Exosomes: a novel therapeutic target for Alzheimer’s disease?
Zhi-You Cai , Ming Xiao, Sohel H. Quazi, Zun-Yu Ke
1 Department of Neurology, Chongqing General Hospital, Chongqing, China
2 Department of Anatomy, Nanjing Medical University, Nanjing, Jiangsu Province, China
3 Department of Biological and Health Sciences, Texas A & M University‐Kingsville, Kingsville, TX, USA
4 Department of Neurology, Renmin Hospital, Hubei University of Medicine, Shiyan, Hubei Province, China
Introduction
Alzheimer’s disease (AD) is the most common cause of de‐mentia, and an age‐related neurodegenerative disease char‐acterized by progressive memory loss and declining cognitive function. Multiple pathogenic hypotheses have been proposed including amyloid, extracellular beta‐amyloid (Aβ) peptide deposition (Lloret et al., 2011; Busche et al., 2016), intracellu‐lar accumulation of hyperphosphorylated tau protein (formation of neurofibrillary tangles) (Lloret et al., 2011; Busche et al., 2016; Panza et al., 2016), cholinergic dysfunction (Picon et al., 2010; Cacabelos et al., 2014), neuroinflammation, and oxidative stress (Latta et al., 2015; Liu et al., 2015). Although several medications are available to treat AD, none of them stop or reverse the disease (de la Torre, 2010). Increased knowledge on available treatments and the existing pathogen‐esis of AD will be bene ficial for reaching the best decisions on medications and preventing progression of AD.
Apart from macromolecular complexes and small molecules,a large number of microvesicles are secreted from cells into the extracellular space (Vlassov et al., 2012; Beach et al., 2014).Microvesicles (also known as circulating microvesicles or mi‐croparticles) are fragments of plasma membrane between 100 nm and 1,000 nm in diameter. Exosomes are microvesicles secreted by all cells. Exosomes first fuse with multivesicular bodies(MVBs) and the cell surface, and are subsequently released from MVBs into the extracellular space (Laulagnier et al., 2004; Vlass‐ov et al., 2012). MVBs are referred to as late endosomes and contain internal vesicles. The most important role of exosomes is to act as intercellular communication messengers by delivering macromolecules between cells (Thery et al., 2002; Beach et al., 2014). Substantial evidence suggests that exosomes serve as active mediators of neurodegenerative disorders, and transport disease particles such as α‐synuclein (Chang et al., 2013; Kong et al., 2014; Tsunemi et al., 2014; Grey et al., 2015), Aβ, and pri‐ons from their cells of origin to other cells (Kalani et al., 2014;Yuyama et al., 2014; Fiandaca et al., 2015; Yuyama et al., 2015).This review discusses the role of exosomes in the pathogenesis of AD, and addresses the association between exosomes and relevant AD pathologies (Aβ, neurofibrillary tangles, oxidative stress, and inflammation). Moreover, this review provides a proposed general role of exosomes in AD pathogenesis, and dis‐cusses novel therapeutic interventions of exosomes for AD.
Exosomes
Exosomes are microvesicles of 30—100 nm in diameter, and small lipid vesicles secreted by all cell types (Kastelowitz and Yin, 2014; Sluijter et al., 2014). Internal vesicles formed by the inward budding of cellular compartments are known as MVBs(Vlassov et al., 2012; Al‐Nedawi, 2014). When MVBs fuse with the plasma membrane, exosomes are released from these internal vesicles. Exosomes exist in blood, saliva, urine, breast milk, and other body fluids (Vlassov et al., 2012; Qin and Xu,2014). Exosomes not only maintain normal cellular functions and cellular viabilityviahousekeeping roles, but also safeguard various functions of multicellular organisms (Ohno, 2006).
