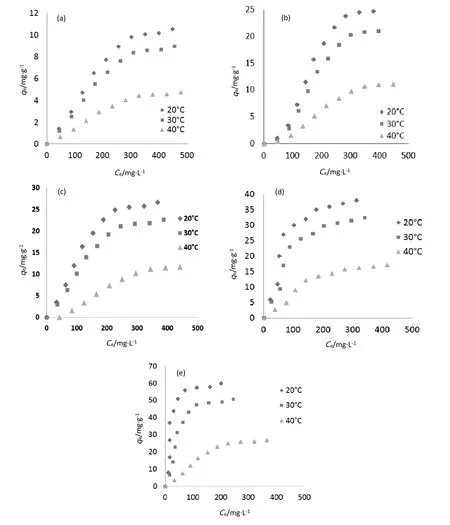
Fig.12.Bio-sorption isotherms of Cr(VI)on naturaland treated sisal fibers:(a)F@N,(b)F@UR,(c)F@TC,(d)F@DCZ,and(E)F@EDTA(adsorbentamount=5 g·L-1,pH 2,contacttime=120 min).
The adsorption process is usually described by the following two widely used isotherms:[31]

where,qeis the constant indicative of the relative adsorption capacity of the adsorbent(mg·g-1),Ceis the equilibrium concentration of chromium(VI)(mg·g-1),KLis the Langmuir coefficient(L·mg-1),andqmis the maximum adsorption capacity(mg·g-1),Kfandnare Freundlich coefficients,and 1/nis the constant indicative of the intensity of the sorption.The modeling of the adsorption isotherm results using the model of Freundlich and Langmuir is given in Table 2.As we have observed,according to the values of the correlation coefficient(R2)of the linear regression,its hows that the adsorption isotherms obey the Langmuir model rather than the Freundlich one.Cr(VI)solution temperature has an important effect on the adsorption mechanism,and the values of Langmuir maximum adsorption capacity(qm)show that the higher chromium adsorption amount on natural and treated sisal fibers is obtained at 20°C.The obtained results from the Langmuir isotherm show that the adsorption processes of Cr(VI)on all adsorbents are favorable at low temperature with a maximum adsorption capacity(qm)up to 61.45 mg·g-1in the case of F@EDTA.

Table 2 Freundlich and Langmuir parameters of Cr(VI)bio-sorption on natural and treated fibers.
3.6.Thermodynamic parameters
The adsorption thermodynamic parameters such as enthalpy(ΔH⊖,kJ·mol-1),Gibbs energy(ΔG⊖,kJ·mol-1),and entropy(ΔS⊖,J·mol-1·K-1),were calculated using the following equations:

Eq.(8)is obtained by combining Eqs.(6)and(7).

whereTis the absolute temperature(Kelvin),Ris the gas constant with a value of 8.314 J·mol-1·K-1,andKis the equilibrium adsorption constants of the isotherm fits(Kequilibrium constant,which must be converted to SI units,by using the chromium(VI)molecular mass)[32,33].Thermodynamic parameters of the chromium(VI)biosorption by sisal fibers can be determined from the plot of lnKto 1/Tusing the data presented in Table 3[32-36].The results of the thermodynamic study show that the Gibbs energy decreases with increasing temperature as shown in Table 3.All ΔG⊖have negative values which indicates that the interactions(Cr(VI)@@Fibers)are spontaneous.Enthalpy(ΔH⊖)negative values are in accordance with the exothermic nature of the interactions of Cr(VI)with the used adsorbents.The decrease of the entropy(ΔS)values indicates the bio-sorption process and has a stable configuration.
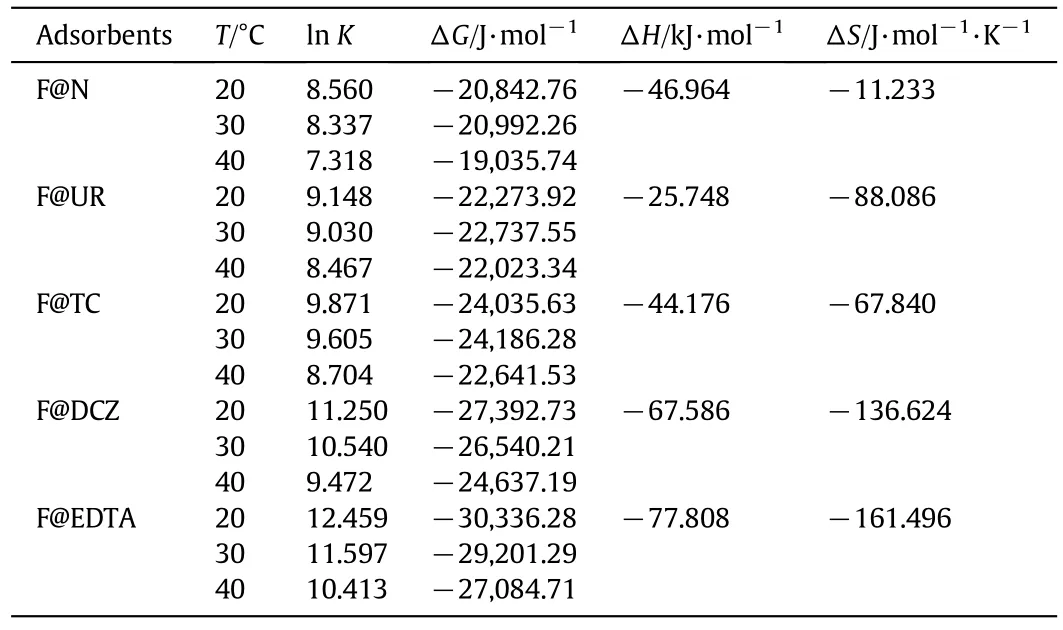
Table 3 Thermodynamic parameters for bio-sorption of Cr(VI)on natural and treated fibers.
