Solubility of red palm oil in supercritical carbon dioxide:Measurement and modelling
Wan Jun Lee ,Chin Ping Tan ,Rabiha Sulaiman ,Gun Hean Chong ,2,*
1 Department of Food Technology,Faculty of Food Science and Technology,Universiti Putra Malaysia,43400 Serdang,Selangor,Malaysia
2 Supercritical Fluid Centre(SFC),Faculty of Food Science and Technology,Universiti Putra Malaysia,43400 Serdang,Selangor,Malaysia
1.Introduction
Red palm oil(RPO)is often regarded as identical to crude palm oil due to the intense red colour of the RPO.Nonetheless,RPO is produced through a process of pretreatment,de-acidification and deodorization using molecular distillation from crude palm oil[1].Unlike palm oil,the absence of bleaching process when producing RPO retains its high concentration of carotenoids(500-700 μg·g-1)and vitamin E(500-1000 μg·g-1)[2],and thus,the red colour of RPO compared to the yellowish palm oil.These components were reported to have the same function as natural antioxidants[3],which aid in the prevention of several diseases[4],and hence,RPO is gaining in popularity as a functional food.In addition to the high concentrations of active components,RPO is also specialin terms of its fatty acid composition;it contains an almost identical percentage of saturated(~50%)and unsaturated(~40%monounsaturated,~10%polyunsaturated)fatty acids[5].
Supercritical carbon dioxide(scCO2),due to its non-toxic and low critical temperature properties,has been widely utilised for different processes on oil samples such as fractionation,extraction,purification,and particle formation.The optimisation and process design of the aforementioned process require information on the solubility data,which will aid in identifying the best operating parameters.Studies relating to the solubility of different oils in scCO2have been carried out by many researchers using the dynamic method[6-8].From similar oil palm plant sources such as palm oil and palm kernel oil,solubility studies have also been reported[9-11].However,there is still a lack of solubility studies on the RPO in scCO2.Different equations for mathematical correlation and prediction of solubility data in scCO2,such as semi-empirical density-based equations(Chrastil,del Valle and Aguilera,and Adachi and Lu models)and theoretical equations of state(EOS)have been developed since the solubility determination by the experimental procedure is time-consuming and costly.
The RPO solubility data obtained will be practically useful for the selection and studies of RPO particle formation processes with scCO2,which will be carried out later on.The supercritical carbon dioxide solution-enhanced dispersion(SEDS)was proposed to be used as the particle formation technology for RPO and for this technique;the scCO2will act as the anti-solvent,and hence,the RPO must be insoluble or have minimum solubility in the scCO2to have maximum loading efficiency in the particle.Thus,this work was conducted under low-pressure conditions to examine the solubility behaviour of RPO prior to particle formation.The solubility of RPO at different pressures(8.5-25 MPa)and temperatures(313.15-333.15 K)was determined.Since there is no available RPO solubility data from the literature,three semi-empirical models and a thermodynamic model will be established for RPO in this work.
2.Materials and Methods
The RPO was supplied by Orifera™(Malaysia).Liquid CO2(purity of 99.99%)was purchased from Mox-Linde Gases Sdn.Bhd.(Malaysia).
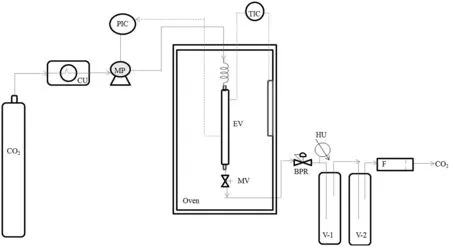
Fig.1.The setup used for determining the red palm oil solubility:CO2—carbon dioxide cylinder;CU—cooler unit;MP—CO2 pump;PIC—pressure indicator;TIC—temperature indicator;EV—extraction vessel;MV—metreing valve;BPR—back pressure regulator;HU—heater unit;V-1/V-2—collection vials;F—bubble flow metre.
