Super-hydrophobic and super-lipophilic functionalized graphene oxide/polyurethane sponge applied for oil/water separation☆
Huiwen Meng,Tao Yan,Jingang Yu,Feipeng Jiao*
School of Chemistry and Chemical Engineering,Central South University,Changsha 410083,China
1.Introduction
Nowadays,oil spills from industrial accidents have led to a serious environmental crisis of the world.To avoid such environmental disasters,therefore there is a growing need for the technological developments that can effectively remove pollutants such as oil spills and organic contaminants from water.Until now,various methods have been extensively used to separate the oils and organic contaminants from water,including mechanical collection,controlled burning,chemical dispersants,and using absorbent materials.However,with the exception of absorbent material,those ways have suffered from problems of high cost,time consuming or secondary pollution.So,absorptive materials are regarded as perfect materials to treat oils and organic contaminants for their low cost and ease of removal and collection.Particularly,with the development of colloid and interface science,and bionics in recent years,the bionic functional materials manufactured by simulating the special in filtration surface properties of various plants and animals in nature have aroused great concern in the field of oil-water separation.Compared to conventional absorptive materials,the bionic 3D super-hydrophobic and super-lipophilic materials such as inorganic based materials[1-4],porous polymers[1],carbon-based composites[5-14]and other materials[15-18]are excellent choices to remove oil pollutions from water,owing to the superiority of low cost,high absorption ability and good compressibility.However,before they gain further applications,there are some drawbacks that should be overcome.For instance,these materials are environmentally incompatible,raw materials are expensive,producing processes are complicated and severe conditions,and their structures are easily damaged during the oil-water separation process.Therefore,developing 3D super-hydrophobic and super-lipophilic materials with inexpensive raw materials,simple fabrication process,excellent flexibility,and environmental friendliness has become the urgent matter.
Commercial PU sponge is a kind of cheap 3D porous material.The porous microstructure of the PU sponge can provide a significantly large space for the storage of oils.Meanwhile,it possesses excellent flexibility.Thus,oils can be removed by simply squeezing,and the PU sponge can be reused.However,the PU sponge is naturally hydrophilic that it is not suitable for the PU sponge to selectively remove oils and organic pollutions from water with efficiency.However,it can be used as a skeleton.Therefore,modifications or physical coatings are required to heighten the hydrophobicity of the PU sponge for a higher efficiency of oil-water separation.Up to now,many materials such as TiO2nanoparticles,rGO,Fe3O4nanoparticles,SiO2and fluoropolymer(FP)have been applied to modify the PU sponges to increase the hydrophobicity of the modified PU sponge[19,20].
Graphene oxide(GO)is a typical two dimensional material with an extended layered structure.Some hydrophilic polar groups,including hydroxyl,carboxyl and epoxy groups,are embedded in its layers,which make GO easily dispersed in polar solvents and possess a rich intercalation chemistry[21].Owing to its low-cost,ease of synthesis,chemical stability,process ability and environmentally friendly properties,GO has attracted a lot of attention in the field of graphene-based functional materials in the last few years[22].Though GO is naturally hydrophilic,there are some ways to effectively adjust the characteristic of GO from hydrophilicity to super-hydrophobicity by grafting long chain alkanes on the GO surface.
In this study,we used a simple and inexpensive method to synthesize super-hydrophobic FGP sponge by coating functionalized graphene oxide nanosheets which were modified byn-Dodecyltrimethoxysilane(DTES)onto commercially available polyurethane sponge skeletons.The works of coating the PU sponge with functionalized GO synergistically constructed a typical nano-micro substructure and formed an alkylated surface chemical composition.This surface structure which was similar with lotus leaf was suitable to substantially enhance the hydrophobicity of the PU sponge.The simple fabricated process avoided the reduction of GO,ensured the integrity of structure of the FGP sponge and effectively enhanced the anti-corrosive properties of the FGP sponge.Besides,provided with high selectivity,excellent capacities of oil absorption and good repeatability,it can be widely applied in absorbing all sorts of oils and organic solvents.The FGP sponge in this work may provide a new and practical approach for large-scale removal of organic contaminants from water.