4.Conclusions
In this study,it has been showed that treatment of sisal fibers with various the chelating agents presents a better uptake capacity in the removal of Cr(VI)from aqueous solutions than natural sisal fibers,with a maximum adsorption capacity up to 61.45 mg·g-1.It has also been shown that the best results for bio-sorption of Cr(VI)on natural and treated sisal fibers were obtained using an adsorbent dose of 5 g·L-1and after a contact time of 120 min.The adsorbed quantities of Cr(VI)decreased with increasing temperature.The suitable biosorption was obtained at pH 2 for a solution temperature of 20°C.The modeling study showed that the adsorption kinetics obey the pseudosecond-order.The adsorption isotherm of Cr(VI)on all adsorbents follows the linear Langmuir model with anR2in the range of 0.991-0.998.The results of the thermodynamic study showed that the chromium adsorption on the natural and treated sisal fibers is exothermic and spontaneous,and has a stable configuration.It is anticipated that this investigation may open the way for another study about the removal of other heavy metals using the natural and/or treated fibers with chelating agents.
[1]A.Han,H.Zhang,J.Sun,G.-K.Chuah,S.Jaenicke,Investigation into bulk liquid membranes for removal of chromium(VI)from simulated wastewater,J.Water Process Eng.17(2017)63-69.
[2]H.Bendjeffal,H.Mamine,A.Djebli,N.Rebbani,Y.Bouhedja,Removal of 4-(2-pyridylazo)-resorcinol from aqueous solution using natural and activated Algerian kaolin,Sens.Lett.15(2017)668-675.
[3]W.N.L.dos Santos,D.D.Cavalcante,E.G.Paranhos da Silva,C.Francisco das Virgens,Biosorption of Pb(II)and Cd(II)ions byAgave sisalana(sisal fiber),Microchem.J.97(2011)269-273.
[4]R.Khalid,Z.Aslam,A.Abbas,W.Ahmad,N.Ramzan,R.Shawabkeh,Adsorptive potential ofAcacia niloticabased adsorbent for Chromium(VI)from an aqueous phase,Chin.J.Chem.Eng.26(3)(2018)614-622.
[5]S.Guendouz,N.Rebbani,K.Guer fi,Y.Bouhedja,Removal of Cr(VI)from an aqueous solution using natural kaolin of Tamazert-east of Algeria,Sens.Lett.14(2016)417-424.
[6]N.Puvaneswari,J.Muthukrishna,P.Gunasekar,Toxicity assessmentand microbialdegradation of azo dyes,Indian J.Exp.Biol.44(2006)618-626.
[7]H.Bendjeffal,K.Guerfi,Y.Bouhedja,N.Rebbani,Phys.Procedia2(2009)889-897.
[8]H.Bendjeffal,Adsorption des complexes organométalliques sur des supports solides,Editions universitaires europeennes,Paris,2017.
[9]L.Assem,H.Zhu,Chromium GeneralInformation,Health Protection Agency,Institute of Environment and Health Cran field University,2007 01-05.
[10]L.S.Zhang,The Advanced Water Treatment and Reuse Technology,Chemical Industry Press,Beijing,2009.
[11]A.K.Bhattacharya,T.K.Naiya,S.N.Mandal,S.K.Das,Adsorption,kinetics and equilibrium studies on removal of Cr(VI)from aqueous solutions using different low-cost adsorbents,Chem.Eng.J.137(2008)529-541.
[12]S.Sugashini,K.M.M.Sheriff Begum,Performance of ozone treated rice husk carbon(OTRHC)for continuous adsorption of Cr(VI)ions from synthetic effluent,J.Environ.Chem.Eng.1(2013)79-85.