2.1.Solubility of red palm oil
RPO solubility was determined using the dynamic method in a laboratory scale apparatus as shown in Fig.1.The apparatus consisted of a CO2pump(P-2024SFC,ChromTech,USA)used to deliver the CO2,an oven(UFE 600,Memmert,Germany)with a PID microprocessor controller and integrated auto-diagnostic system to control the temperature,a preheating coil to ensure the CO2delivered reached the system temperature before coming into contact with the sample,an extraction vessel(110 cm3)where the sample was loaded,a back pressure regulator(26-1700 Tescom,USA)to control the pressure,collection vials to collect the solubilised oil and a bubble flow metre(#20136,Restek,USA)to measure the CO2flow rate.
In each experiment,the extraction vessel was loaded with 2 mm solid glass beads(Z273627,Sigma-Aldrich,USA)wet with approximately 8.0 g of RPO.The amount of RPO loaded was in excess to ensure that the exit stream of CO2was always saturated with the oil.However,the maximum oil feed was limited to 8.0 g as it was preliminarily determined that too much oil will cause the oil to flow down from the extraction vessel,leading to less accurate results.Glass wool(#20411,Supelco,USA)was placed at both ends of the extraction vessel to prevent liquid entrainment[12].The solubilised oil was precipitated over time in the collection vials and oil was weighed immediately every 15-30 min until constant solubility readings were recorded,indicating that the solubility readings were taken at equilibrium conditions.Deposited oil in the valves was washed with methanol,evaporated,weighed and included in the total weight of the solubilised oil.The RPO solubility in scCO2was defined by the equation:
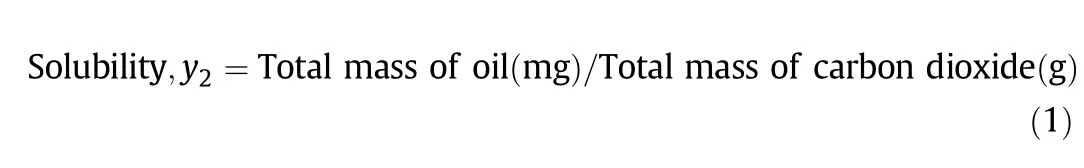
The reliability of the apparatus and measurement method was validated with grape seed oil solubility from Sovováet al.[12]prior to determining the solubility of RPO.Additionally,the validity of using the dynamic method for solubility measurement is also dependent on the CO2flow rate.The suitable CO2flow rate,which allows for the flowing scCO2to be saturated with oil,was determined by the trial-and-error method[6],and the results are discussed in Section 3.1.The solubility of RPO was determined at eight different pressures of 8.5,10.0,12.5,15.0,17.5,20.0,22.5 and 25.0 MPa and three different temperatures of 313.15,323.15 and 333.15 K using full factorial designs at a fixed CO2flow rate of 2.90 g·min-1.All measurements were performed in triplicate and the average results are reported.
2.2.Data correlation with density-based models and a theoretical model
2.2.1.Semi-empirical models
Chrastil model:

wherey(g·L-1)is the solubility of RPO and density of scCO2,ρ (g·L-1),at temperatureT(K).The linear correlation slope is represented by the constantk,whereas constantais the total enthalpies of solvation and vaporisation,and constantbrelates to the molecular mass of the RPO and scCO2[6,13].
del Valle and Aguilera model:

wherey,k,ρ andTdenote the similar parameters as proposed by Chrastil.The solubilisation process' thermal effects are represented by constantsaandb,while the molecular mass of RPO and scCO2is represented by constantc[6,14].
Adachi and Lu:
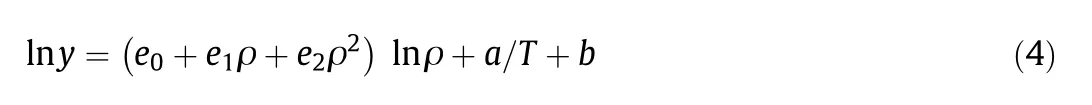
wherey,ρ,T,aandbrepresent the similar parameters as in Chrastil.The constantkis the exponent of the density and is introduced to the dependency of solubility on density[6,15].
The parameters for all the semi-empirical models were estimated using the multiple regression function in Microsoft Excel®.The ability of regression to estimate the parameters for the semi-empirical models was reported[16]and this method was further validated with reported solubility data from the literature[6,17].