Exosomes contain various substances, including small RNAs, lipids, and a variety of proteins (Vitek et al., 1994;Beach et al., 2014). MicroRNAs (miRNAs) target messenger RNAs for degradation and prevent translation. It has been shown that exosome‐released miRNAs regulate the inflam‐matory response to external environmental changes. For example, exosome‐delivered miR‐155 increases expression of inflammatory genes, while miR‐146a decreases their expression (Gao et al., 2016). The most intriguing role of exosomes are as conveyors of proteins and lipids, which affect down‐stream signaling events in recipient cells and influence various aspects of cell behavior and physiology, including nerve regeneration, and synaptic function and behavior (Arscott et al., 2013; Chen et al., 2014). Exosomes may serve as vesicular carriers for intercellular communication in neurodegenerative disorders (Schneider and Simons, 2013). Further, they play an ominous role in propagation of toxic Aβ pathology(enhancing Aβ generation and deposition, and inhibiting Aβ clearance), abnormal tau phosphorylation, and triggering neuroinflammation and oxidative stress by exchange of information between neurons and glia in neurodegenerative AD conditions (Saman et al., 2014; Yuyama et al., 2014, 2015;Fiandaca et al., 2015; Goetzl et al., 2016; Shi et al., 2016).
Exosomes and Neurodegeneration
Neurodegeneration encompasses a series of diseases due to loss of structure and function of nerve cells in the brain.Most attention has been focused on Parkinson’s disease (PD),Huntington’s disease, and AD (Tonekaboni and Mollamo‐hammadi, 2014; Mouton‐Liger et al., 2015). Indeed, a large proportion of less popular diseases have been ignored (Kerner,2014), such as multiple sclerosis (Orack et al., 2015), epilepsy(Aboud et al., 2013; Pottoo et al., 2014), and stroke (Seifert et al., 2014; Walberer et al., 2014). Increasing evidence indicates that exosomes are involved in neurodegenerative disorders as potential carriers of misfolded proteins (Russo et al., 2012;Candelario and Steindler, 2014; Kalani et al., 2014).
Two of the most common neurodegenerative diseases are AD and PD. The main cause of PD is death of dopaminergic cells in the substantia nigra. Emerging studies have shown that progression of neurodegeneration in PD may involve release of toxic forms of α‐synuclein, which are taken up by neighboring neurons and trigger dysfunction (Russo et al.,2012). Several studies have noted that exosomes are involved in PD pathogenesis such as acceleration of α‐synuclein ag‐gregation (Tsunemi et al., 2014; Grey et al., 2015). Molecular biology data support the proposition that lysosomal dys‐function leads to increased α‐synuclein release in exosomes,and a concomitant increase in α‐synuclein transmission to recipient cells (Alvarez‐Erviti et al., 2011). Additionally, anin vitrostudy suggested involvement of ATP13A2/(PARK9)in exosome biogenesis and α‐synuclein secretion (Tsunemi et al., 2014). While PD‐linked human ATP13A2/(PARK9)promotes α‐synuclein externalizationviaexosomes (Kong et al., 2014). Furthermore, exosomes secreted from activated microglia are important mediators of α‐synuclein‐induced neurodegeneration (Chang et al., 2013).
Exosomes and Amyloid Pathology
Senile plaques produced by accumulation of Aβ are a classical hallmark of AD. Aβ originates from sequential cleavage of amyloid precursor protein (APP) (Tam et al., 2014; Agostin‐ho et al., 2015). Cleavage by β‐secretase within the luminal/extracellular domain leads to generation of β‐carboxyl‐ter‐minal fragments (CTFs) (Cai et al., 2012; Ortega et al., 2013).Following β‐secretase cleavage, γ‐secretase processes APP at the carboxyl‐terminus to produce Aβ. CTFs of APP can ac‐cumulate in MVBs and be released from the cell in exosomes(Sharples et al., 2008). Exosomes also contain CTFs and β‐and γ‐secretases (Sharples et al., 2008), indicating a wider role in APP metabolism.
Formation and clearance of Aβ are associated with endo‐somal compartments as Aβ and CTFs are secreted from exo‐somes (Rajendran et al., 2006). Cleavage of APP by β‐secretase occurs in early endosomes (Rajendran et al., 2006).Exosome‐associated Aβ levels increased more significantly in the cerebrospinal fluid of younger cynomolgus monkeys and APP transgenic mice compared with older animals(Yuyama et al., 2015). Additional evidence has con firmed that exosomes promote Aβ aggregation and accelerate amyloid plaque formation (Dinkins et al., 2014). Meanwhile,in vivoexosome reduction contributes to lower amyloid plaque load in the 5xFAD mouse model, a mouse line that expresses five mutations of familial AD (Dinkins et al., 2014).