[13]N.Rajesh,B.G.Mishra,P.K.Pareek,Preparation,UV-vis,IR,EPR and resonance Raman study of Fe,Ni,Co and Zn dioxolene complexes,Spectrochim.Acta A69(2007)612-618.
[14]Ashraf Ali,Khalid Saeed,Fazal Mabood,Removal of chromium(VI)from aqueous medium using chemically modified banana peels as efficient low-cost adsorbent,Alex.Eng.J.55(2016)2933-2942.
[15]Y.Gong,Q.Hu,Y.Guo,L.Yu,Photocatalyzed reduction of chromium(VI)and thermal-driven heterogeneous separation,ACS Sustain.Chem.Eng.5(6)(2017)4511-4516.
[16]C.E.Barrera-Draza,V.Lugo-Lugo,B.Bilyeu,A review of chemical,electrochemical and biological methods for aqueous Cr(VI)reduction,J.Hazard.Mater.223(2012)1-12.
[17]B.Galan,D.Castaneda,I.Ortiz,Water Res.39(2005)4317-4324.
[18]A.Çimen,Removal of chromium from wastewater by reverse osmosis,Russ.J.Phys.Chem.89(2015)1238-1243.
[19]N.DalhatMu'azu,A.Usman,N.Jarrah,Pulsed electrokinetic removal of chromium,mercury and cadmium from contaminated mixed clay soils,Soil Sediment Contam.Int.J.25(2016)757-775.
[20]M.X.Loukidou,A.I.Zouboulis,T.D.Karapantsios,K.A.Matis,Equilibrium and kinetic modeling of chromium(VI)biosorption byAeromonas caviae,Colloids Surf.A Physicochem.Eng.Asp.242(2004)93-104.
[21]E.Padmini,M.Helen Kalavathy,M.Lima Rose,Surface modifiedAgave sisalanaas an adsorbent for the removal of nickel from aqueous solutions—Kinetic and Equilibrium studies,Carbon Lett.9(2008)97-104.
[22]T.Hajeeth,P.N.Sudha,K.Vijayalakshmi,Removal of Cr(Vi)from aqueous solution using graft copolymer of cellulose extracted from sisal fibre with acrylic acid monomer,Cellul.Chem.Technol.49(2015)891-900.
[23]D.Kumar Singh,V.Kumar,S.Mohan,Polylysine functionalized graphene aerogel for the enhanced removal of Cr(VI)through adsorption:Kinetic,isotherm,and thermodynamic modeling of the process,J.Chem.Eng.Data62(2017)1732-1742.
[24]Y.S.Ho,J.C.Y.Ng,G.McKay,Kinetics of pollutant sorption by biosorbents:Review,Sep.Purif.Methods29(2000)189-232.
[25]Z.Hattab,N.Filali,R.Mazouz,K.Guerfi,N.Rebbani,Adsorption of cyanide ions in aqueous solution using raw and oxidized coke,Desalin.Water Treat.57(2016)3522-3531.
[26]A.Shukla,Y.H.Zhang,P.Dubey,The role of sawdust in the removal of unwanted materials from water,J.Hazard.Mater.95(2002)137-152.
[27]J.Ma,Y.Jia,Y.Jing,Y.Yao,Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite,Dyes Pigments93(2012)1441-1446.
[28]G.Absalan,M.Asadi,S.Kamran,L.Sheikhian,Removal of reactive red-120 and 4-(2-pyridylazo)resorcinol from aqueous samples by Fe3O4magnetic nanoparticles using ionic liquid as modifier,J.Hazard.Mater.192(2011)476-481.
[29]C.H.Giles,T.H.MacEwan,S.N.Nakhwa,D.Smith,Studies in adsorption.Part XI.A system of classification of solution adsorption isotherms,and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids,J.Chem.Soc.0(1960)3973-3993.
[30]B.K.G.Theng,Formation and Properties of Clay-Polymer Complexes,Elsevier Amsterdam,2012.
[31]E.Eren,Adsorption performance and mechanism in binding of azo dye by raw bentonite,Clean Soil Air Water38(8)(2010)758-763.
[32]Liu Yu,Is the free energy change ofadsorption correctly calculated,J.Chem.Eng.Data54(2009)1981-1985.
[33]D.Castro dos Santos,et al.,New carbon composite adsorbents for the removal of textile dyes from aqueous solutions:Kinetic,equilibrium,and thermodynamic studies,Korean J.Chem.Eng.31(8)(2014)1470-1479.
[34]C.P.Bergmann,F.M.Machado,Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications,Springer,Porto Alegre,2015.
[35]Y.Liu,H.Xu,Equilibrium,thermodynamics and mechanisms of Ni2+biosorption by aerobic granules,Biochem.Eng.J.35(2007)174-182.
[36]Y.Liu,Y.-J.Liu,Biosorption isotherms,kinetics and thermodynamics,Sep.Purif.Technol.61(2008)229-242.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