The discrepancies between experimental oil solubility and model predictions were then evaluated according to the absolute average relative deviation(AARD)and coefficient of determination(R2):
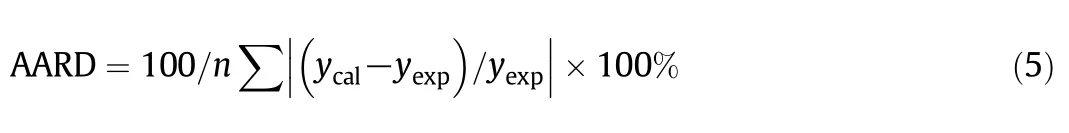
wherenis the number of solubility experimental data,ycalis the calculated solubility,andyexpis the solubility obtained from the experiment.
2.2.2.Thermodynamic model
Peng-Robinson equation of state(PR-EOS):
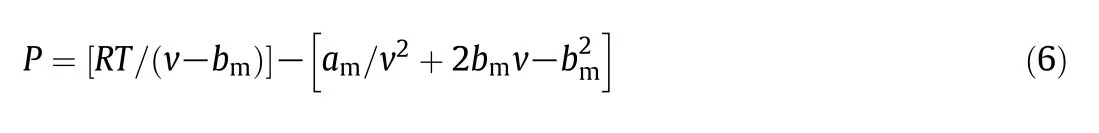
In this equation,P,R,Tandvare the pressure(MPa),gas constant(8.314 J·K-1·mol-1),temperature (K),and molar volume(m3·mol-1),respectively.The constantsamandbmare parameters obtained from the van der Waals mixing rules[18],with the interaction parameters given as:
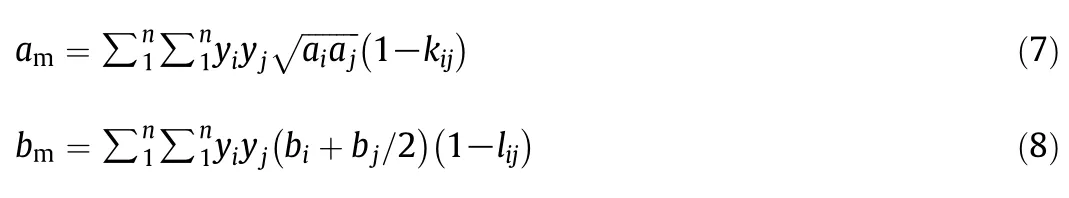
The number of components present in the system is denoted byn.Theyiandyjrepresented the mole fraction componentiandj,respectively.Binary interaction parameters are denotedkijandlij.The parametersaiandbifor componentiare obtained from these equations:

The critical temperature,Tci(K),critical pressure,Pci(MPa)and acentric value ω,are the estimated parameters for PR-EOS.The RPO is made up largely of triglycerides,and triolein was chosen to represent RPO.Similar assumptions were made by other authors for oils for which triolein is the most abundant component[6,19].The vapour pressure data of triolein were estimated by extrapolating the results reported by Perryet al.[20],and the values ofTci,Pci,ω,andvof triolein and CO2were adapted from Zhao and Zhang[6].
The equation to obtain the solubility of a solute by PR-EOS is as follows:

where
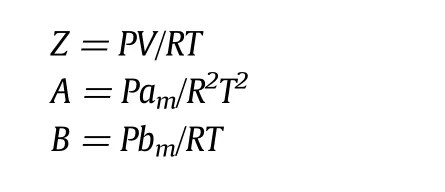
The estimated best- fitted constant values with minimised AARD were carried out using Microsoft Excel®2010 Solver's function.The calculation method was validated based on the literature[6,21-23]before applying it to RPO solubility.
2.3.Statistical analysis
All analyses were performed in triplicate,and the results are presented as average values.Analysis of variance(ANOVA)was performed to evaluate the significance of pressure and temperature effects on solubility and was carried out using Minitab 16 statistical software(Minitab Inc.,Pennsylvania),where apvalue of<0.05 was reported to have a significant difference.