Recent evidence revealed that infusion of neuronal exosomes into the brain of APP transgenic mice decreased Aβ generation and deposition, which was not observed with glial exosomes(Yuyama et al., 2015). This finding highlights the role of neuronal exosomes in Aβ clearance (Yuyama et al., 2015), and suggests that diminished secretion of neuronal exosomes may relate to Aβ accumulation, and ultimately, development of AD pathology (Figure 1). Indeed, it appears that improving Aβ clearance by exosome administration may provide a novel therapeutic intervention for AD (Yuyama et al., 2014).
Exosomes and Neuro fibrillary Tangles
Neurofibrillary tangles are aggregates of hyperphosphorylated tau protein (Gendreau and Hall, 2013). Definitive diagnosis of AD requires postmortem identification of amyloid plaques and neurofibrillary tangles. Several studies suggest that tau can be secreted from neuronsviaexosomes, and exosomerelated tau may be an important contributor to spreading neu‐rofibrillary lesions (Vingtdeux et al., 2012; Saman et al., 2014).Exosomes as a novel way of interneuronal communication,participate in spreading pathological proteins (such as APP fragments, phosphorylated tau, or α‐synuclein) across the nervous system (Chivet et al., 2012, 2013). There is signifi‐cant correlation between multiple genes of AD and proteins recruited to exosomes by tau overexpression (Saman et al.,2014). A clinical study showed that exosome levels of total tau (pT181‐tau and pS396‐tau) were significantly higher in AD patients than case‐controls, both 1—10 years before and at AD diagnosis, suggesting that pS396‐tau and pT181‐tau levels in extracts of neutrally‐derived blood exosomes predict AD development before clinical onset (Fiandaca et al., 2015).In addition, exosome‐associated tau phosphorylated at Thr‐181 (AT270) is present in human cerebrospinal fluid samples,suggesting that phosphorylated tau induced by exosome se‐cretion may contribute to abnormal tau processing (Saman et al., 2012).
Exosomes as Mediators of Neuroin flammation Associated with AD
Inflammation represents a response induced by injury or destruction of tissues, which enables removal, dilution, or isolation of both injurious substances and injured tissue.Neuroinflammation is inflammation of nervous tissue, and is a pathological and physiological process in response to a variety of events (Cai et al., 2013a, 2014), including microbial infections (Cox et al., 2013), chemical substances (de Rivero Vaccari et al., 2016), tissue necrosis from ischemia and an‐oxia (Maddahi and Edvinsson, 2010), traumatic brain injury(Lozano et al., 2015; de Rivero Vaccari et al., 2016), toxic metabolites (Butterworth, 2011; McMillin et al., 2014), and autoimmunity (Liu et al., 2014; Morales et al., 2014). It is well known that inflammation can be classified as either acute or chronic. As a common inflammatory process, acute neu‐roinflammation occurs immediately following injury to the central nervous system. It is characterized by the release of in flammatory molecules, glial cell activation, endothelial cell activation, platelet deposition, and tissue edema. Meanwhile,chronic neuroinflammation is of longer duration, with main‐tained glial cell activation and recruitment of other immune cells in the brain (Millington et al., 2014; Phillips et al., 2014).Neuroinflammation is regarded as chronic inflammation of the central nervous system. Mounting evidence shows that AD is associated with chronic inflammatory responses, with sustained presence of inflammatory cytokines from activated microglia and astrocytes, free radicals, and oxidative stress(Kaur et al., 2015; Latta et al., 2015; Zhang and Jiang, 2015).Exosomes are emerging as important inflammatory media‐tors because of their role as cargo of inflammatory molecules,and thereby induce neuroinflammation by exchange of information between neurons and glia (Gupta and Pulliam, 2014;Kore and Abraham, 2014; Rajendran et al., 2014; Fernan‐dez‐Messina et al., 2015; de Rivero Vaccari et al., 2016). Aβ is effectively packaged into exosomes and spread from one cell to another, initiating an inflammatory cascade (Gupta and Pulliam, 2014). In addition to releasing inflammatory factors,exosomes secreted by dead brain cells can influence bystander cells by the transfer of inflammatory mediators in response to pathogenic stimuli (Prado et al., 2010; Sun et al., 2010; Gupta and Pulliam, 2014). Extracellular exosomes release Aβ and accelerate amyloid plaque formation, which are important causes of neuroinflammation (Engel, 2014). Considering their ability to mediate intercellular communication between cells(Record, 2014; Salido‐Guadarrama et al., 2014; Zhang and Grizzle, 2014), exosomes represent one of the key players in transporting neurotoxic inflammatory agents and spreading progression of inflammation in brain cells. Oversecretion of exosomes is harmful and can strengthen progression of inflammation in the extracellular microenvironment. Nonetheless, despite abundant evidence demonstrating a role for exosomes in regulating the inflammatory response, the exact mechanisms remain unclear. Therefore, improved under‐standing of the role of exosomes in inflammation at different stages of AD will benefit prevention and treatment of AD.