2.Experimental
2.1.Materials
All chemical reagents were of analytical grade level and used without further purification.The deionized water was used to prepare all solutions.PU sponges were purchased from Alibaba Enterprise.DTES and xylene were supplied from Shandong Xiya Reagent.Tetrachloromethane and dichloromethane were obtained from Sinopharm Chemical Reagent Co.,Ltd.n-Hexane was purchased from Shanghai Titan Scientific Co.,Ltd.Hydrogen peroxide was selected from Tianjin Fuqi Chemical Co.,Ltd.Hydrochloric acid and sulfuric acid were obtained from Zhuzhou Starry Sky Glass Co.,Ltd.Acetic acid was supplied from Sinopharm Chemical Reagent Co.,Ltd.Kerosene and sesame oil were purchased from nearby stores.
2.2.Preparation of silane-functionalized graphene oxide nanosheets(FGO)
GO powders were synthesized from graphite by the modified Hummers method.As is known to all,hydroxyl functional groups on GO could dehydrate with silane coupling agents.Brie fly,3 ml of DTES was mixed with 2 ml of water and 38 ml of ethanol,then 1 mol·L-1acetic acid solution was added into the mixture until a pH between 4 and 5 was reached.Then,the mixture was stirred with a magnetic stirrer for 1 h.Finally,100 mg GO and the DTES solution were poured into a round-bottom flask to heat at 65°C for6 h.Then FGO was ultrasonically treated with ethanol for 3 times,followed by drying in the vacuumoven at 60°C overnight.
2.3.Preparation of silane-functionalized graphene oxide/polyurethane sponge
In the typical process,the PU sponge was cut into cubes and ultrasonically treated with ethanol and deionized water for 30 min and dried at 60°C.The as-dried sponge was then immersed into a FGO suspension(the solvent is ethanol),and finally dried in the oven for 2 h at 120°C.The whole process was displayed in Fig.1.
2.4.Oil absorption of the FGP sponge
The absorption capacities(Q)of the FGP sponges for six types of oils and organic solvents(tetrachloromethane,dichloromethane,sesame oil,xylene,kerosene andn-Hexane)were tested.All tests were performed at room temperature.A block of the FGP sponge was put into a 200 ml glass beaker filled with enough oil for 5 min.Then the soaked FGP sponge was taken out and the mass was recorded.TheQvalue was calculated with the equation:

whereM0andM1are the mass of the sponge before and after the absorption of oil.
2.5.Characterization

Fig.1.Schematic illustration of the fabrication procedure and mechanism for the FGP sponge.
A Nicolet-Avatar 360 FTIR spectrometer within the wave number from 400 to 4000 cm-1measured the surface functional groups of samples.The phase and crystal structures of the specimens were checked by an X-ray diffractometer(XRD,Bruker D8)using Cu Kα radiation(λ=0.15406 nm,40 kV,40 mA).The morphology of the samples was examined by Scanning electron microscopy(SEM)using a TESCAN MIRA3 LMU microscope.The water contact angles(WCAs)were measured by the instrument(JC2000D1,Powereach Co.Ltd.)at room temperature.WCAs on all samples were measured for three times and average value was taken.
3.Results and Discussion
3.1.Characterization of products
3.1.1.FT-IR
The FT-IR spectra of GO,the pure PU sponge and the FGP sponge were displayed in Fig.2.The four absorption peaks at 1141(alkoxy C--O),1620(C=C stretching),1734(C=O stretching),and 3402(--OH stretching)cm-1were in good agreement with the GO spectra[23].As compared with the pristine GO,the intensity of the characteristic absorption peaks of the FGP sponge was remarkably reduced,proving that most of the oxygen-containing groups were removed.The absorption peaks at 1123 and 1062 cm-1were the characteristic peaks of the Si--O,and the absorption peaks at1401 cm-1(C--Hbending),1464(C--H bending),2852(C--H stretching)and 2922(C--H stretching)were ascribed to the alkyl group of DTES,offering further evidence for chemical functionalization of the GO[24].Apparently,it can be found that mostly typical absorption peaks of the PU and FGP sponges between 600 and 1800 cm-1were similar[25].Otherwise,the absorption peak at 1547 cm-1belongs to the amide II band,suggesting that the ternary composite network structure of the PU sponge retained in the FGP sponge[26].