3.Results and Discussion
3.1.Effect of scCO2flow rate
Fig.2 shows the effect of a flow rate of 1.17-6.70 g·min-1on RPO solubility determined at 20.0 MPa and 313.15 K.When the CO2flow rate increased from 1.17 to 2.12 g·min-1CO2,the RPO solubility increased as more CO2molecules were available to come into contact with the RPO and enhance the solubility.However,the solubility decreased at a flow rate beyond 4.57 g·min-1and when far too much CO2was pumped into the vessel,the resident time and contact time of CO2with RPO were shortened.At a flow rate of 2.12-4.57 g·min-1CO2,the flow rates were considered to be at equilibrium with a nonsignificant difference(pvalue>0.05)in the solubility readings.These flow rates were hence considered to be able to assure saturation for the RPO solubility measurement and the flow rate was fixed at 2.90 g·min-1throughout the experiment.

Fig.2.Red palm oil solubility determined at constant pressure and temperature of 20.0 MPa and 313.15 K and at different carbon dioxide flow rates.
3.2.Solubility of red palm oil
The solubility of RPO in scCO2ranged from 0.5 to 11.3 mg·(g CO2)-1,as shown in Table 1.The magnitude ofRPO solubility was in good agreement with other vegetable oil solubility data from the literature[6,24].Comparing the solubility of RPO to oil of similar palm plant sources such as palm oil(PO),palm-pressed mesocarpfibre oil(PPO)and palmkerneloil(PKO),it was found that the solubility ofRPOwas higher than PO(5.0 mg·(g CO2)-1at 24.0 MPa,313.15 K)[25]and PPO(0.26 mg·(g CO2)-1at 10.0 MPa,313.15 K)[10]but lower than PKO(16 mg·(g CO2)-1at20.7 MPa,313.15 K)[26],at almost similar operating conditions.It has been reported that oil consisting of more long chain fatty acids has lower solubility[6,7,27],and hence,the solubility of RPO with a high percentage of long chain oleic acid(~60%)was lower than PKO,which is rich in medium chain lauric acid.PPO and PO consisted of almost identical fatty acid compositions to RPO and the solubility of both oils was lower,which might be due to thedifference in the state of raw material used.The glass beads were wet with RPO in this work whereas,in the work for both POand PPO,the experiments were conducted using the mesocarp and palm-pressed fibre,respectively.A lower solute-solvent contact surface was created in the presence of plant tissues and water content in plant matrixes,which then hindered the flow of scCO2through the solute,and the diffusion resistance was increased[28].

Table 1 Red palm oil(RPO)solubility in supercritical carbon dioxide
3.3.Effect of pressure and temperature
The effect of pressure and temperature on the solubility of RPO in scCO2is shown in Table 1.The RPO solubility significantly(p<0.05)increased when the operating pressure was increased.The solvating power of scCO2became greater at higher pressure due to the increment in the CO2density as in Table 1,and thus more oil was solubilised in the scCO2[6,8].The solubility of RPO decreased significantly when the operating temperature was increased at a constant pressure.The solvent power of scCO2decreased when the temperature was higher due to the decrease in the density and hence lowered the solubility[7].In this work,the phenomenon of the crossover effect was not observed and it was postulated that the effect of reduction in scCO2density was more distinctive than the solute vapour pressure effect.Hence,the RPO solubility diminished with increasing temperature.The crossover would have occurred at a higher pressure region(above 25.0 MPa).Similarly,the crossover region for oil that comprises of almost identical fatty acid compositions to RPO(high percentage of long chain fatty acids)was reported to be beyond 25.0 MPa[6,24,25,27,29].Our findings were consistent with the results in the literature.
3.4.Mathematical data correlation
The fitting constants together with the AARD andR2for the semiempirical model correlation for RPO solubility are shown in Table 2.The correlations between experimental data against the calculated data for all three models were plotted as in Fig.3.From Table 2,the best mathematical correlation with an AARD of 13.57%andR2of 0.9667 was attained by the Adachi-Lu model,whereas the Chrastil and del Valle and Aguilera models did not show good correlationswith a high AARD and lowR2.The ability of the Adachi-Lu model to correlate the RPO data was due to the equation originally being modified for triglyceride solubility[30]and the high dependency of solubility on solvent density[31].The Adachi-Lu model showed a high correlation with the oil solubility data,as reported in the literature[32-34].For the data correlation with PR-EOS,the optimal binary interaction parameterskijandlijand the AARD of the correlation are shown in Table 3.The experimental results against predicted solubility of RPO with the PR-EOS are plotted in Fig.4.The Adachi-Lu model provided a better correlation with RPO solubility in comparison to the PR-EOS.The higher deviation by PR-EOS was due to the possibilities of errors when estimating the thermophysical parameters for the oil.RPO is a multi-component system,containing triglycerides and other minor components,while for the estimations of the parameters,triolein was chosen to represent the RPO as a whole.A similar problem was observed in the correlation of sacha inchi oil by do Pradoet al.[35],showing a relatively high AARD of 34%-40%.