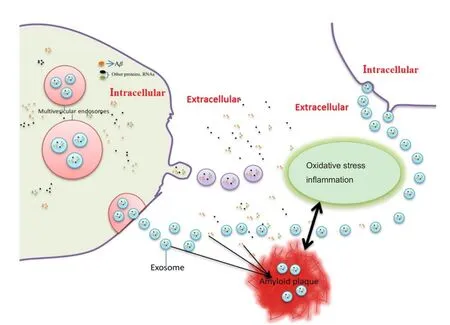
Figure 1 Schematic diagram of the emerging role of exosomes in betaamyloid peptide pathology.
Oxidative Stress: A Direct Mediator of Exosome Release in AD?
Extensive research has shown that oxidative stress is strongly linked to AD pathogenesis (Cai et al., 2011, 2013b; Ferreira et al., 2015). An important feature of AD is an active and self‐perpetuating cycle of chronic neuroinflammation and oxidative stress that may contribute to irreversible neuronal dysfunction and cell death (Cai, 2014). Oxidative stress is proposed to contribute to Aβ generation and formation of neurofibrillary tangles (Santos et al., 2014; Kanamaru et al.,2015; Kamat et al., 2016). Many results show that neuroin‐flammation‐induced oxidative stress increases Aβ generation by enhancing β‐ and γ‐secretase activity (Cai et al., 2011;Bonda et al., 2014; Chang et al., 2014). In addition, intracellular Aβ accumulation promotes significant oxidative and inflammatory mechanisms that generate a vicious cycle of Aβ generation and oxidation, each accelerating the other(Luque‐Contreras et al., 2014; Persson et al., 2014).
Many studies have noted that exosome release from MVBs are induced and accelerated by oxidative stress (Soderberg et al., 2007; Eldh et al., 2010; Zhou et al., 2013; Tsanova et al.,2014). Previous studies have indicated that exosome release from MVBs is associated with the pathogenesis of many dis‐eases involved in oxidative stress (Tsanova et al., 2014), such as multiple sclerosis and dysmyelinating syndromes (Pusic et al., 2014), cancer (Goldkorn et al., 2013; Meseure et al., 2014),cerebral ischemia disease (DeGracia et al., 2008; Fröhlich et al., 2014), as well as cardiovascular disease (Fleury et al., 2014;Yamaguchi et al., 2015). However, many questions have not been answered: what is the exact role of exosome release mediated by oxidative damage in AD pathogenesis? Is release of exosomes from MVBs a cause or consequence of oxidative stress in AD? What is the relationship between oxidative‐mediated release of exosomes and AD pathology?
Exosomes: A Novel Therapeutic Strategy for AD?
AD is a progressive brain disorder and the most common form of dementia. To date, there is still no cure for AD that can reverse or halt its progress, although there are medications that can help improve symptoms in some cases. Exosomes are extracellular vesicles that transport different molecules between cells. They are formed and stored inside MVBs until they are released to the extracellular environment. It is apparent that the brain microenvironment correlates with neurodegeneration, and brain intercellular communication induced by exosomes is necessary for this to occur. In the past decade, exosomes have been shown to be efficient carriers of genetic information, which can be transferred between cells to regulate gene expression and function of recipient cells (Fer‐nandez‐Messina et al., 2015). Hence, they may be an important means of regulating the neurodegenerative process underlying AD, and improve the brain microenvironment by affecting the intercellular communication induced by exosomes.