Fig.2.FTIR spectra for(a)GO,(b)PU sponge and(c)FGP sponge.
3.1.2.XRD
Fig.3 showed the XRD diffraction patterns of GO,pure PU sponge and FGP sponge.A typical sharp peak at 9.96°appeared in Fig.3(a),which was a characteristic peak of GO.Besides,it was in accordance with the previously reported results[27].As shown in Fig.3(b),the PU sponge had a typical broad characteristic,it was a typical characteristic peak of the polymer.As shown in Fig.3(c),the peak of GO at 9.96°disappeared in the XRD diffraction patterns of the FGP sponge,which indicated that GO was completely reduced.Otherwise,as shown in Fig.3(c),the FGP sponge possessed an obvious but dispersive peak centered at 19.5°,owing to the joint operation of polymer matrix and graphene sheets[28].We can conclude that the process of modification was sufficient to modify GO and permitted FGO to be well dispersed and adherent to the reticular matrix of the PU sponge.

Fig.3.XRD patterns for(a)GO,(b)the PU sponge and(c)the FGP sponge.
3.1.3.FESEM
FESEM is used to inspect the morphological evolution of the pure PU sponge and FGP sponge,successively.The morphology of the pure PU sponges was displayed in Fig.4(a),b and c.It can be observed that the PU sponges had three-dimensional porous network structures,and the skeletons of the PU sponge were completely smooth.The porous structure offered enough space to store the absorbed oils.The morphology of the FGP sponges was shown in Fig.4(d),e and f.The FGP sponges had similar three-dimensional porous network structures with the pure PU sponges,indicating that the process of coating in this work allowed three-dimensional porous network structures of the PU sponge to be retained in the FGP sponge.However,the skeletons of the FGP sponge exhibited a rough texture.It was believed that the PU sponge provided a substrate for FGO sheets to attach to the skeletons of the pure PU sponge.Meanwhile,the surface structure of the FGP sponge looked quite similar to the lotus.Their surface both possesses micro-nano structure and low surface energy,providing further evidence for the super-hydrophobic characteristic of the FGP sponge.
3.1.4.WCA
As displayed in Fig.5(a),a FGP sponge(black color) floated on the surface of water while a pure sponge(yellow color)suspended in the water.It can be kwon that the FGP sponge was hydrophobic.Furthermore,the FGP sponge can be immersed into water by an outside force,but when the outside force was released,it immediately floated on the surface of the water,and no water was found by squeezing the sponge.A photograph was displayed in Fig.5(b),where the FGP sponge surface looked like a silver mirror when it was immersed in water by an outside force,which was ascribed to the whole FGP sponge being wrapped around a thick layer of bubble,which was referred to so-called non-wetting Cassie-Baxter surfaces[29].As shown in Fig.5(c),a water droplet(dyed with Congo Red)maintained nearly a spherical shape when it was placed on the FGP sponge surface.The reason was that a solidliquid-air interface formed under the water.On the contrary,when a kerosene droplet was dropped on the surface of the FGP sponge,it quickly spread over the surface and was instantaneously sucked by the FGP sponge,as shown in the circle(Fig.5(c)).Due to the properties of super-lipophilic of FGP sponge.Besides,the hydrophobic properties of the materials were often evaluated by the water contact angle(WCA).An optical image of a water droplet(3 μl)on the surfaces of the FGP sponge was shown in Fig.5d.We can see that the WCA is 152°± 1°.The strong hydrophobicity can be attributed to the combination of the relatively low surface energy of the FGO on the skeletons of the FGP sponge and the micro/nano-textured structure which was caused by the double effect of wrinkled and folded FGO and micro-porous structure of the sponge.

Fig.4.SEM images with different magnifications for pure PU sponge(a),(b)and(c)and(d),(e)and(f)FPG sponge.