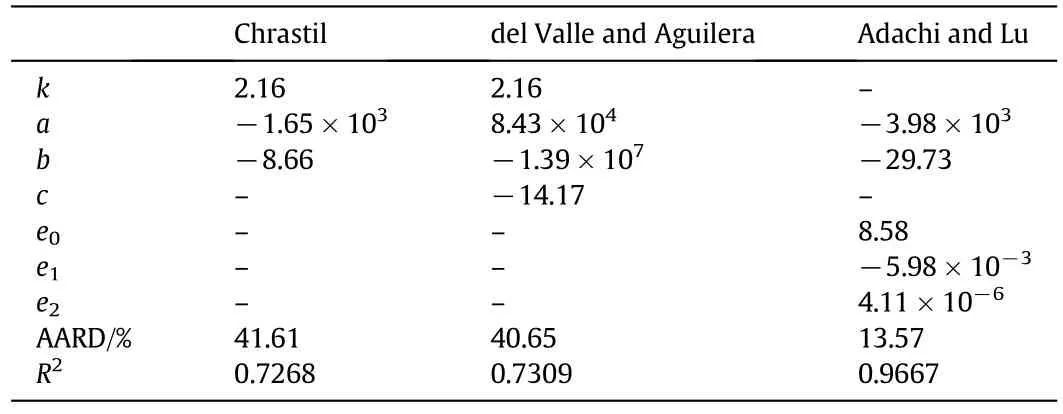
Table 2 Fitting constants and the average absolute deviations for semi-empirical model correlation

Fig.3.The experimental results against calculated red palmoil solubility data using the(a)Chrastil model;(b)del Valle and Aguilera model;and(c)Adachi and Lu model.
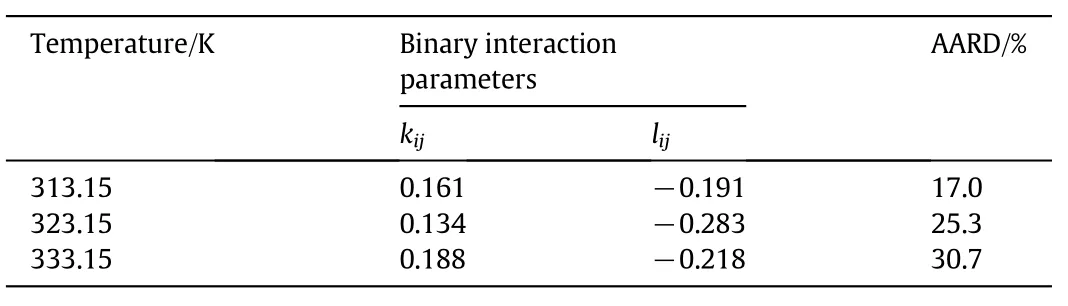
Table 3 Optimal binary interaction parameters of red palm oil(predicted with triolein)for the Peng-Robinson equation of state model

Fig.4.Experimental and predicted solubility data for red palm oil with the Peng-Robinson equation of state.
4.Conclusions
The solubility of RPO in scCO2was determined using a dynamic method.The solubility of RPO was significantly affected by the pressure and temperature.There was a positive relationship between the pressure and RPO solubility,whereby the solubility of RPO increased with pressure.Generally,temperatures exhibited a negative relationship with RPO solubility.The Adachi-Lu semi-empirical model was the best correlating model for RPO solubility in comparison to Chrastil,del Valle and Aguilera and Peng-Robinson equation of state.The solubility of RPO is generally low in scCO2and is suggested to be suitable for particle formation using scCO2as the anti-solvent.
Acknowledgements
This work was supported by Geran Putra IPS(Vote No.:9469400),University Putra Malaysia.Gratitude is expressed to Orifera™(Selangor,Malaysia)for the free supply of red palm oil for this work.