Exosomes can cross the blood‐brain barrier and therefore be used as delivery vehicles of drugs and genetic elements for treatment of neurological disorders. Several studies have suggested that exosomes derived from multipluripotent mesen‐chymal stromal cells play a neuroprotective role by promoting functional recovery (Xin et al., 2014), neurovascular plasticity(Xin et al., 2013a, b; Zhang et al., 2015), and repairing injured tissue in traumatic brain injury and neurodegenerative disorders. Thus, it may be possible to use mesenchymal stromal cell exosomes in therapies for AD (Katsuda et al., 2015). Further‐more, intracerebrally administered exosomes can act as potent Aβ scavengers by binding to Aβ through enriched glycans on glycosphingolipids on the exosome surface, suggesting a role for exosomes in Aβ clearance in the central nervous system(Yuyama et al., 2014). Improving Aβ clearance by exosome administration provides a novel therapeutic intervention for AD.
Ambiguous knowledge of the underlying mechanisms responsible for causing AD and its progression is the major impediment to therapeutic advances. The potential role of exosomes in neurological disorders and knowledge of their biology show promising leads that are close to clinical translation. Regulating the status and state of exosomes may be a‘Trojan‐horse’ approach to deliver drugs into the brain and treat neurodegenerative and other disorders.
Author contributions:ZYC, MX, SHQ and ZYK drafted and reviewed the paper and ZYC finalized the paper. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Sage Arbor, Marian University College of Osteopathic Medicine, USA.
Aboud O, Mrak RE, Boop FA, Griffin WS (2013) Epilepsy: neuroinflammation, neurodegeneration, and APOE genotype. Acta Neuropathol Com‐mun 1:41.
Agostinho P, Pliassova A, Oliveira CR, Cunha RA (2015) Localization and trafficking of amyloidbeta protein precursor and secretases: impact on Alzheimer’s disease. J Alzheimers Dis 45:329‐347.
Al‐Nedawi K (2014) The yin‐yang of microvesicles (exosomes) in cancer biology. Front Oncol 4:172.
Alvarez‐Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ,Cooper JM (2011) Lysosomal dysfunction increases exosome‐mediated alpha‐synuclein release and transmission. Neurobiol Dis 42:360‐367.
Arscott WT, Tandle AT, Zhao S, Shabason JE, Gordon IK, Schlaff CD,Zhang G, To filon PJ, Camphausen KA (2013) Ionizing radiation and glio‐blastoma exosomes: implications in tumor biology and cell migration.Transl Oncol 6:638‐648.
Beach A, Zhang HG, Ratajczak MZ, Kakar SS (2014) Exosomes: an over‐view of biogenesis, composition and role in ovarian cancer. J Ovarian Res 7:14.
Bonda DJ, Wang X, Lee HG, Smith MA, Perry G, Zhu X (2014) Neuronal failure in Alzheimer’s disease: a view through the oxidative stress look‐ing‐glass. Neurosci Bull 30:243‐252.
Busche MA, Staufenbiel M, Willem M, Haass C, Förstl H (2016) Mecha‐nisms of Alzheimer’s disease : Neuronal hyperactivity and hypoactivity as new therapeutic targets. Nervenarzt 87:1163‐1174.
Butterworth RF (2011) Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology 53:1372‐1376.
Cacabelos R, Cacabelos P, Torrellas C, Tellado I, Carril JC (2014) Pharma‐cogenomics of Alzheimer’s disease: novel therapeutic strategies for drug development. Methods Mol Biol 1175:323‐556.
Cai Z (2014) Monoamine oxidase inhibitors: promising therapeutic agents for Alzheimer’s disease (Review). Mol Med Rep 9:1533‐1541.
Cai Z, Zhao B, Ratka A (2011) Oxidative stress and betaamyloid protein in Alzheimer’s disease. Neuromolecular Med 13:223‐250.