3.2.The oil absorption capacity of the FGP sponge
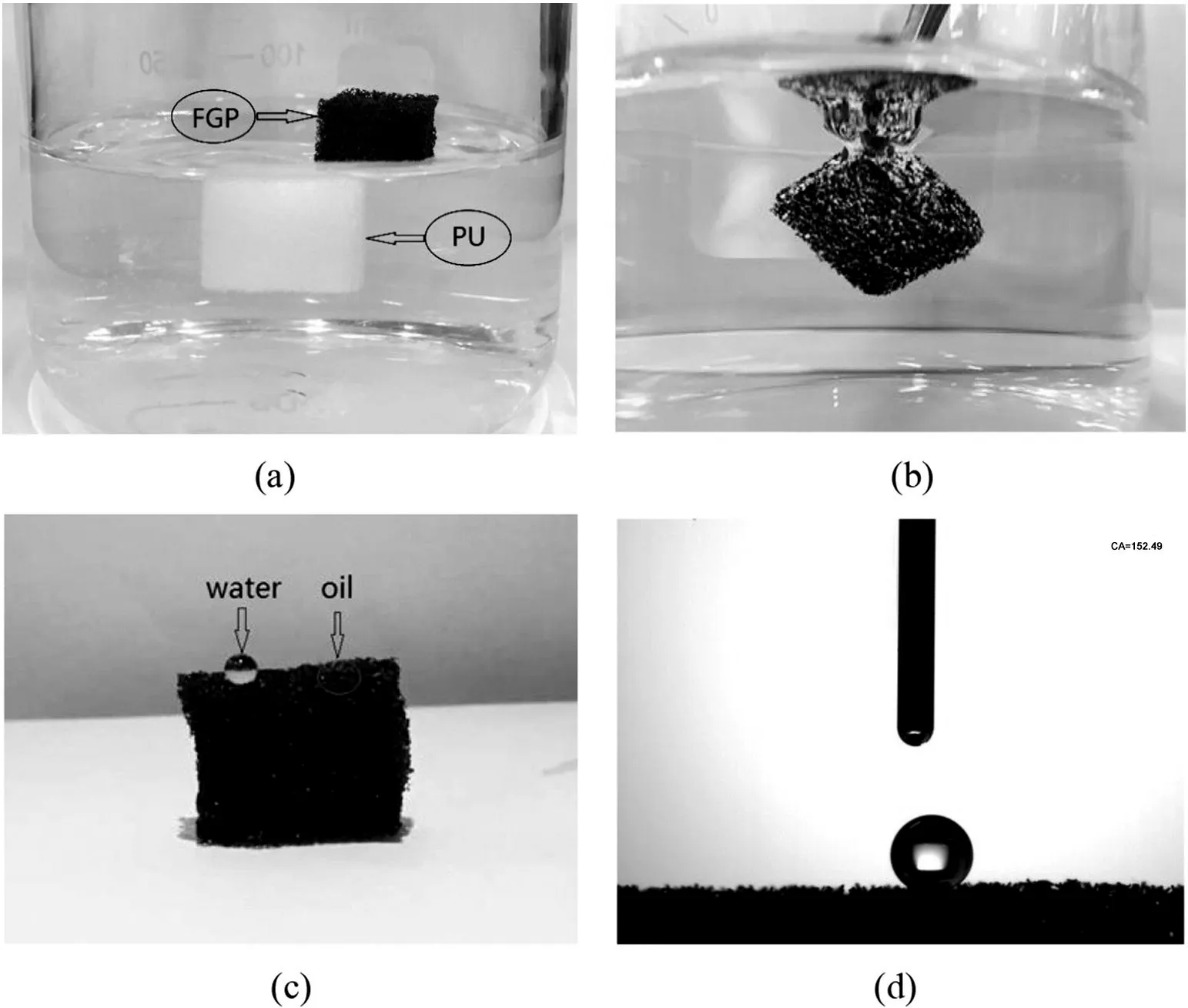
Fig.5.(a)Photograph of pure(yellow color)and FGP sponge(black color)after being placed on water.(b)Photograph of the FGP sponge immersed in water by a force.(c)Water droplets and kerosene trace on the surface of the FGP sponge.(d)Photograph of water contact angle(CA)on the surface of the FGP sponge.