[1]J.Van Rooyen,A.J.Esterhuyse,A.M.Engelbrecht,E.F.Du Toit,Health benefits of a natural carotenoid rich oil:a proposed mechanism of protection against ischaemia/reperfusion injury,Asia Pac.J.Clin.Nutr.17(2008)316-319.
[2]J.Boateng,M.Verghese,C.B.Chawan,L.Shackelford,L.T.Walker,J.Khatiwada,D.S.Williams,Red palm oil suppresses the formation of azoxymethane(AOM)induced aberrant crypt foci(ACF)in Fisher 344 male rats,Food Chem.Toxicol.44(2006)1667-1673.
[3]J.V.Woodside,A.J.McGrath,N.Lyner,M.C.McKinley,Carotenoids and health in older people,Maturitas80(2015)63-68.
[4]M.L.Colombo,An update on vitamin E,tocopherol and tocotrienol—perspectives,Molecules15(2010)2103-2113.
[5]D.O.Edem,Palm oil:biochemical,physiological,nutritional,hematological,and toxicological aspects:a review,Plant Foods Hum.Nutr.57(2002)319-341.
[6]S.Zhao,D.Zhang,An experimental investigation into the solubility ofMoringa oleiferaoil in supercritical carbon dioxide,J.Food Eng.138(2014)1-10.
[7]M.H.Zuknik,N.A.Nik Norulaini,W.S.Wan Nursyazreen Dalila,N.R.Ali,A.K.M.Omar,Solubility of virgin coconut oil in supercritical carbon dioxide,J.Food Eng.168(2016)240-244.
[8]J.M.Danlami,M.A.A.Zaini,A.Arsad,M.A.C.Yunus,Solubility assessment of castor(Ricinus communisL)oil in supercritical CO2at different temperatures and pressures under dynamic conditions,Ind.Crop.Prod.76(2015)34-40.
[9]S.-A.Hong,J.-D.Kim,J.Kim,J.W.Kang,I.-J.Kang,Phase equilibria of palm oil,palm kernel oil,and oleic acid+supercritical carbon dioxide and modeling using Peng-Robinson EOS,J.Ind.Eng.Chem.16(2010)859-865.
[10]H.Lik Nang Lau,Y.M.Choo,A.N.Ma,C.H.Chuah,Selective extraction of palm carotene and vitamin E from fresh palm-pressed mesocarp fiber(Elaeis guineensis)using supercritical CO2,J.Food Eng.84(2008)289-296.
[11]Vânia M.B.Cunha,Marcilene P.Silva,Raul N.Carvalho Jr.,M.Angela A.Meireles,Nelio T.Machado,Marilena E.Araújo1,Lauric acid rich oil supercritical extraction and methodology to predict solubility,Food Public Health6(2016)26-32.
[12]H.Sovová,M.Zarevúcka,M.Vacek,K.Stránský,Solubility of two vegetable oils in supercritical CO2,J.Supercrit.Fluids20(2001)15-28.
[13]J.Chrastil,Solubility of solids and liquids in supercritical gases,J.Phys.Chem.86(1982)3016-3021.
[14]J.M.Del Valle,J.M.Aguilera,An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide,Ind.Eng.Chem.Res.27(1988)1551-1553.
[15]Y.Adachi,B.C.-Y.Lu,Supercritical fluid extraction with carbon dioxide and ethylene,Fluid Phase Equilib.14(1983)147-156.
[16] Ö.Güçlü-Üstündağ,F.Temelli,Correlating the solubility behavior of minor lipid components in supercritical carbon dioxide,J.Supercrit.Fluids31(2004)235-253.
[17]B.L.F.Lopes,A.P.Sánchez-Camargo,A.L.K.Ferreira,R.Grimaldi,L.C.Paviani,F.A.Cabral,Selectivity of supercritical carbon dioxide in the fractionation of fish oil with a lower content of EPA+DHA,J.Supercrit.Fluids61(2012)78-85.
[18]D.-Y.Peng,D.B.Robinson,A new two-constant equation of state,Ind.Eng.Chem.Fundam.15(1976)59-64.