Cai Z, Yan Y, Wang Y (2013a) Minocycline alleviates beta‐amyloid protein and tau pathology via restraining neuroinflammation induced by diabetic metabolic disorder. Clin Interv Aging 8:1089‐1095.
Cai Z, Yan LJ, Ratka A (2013b) Telomere shortening and Alzheimer’s dis‐ease. Neuromolecular Med 15:25‐48.
Cai Z, Hussain MD, Yan LJ (2014) Microglia, neuroinflammation, and be‐ta‐amyloid protein in Alzheimer’s disease. Int J Neurosci 124:307‐321.
Cai Z, Zhao B, Li K, Zhang L, Li C, Quazi SH, Tan Y (2012) Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer’s disease? J Neurosci Res 90:1105‐1118.
Candelario KM, Steindler DA (2014) The role of extracellular vesicles in the progression of neurodegenerative disease and cancer. Trends Mol Med 20:368‐374.
Chang C, Lang H, Geng N, Wang J, Li N, Wang X (2013) Exosomes of BV‐2 cells induced by alpha‐synuclein: important mediator of neurode‐generation in PD. Neurosci Lett 548:190‐195.
Chang YT, Chang WN, Tsai NW, Huang CC, Kung CT, Su YJ, Lin WC,Cheng BC, Su CM, Chiang YF, Lu CH (2014) The roles of biomarkers of oxidative stress and antioxidant in Alzheimer’s disease: a systematic review. Biomed Res Int 2014:182303.
Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, Tsukamoto H, Lee LJ, Paulaitis ME, Brigstock DR (2014) Epigenetic regulation of connec‐tive tissue growth factor by MicroRNA‐214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 59:1118‐1129.
Chivet M, Hemming F, Pernet‐Gallay K, Fraboulet S, Sadoul R (2012)Emerging role of neuronal exosomes in the central nervous system.Front Physiol 3:145.
Chivet M, Javalet C, Hemming F, Pernet‐Gallay K, Laulagnier K, Fraboulet S, Sadoul R (2013) Exosomes as a novel way of interneuronal communi‐cation. Biochem Soc Trans 41:241‐244.
Cox GM, Kithcart AP, Pitt D, Guan Z, Alexander J, Williams JL, Shawler T, Dagia NM, Popovich PG, Satoskar AR, Whitacre CC (2013) Macro‐phage migration inhibitory factor potentiates autoimmune‐mediated neuroinflammation. J Immunol 191:1043‐1054.
de la Torre JC (2010) Alzheimer’s disease is incurable but preventable. J Alzheimers Dis 20:861‐870.
de Rivero Vaccari JP, Brand F, 3rd, Adamczak S, Lee SW, Perez‐Barcena J,Wang MY, Bullock MR, Dietrich WD, Keane RW (2016) Exosome‐me‐diated inflammasome signaling after central nervous system injury. J Neurochem 136 Suppl 1:39‐48.
DeGracia DJ, Jamison JT, Szymanski JJ, Lewis MK (2008) Translation arrest and ribonomics in post‐ischemic brain: layers and layers of players.J Neurochem 106:2288‐2301.
Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E (2014) Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 35:1792‐1800.
Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernås M, Lötvall J(2010) Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One 5:e15353.
Engel PA (2014) Does metabolic failure at the synapse cause Alzheimer’s disease? Med Hypotheses 83:802‐808.
Fernandez‐Messina L, Gutierrez‐Vazquez C, Rivas‐Garcia E, Sanchez‐Ma‐drid F, de la Fuente H (2015) Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol Cell 107:61‐77.
Ferreira ME, de Vasconcelos AS, da Costa Vilhena T, da Silva TL, da Silva Barbosa A, Gomes AR, Dolabela MF, Percario S (2015) Oxidative stress in Alzheimer’s disease: should we keep trying antioxidant therapies? Cell Mol Neurobiol 35:595‐614.
Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB,Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ (2015) Iden‐tification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case‐control study. Alz‐heimers Dement 11:600‐607.e1.
Fleury A, Martinez MC, Le Lay S (2014) Extracellular vesicles as therapeu‐tic tools in cardiovascular diseases. Front Immunol 5:370.