Fig.6.Snapshots of the removal process of n-Hexane(dyed with Sudan II for clear observation)(a)and dichloromethane(b)from water using the FGP sponges.(c)Oil and organic solvent absorption capacities of the FGP sponge and PU sponge.(d)Absorption recycl ability of the FGP sponges for oils and organic solvents.
To evaluate the separation ability of the FGP sponge,the removal of oil on the surface of water wastested.As exhibited in Fig.6(a),a piece of the FGP sponge was forced to contact with a layer of the kerosene(dyed with Sudan II)on the surface of the water,it absorbed the oil completely from the oil/water mixture within a few seconds.Then,taking the FGP sponge out to separate the oil from the water,it cleanly provided a useful method to remove oils and organic solvents from a polluted area.The removal of organic solvent on the bottom of water was also tested.Similarly,the FGP sponge was forced to approach the dichloromethane in the bottom of water,it can be seen that the dichloromethane(dyed with Sudan II)droplet was rapidly absorbed when the FGP sponge contacted the droplet(Fig.6(b)).To further evaluate the absorption capacity and selectivity of the FGP sponge.The FGP sponges were placed on the surface of a variety of oils and organic solvents(tetrachloromethane,dichloromethane,sesame oil,xylene,kerosene andn-Hexane).As depicted in Fig.6(c),the absorption capacities of the FGP sponge range from 16 to 35 times of original mass for a range of oils and solvents.The changes of the absorption capacity probably depended upon the density of oils and organic solvents.Besides,the absorption capacities of the FGP sponge were similar with the pure PU sponge.It was believed that the absorption capacities of the PU sponge were not weakened after coating with functionalized graphene oxide nanosheets.The absorption capacities were higher than nanowire membranes(<20 times)[1],and were similar with micro-porous polymers(<33 times)[31].However,in this study,the FGP sponge used cheap commercial polyurethane sponge as skeleton,so it possesses excellent flexibility,low density and easily scalable fabrication.Besides,the fabrication of the FGP sponge was more facile,the oils sucked in the FGP sponge were easily gathered by squeezing,and the FGP sponge can be reused after being washed and dried.The recyclability of the FGP sponge is also a key factor in oil or chemical cleanup applications.The recyclability of the FGP sponge for oil-water separation was shown in Fig.6(d).The FGP sponge can be reused into the oil absorption procedure after being washed in acetone and dried in an oven.Otherwise,the absorption capacity of the FGP sponge was higher than 90%of the first maximum 10 cycles of testing.
4.Oil-Water Separation Mechanism
When water droplets were placed on the FGP sponge surface,a solid-liquid-air interface formed under the water,as shown in Fig.7(a),a lot of air filled in the pore of the composite material surface,the real contact area of solid surface and water is far less than the apparent area.The super-hydrophobicity and high WCAs of the FGP sponge can be described by the Wenzel equation and Cassie-Baxter equation.


Fig.7.Scheme for liquid droplets placed on rough surfaces of the superhydrophobic PU/Cu composite(a)water droplet(b)oil droplet.(c)Photograph of water contact angle(CA)on the surface of the PU sponge.
where θrand θ are the water contact angles on the FGP sponge and PU sponge surfaces,respectively.For the FGP sponge surface in this study,θrand θ were 152°(Fig.5(d))and 96°(Fig.7(c)),respectively.The variablesf1andf2are the solid and air fractions,respectively,under the water droplet;hence,f1+f2=1.Solving Eq.(1)gives anf2value of 0.8692.Which means that about 86.92%contact area is the water/solid contact interface.Therefore,super-lipophobicity can be observed.
When oil droplets contact with the FGP sponge,oil droplets can in filtrate material surface,an oil-solid interface formed under the oil,as shown in Fig.7(b).According to the Wenzel equation,the surface-lipophilicity was enhanced by the high surface roughness,resulting in a super-lipophilic surface.Capillary channel was formed on the porous structure of the FGP sponge surface by DTES.When oil droplets contacted the FGP sponge,they rapidly spread on the surface of materials by capillary force and the force of in filtration and spread of the material.Therefore,when the oil drops contact the FGP sponge,the oil drops are absorbed quickly.