[19]Z.-R.Yu,B.Singh,S.S.H.Rizvi,J.A.Zollweg,Solubilities of fatty acids,fatty acid esters,triglycerides,and fats and oils in supercritical carbon dioxide,J.Supercrit.Fluids7(1994)51-59.
[20]E.S.Perry,W.H.Weber,B.F.Daubert,Vapor pressures of phlegmatic liquids.I.Simple and mixed triglycerides,J.Am.Chem.Soc.71(1949)3720-3726.
[21]P.Coimbra,C.M.M.Duarte,H.C.De Sousa,Cubic equation-of-state correlation of the solubility of some anti-inflammatory drugs in supercritical carbon dioxide,Fluid Phase Equilib.239(2006)188-199.
[22]P.Coimbra,D.Fernandes,P.Ferreira,M.H.Gil,H.C.de Sousa,Solubility of Irgacure®2959 photoinitiator in supercritical carbon dioxide:experimental determination and correlation,J.Supercrit.Fluids45(2008)272-281.
[23]A.R.C.Duarte,P.Coimbra,H.C.de Sousa,C.M.M.Duarte,Solubility of flurbiprofen in supercritical carbon dioxide,J.Chem.Eng.Data49(2004)449-452.
[24]B.M.C.Soares,F.M.C.Gamarra,L.C.Paviani,L.A.G.Gonçalves,F.A.Cabral,Solubility of triacylglycerols in supercritical carbon dioxide,J.Supercrit.Fluids43(2007)25-31.
[25]H.L.N.Lau,Y.M.Choo,A.N.Ma,C.H.Chuah,Production of refined carotene-rich palm oil from palm mesocarp(Elaeis guineensis)using supercritical carbon dioxide,J.Food Lipids14(2007)396-410.
[26]W.B.Setianto,P.Atmaji,D.D.Anggoro,Solubility examination of palm kernel oil in supercritical CO2and its correlation with solvent density based model,Proc.Int.Conf.Chem.Mater.Eng,Semarang,Indonesia,2012(p.SPE.10-1-6).
[27]L.A.Follegatti-Romero,C.R.Piantino,R.Grimaldi,F.A.Cabral,Supercritical CO2extraction of omega-3 rich oil from Sacha inchi(Plukenetia volubilisL.)seeds,J.Supercrit.Fluids49(2009)323-329.
[28]M.Durante,M.Lenucci,G.Mita,Supercritical carbon dioxide extraction of carotenoids from pumpkin(Cucurbitaspp.):a review,Int.J.Mol.Sci.15(2014)6725-6740.
[29]S.Jokić,S.Svilović,Z.Zeković,S.Vidović,Mathematical modelling of soybean oil solubility in supercritical carbon dioxide,Int.J.Food Sci.Technol.46(2011)1031-1037.
[30]D.L.Sparks,R.Hernandez,L.A.Estévez,Evaluation of density-based models for the solubility of solids in supercritical carbon dioxide and formulation of a new model,Chem.Eng.Sci.63(2008)4292-4301.
[31]J.M.del Valle,J.C.de la Fuente,E.Uquiche,A refined equation for predicting the solubility of vegetable oils in high-pressure CO2,J.Supercrit.Fluids67(2012)60-70.
[32]S.G.Özkal,U.Salgın,M.E.Yener,Supercritical carbon dioxide extraction of hazelnut oil,J.Food Eng.69(2005)217-223.
[33]A.de Lucas,I.Gracia,J.Rincón,M.T.García,Solubility determination and model prediction of olive husk oil in supercritical carbon dioxide and cosolvents,Ind.Eng.Chem.Res.46(2007)5061-5066.
[34]H.N.Nguyen,P.D.Gaspillo,J.B.Maridable,R.M.Malaluan,H.Hinode,C.Salim,H.K.P.Huynh,Modeling ofMoringa oleiferaoil solubility in supercritical carbon dioxide,J.Chem.Eng.Process Technol.2(2011)114-118.
[35]I.M.do Prado,W.M.Giufrida,V.H.Alvarez,V.F.Cabral,S.Quispe-Condori,M.D.A.Saldaña,L.Cardozo-Filho,Phase equilibrium measurements of Sacha Inchi oil(Plukenetia volubilis)and CO2at high pressures,J.Am.Oil Chem.Soc.88(2011)1263-1269.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