Fröhlich D, Kuo WP, Frühbeis C, Sun JJ, Zehendner CM, Luhmann HJ,Pinto S, Toedling J, Trotter J, Krämer‐Albers EM (2014) Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci 369:20130510.
Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, Zhu J, Ma L, Guo J, Shi H, Zou Y, Ge J (2016) Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF‐alpha mediated NF‐kappaB pathway. J Cell Mol Med 20:2318‐2327.
Gendreau KL, Hall GF (2013) Tangles, toxicity, and Tau secretion in AD ‐new approaches to a vexing problem. Front Neurol 4:160.
Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L,Schwartz JB, Miller BL (2016) Cargo proteins of plasma astrocyte‐de‐rived exosomes in Alzheimer’s disease. FASEB J 30:3853‐3859.
Goldkorn T, Chung S, Filosto S (2013) Lung cancer and lung injury: the dual role of ceramide. Handb Exp Pharmacol:93‐113.
Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S (2015)Acceleration of alpha‐synuclein aggregation by exosomes. J Biol Chem 290:2969‐2982.
Gupta A, Pulliam L (2014) Exosomes as mediators of neuroinflammation. J Neuroinflammation 11:68.
Kalani A, Tyagi A, Tyagi N (2014) Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol 49:590‐600.
Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N (2016)Mechanism of oxidative stress and synapse dysfunction in the pathogen‐esis of Alzheimer’s disease: understanding the therapeutics strategies.Mol Neurobiol 53:648‐661.
Kanamaru T, Kamimura N, Yokota T, Iuchi K, Nishimaki K, Takami S,Akashiba H, Shitaka Y, Katsura K, Kimura K, Ohta S (2015) Oxidative stress accelerates amyloid deposition and memory impairment in a double‐transgenic mouse model of Alzheimer’s disease. Neurosci Lett 587:126‐131.
Kastelowitz N, Yin H (2014) Exosomes and microvesicles: identification and targeting by particle size and lipid chemical probes. ChemBioChem 15:923‐928.
Katsuda T, Oki K, Ochiya T (2015) Potential application of extracellular vesicles of human adipose tissue‐derived mesenchymal stem cells in Alzhei‐mer’s disease therapeutics. Methods Mol Biol 1212:171‐181.
Kaur U, Banerjee P, Bir A, Sinha M, Biswas A, Chakrabarti S (2015) Reactive oxygen species, redox signaling and neuroinflammation in Alzheimer’s disease: the NF‐kappaB connection. Curr Top Med Chem 15:446‐457.
Kerner B (2014) Psychiatric genetics, neurogenetics, and neurodegeneration.Front Genet 5:467.
Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, Farghaian H, Cole AR, Lay PA, Sue CM, Cooper AA (2014) Parkinson’s disease‐linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha‐Synu‐clein externalization via exosomes. Hum Mol Genet 23:2816‐2833.
Kore RA, Abraham EC (2014) In flammatory cytokines, interleukin‐1 beta and tumor necrosis factor‐alpha, upregulated in glioblastoma multiforme,raise the levels of CRYAB in exosomes secreted by U373 glioma cells. Bio‐chem Biophys Res Commun 453:326‐331.
Latta CH, Brothers HM, Wilcock DM (2015) Neuroinflammation in Alzhei‐mer’s disease; A source of heterogeneity and target for personalized thera‐py. Neuroscience 302:103‐111.
Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T,Salles JP, Perret B, Bonnerot C, Record M (2004) Mast cell‐ and dendritic cell‐derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J 380:161‐171.
Liu C, Cui G, Zhu M, Kang X, Guo H (2014) Neuroinflammation in Alzhei‐mer’s disease: chemokines produced by astrocytes and chemokine receptors. Int J Clin Exp Pathol 7:8342‐8355.
Liu Z, Li T, Li P, Wei N, Zhao Z, Liang H, Ji X, Chen W, Xue M, Wei J (2015)The ambiguous relationship of oxidative stress, tau hyperphosphorylation,and autophagy dysfunction in Alzheimer’s disease. Oxid Med Cell Longev 2015:352723.