5.Conclusions
In conclusion,this study reported a facile and cheap approach to fabricate a silane-functionalized graphene oxide/polyurethane sponge.Functionalized graphene oxide nanosheets which were modified byn-Dodecyltrimethoxysilane were controllably anchored on a commercially available polyurethane sponge.The results expressed that FGP sponge exhibited a similar surface structure to that of a lotus leaf,and possessed super-hydrophobic and super-lipophilic characteristics with the WAC of 152°.The FGP sponge can quickly absorb tetrachloromethane,dichloromethane,sesame oil,xylene,kerosene andn-Hexane.More importantly,the FGP sponge possessed high absorption capacities(up to 35 times of its own mass)and could still maintain its super-hydrophobicity and oil absorption capacity even after ten cycles of absorption-desorption.Furthermore,the cost-effective,simple,and scalable fabricated strategy for the FGP sponge is of great significance for the large-scale production of adsorbents.We believed that the FGP sponge in this work may present a new and practical method for large-scale removal of organic contaminants from water.
[1]J.Yuan,X.Liu,O.Akbulut,et al.,Superwetting nanowire membranes for selective absorption,Nat.Nanotechnol.3(6)(2008)332-336.
[2]Z.C.Ma,L.M.Wang,D.Q.Chu,et al.,Fabrication of an α-MoO3nanobelt membrane showing a three-dimensional cross-linked nano-scale network structure for water and oil mixture separation,RSC Adv.5(35)(2015)27398-27401.
[3]R.Arora,K.Balasubramanian,Hierarchically porous PVDF/nano-SiC foam for distant oil-spill cleanups,RSC Adv.4(96)(2014)53761-53767.
[4]W.Liang,Y.Liu,H.Sun,et al.,Robust and all-inorganic absorbent based on natural clay nanocrystals with tunable surface wettability for separation and selective absorption,RSC Adv.4(24)(2014)12590-12595.
[5]X.Gui,H.Li,K.Wang,et al.,Recyclable carbon nanotube sponges for oil absorption,Acta Mater.59(12)(2011)4798-4804.
[6]H.Wang,Y.Gong,Y.Wang,Cellulose-based hydrophobic carbon aerogels as versatile and superior adsorbents for sewage treatment,RSC Adv.4(86)(2014)45753-45759.
[7]C.F.Wang,S.J.Lin,Robust superhydrophobic/superoleophilic sponge for effective continuous absorption and expulsion of oil pollutants from water,ACS Appl.Mater.Interfaces5(18)(2013)8861-8864.
[8]Z.Y.Wu,C.Li,H.W.Liang,et al.,Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions,Sci.Rep.4(2014)4079.
[9]Y.Gao,Y.S.Zhou,W.Xiong,et al.,Highly efficient and recyclable carbon soot sponge for oil cleanup,ACS Appl.Mater.Interfaces6(8)(2014)5924-5929.
[10]H.Bi,X.Huang,X.Wu,et al.,Carbon microbelt aerogel prepared by waste paper:an efficient and recyclable sorbent for oils and organic solvents,Small10(17)(2014)3544-3550.
[11]Y.Yang,Q.Zhang,S.Zhang,et al.,Triphenylamine-containing microporous organic copolymers for hydrocarbons/water separation,RSC Adv.4(11)(2014)5568-5574.
[12]L.Peng,H.Li,Y.Zhang,et al.,A superhydrophobic 3D porous material for oil spill cleanup,RSC Adv.4(87)(2014)46470-46475.
[13]X.Gui,Z.Zeng,Z.Lin,et al.,Magnetic and highly recyclable macroporous carbon nanotubes for spilled oil sorption and separation,ACS Appl.Mater.Interfaces5(12)(2015)5845-5850.
[14]J.N.Wang,Y.L.Zhang,Y.Liu,et al.,Recent developments in superhydrophobic graphene and graphene-related materials:from preparation to potential applications,Nano7(16)(2015)7101-7114.
[15]F.Zhao,L.Liu,F.Ma,et al.,Candle soot coated nickel foam for facile water and oil mixture separation,RSC Adv.4(14)(2014)7132-7135.
[16]L.Zhang,J.Wu,X.Zhang,et al.,Multifunctional,marvelous polyimide aerogels as highly efficient and recyclable sorbents,RSC Adv.5(17)(2015)12592-12596.
[17]C.Chen,R.Li,L.Xu,et al.,Three-dimensional superhydrophobic porous hybrid monoliths for effective removal of oil droplets from the surface of water,RSC Adv.4(33)(2014)17393-17400.
[18]P.Xi,L.Huang,Z.Xu,etal.,Low cost and robust soot dipped polyurethane sponge for highly efficient and recyclable oil and organic solvent cleanup,RSC Adv.4(103)(2014)59481-59485.
[19]D.Wu,W.Wu,Z.Yu,et al.,Facile preparation and characterization of modified polyurethane sponge for oil absorption,Ind.Eng.Chem.Res.53(52)(2014)20139-20144.
[20]Y.Liu,J.Ma,T.Wu,et al.,Cost-effective reduced graphene oxide-coated polyurethane sponge as a highly efficient and reusable oil-absorbent,ACS Appl.Mater.Interfaces5(20)(2013)10018-10026.
[21]A.K.Geim,K.S.Novoselov,The rise of grapheme,Nat.Mater.6(3)(2007)183-191.
[22]S.Pei,H.M.Cheng,The reduction of graphene oxide,Carbon50(9)(2012)3210-3228.
[23]A.Mariconda,P.Longo,A.Agovino,et al.,Synthesis of ruthenium catalysts functionalized graphene oxide for self-healing applications,Polymer69(2015)330-342.
[24]P.Singhal,W.Small,E.Cosgriff-Hernandez,et al.,Low density biodegradable shape memory polyurethane foams for embolic biomedical applications,Acta Biomater.10(1)(2014)67-76.
[25]B.Yu,X.Wang,W.Xing,et al.,Enhanced thermal and mechanical properties of functionalized graphene/thiol-ene systems by photopolymerization technology,Chem.Eng.J.228(2013)318-326.
[26]D.A.Middleton,J.Madine,V.Castelletto,etal.,Insights into the molecular architecture of a peptide nanotube using FTIR and solid-state NMR spectroscopic measurements on an aligned sample,Angew.Chem.Int.Ed.52(40)(2013)10537-10540.
[27]S.Dai,Q.Ma,T.Andersen,et al.,Subdiffractional focusing and guiding of polaritonic rays in a natural hyperbolic material,Nat.Commun.6(2015).
[28]V.Chabot,D.Higgins,A.Yu,et al.,A review of graphene and graphene oxide sponge:material synthesis and applications to energy and the environment,Energy Environ.Sci.7(5)(2014)1564-1596.
[29]I.A.Larmour,S.E.J.Bell,G.C.Saunders,Remarkably simple fabrication of superhydrophobic surfaces using electroless galvanic deposition,Angew.Chem.Int.Ed.46(10)(2007)1710-1712.
[30]A.Li,H.X.Sun,D.Z.Tan,et al.,Superhydrophobic conjugated microporous polymers for separation and adsorption,Energy Environ.Sci.4(6)(2011)2062-2065.
 Chinese Journal of Chemical Engineering2018年5期
Chinese Journal of Chemical Engineering2018年5期
- Chinese Journal of Chemical Engineering的其它文章
- Bioregeneration of spent activated carbon:Review of key factors and recent mathematical models of kinetics
- CFD simulations of quenching process for partial oxidation of methane:Comparison of jet-in-cross- flow and impinging flow configurations☆
- Quantifying growth and breakage of agglomerates in fluid-particle flow using discrete particle method☆
- Coupling simulation of fluid structure interaction in the stirred vessel with a pitched blade turbine☆
- An integrated model for predicting the flame propagation in crimped ribbon flame arresters☆
- Assessment of k-ε models using tetrahedral grids to describe the turbulent flow field of a PBT impeller and validation through the PIV technique