Lloret A, Badia MC, Giraldo E, Ermak G, Alonso MD, Pallardo FV, Davies KJ, Vina J (2011) Amyloid‐beta toxicity and tau hyperphosphorylation are linked via RCAN1 in Alzheimer’s disease. J Alzheimers dis 27:701‐709.
Lozano D, Gonzales‐Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y,Borlongan CV (2015) Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities.Neuropsychiatr Dis Treat 11:97‐106.
Luque‐Contreras D, Carvajal K, Toral‐Rios D, Franco‐Bocanegra D, Cam‐pos‐Peña V (2014) Oxidative stress and metabolic syndrome: cause or consequence of Alzheimer’s disease? Oxid Med Cell Longev 2014:497802.
Maddahi A, Edvinsson L (2010) Cerebral ischemia induces microvascular pro‐inflammatory cytokine expression via the MEK/ERK pathway. J Neu‐roinflammation 7:14.
McMillin M, Frampton G, Thompson M, Galindo C, Standeford H, Whit‐tington E, Alpini G, DeMorrow S (2014) Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J Neuroin flammation 11:121.
Meseure D, Drak Alsibai K, Nicolas A (2014) Pivotal role of pervasive neo‐plastic and stromal cells reprogramming in circulating tumor cells dis‐semination and metastatic colonization. Cancer Microenviron 7:95‐115.
Millington C, Sonego S, Karunaweera N, Rangel A, Aldrich‐Wright JR,Campbell IL, Gyengesi E, Münch G (2014) Chronic neuroinflammation in Alzheimer’s disease: new perspectives on animal models and promising candidate drugs. Biomed Res Int 2014:309129.
Morales I, Guzmán‐Martínez L, Cerda‐Troncoso C, Farias GA, Maccioni RB(2014) Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci 8:112.
Mouton‐Liger F, Rebillat AS, Gourmaud S, Paquet C, Leguen A, Dumurgier J, Bernadelli P, Taupin V, Pradier L, Rooney T, Hugon J (2015) PKR down‐regulation prevents neurodegeneration and beta‐amyloid production in a thiamine‐deficient model. Cell Death Dis 6:e1594.
Ohno H (2006) Overview: membrane traffic in multicellular systems: more than just a housekeeper. J Biochem 139:941‐942.
Orack JC, Deleidi M, Pitt D, Mahajan K, Nicholas JA, Boster AL, Racke MK,Comabella M, Watanabe F, Imitola J (2015) Concise review: modeling multiple sclerosis with stem cell biological platforms: toward functional validation of cellular and molecular phenotypes in inflammation‐induced neurodegeneration. Stem Cells Transl Med 4:252‐260.
Ortega F, Stott J, Visser SA, Bendtsen C (2013) Interplay between alpha‐, beta‐, and gamma‐secretases determines biphasic amyloid‐beta protein level in the presence of a gamma‐secretase inhibitor. J Biol Chem 288:785‐792.
Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Lozupone M, Santamato A, Zecca C, Barulli MR, Bellomo A, Pilotto A, Daniele A, Greco A, Logroscino G(2016) Tau‐centric targets and drugs in clinical development for the treat‐ment of Alzheimer’s disease. Biomed Res Int 2016:3245935.
Persson T, Popescu BO, Cedazo‐Minguez A (2014) Oxidative stress in Alz‐heimer’s disease: why did antioxidant therapy fail? Oxid Med Cell Longev 2014:427318.
Phillips EC, Croft CL, Kurbatskaya K, O’Neill MJ, Hutton ML, Hanger DP,Garwood CJ, Noble W (2014) Astrocytes and neuroinflammation in Alz‐heimer’s disease. Biochem Soc Trans 42:1321‐1325.
Picon PD, Camozzato AL, Lapporte EA, Picon RV, Moser Filho H, Cerveira MO, Chaves ML (2010) Increasing rational use of cholinesterase inhibitors for Alzheimer’s disease in Brazil: public health strategy combining guideline with peer‐review of prescriptions. Int J Technol Assess Health Care 26:205‐210.
Pottoo FH, Bhowmik M, Vohora D (2014) Raloxifene protects against seizures and neurodegeneration in a mouse model mimicking epilepsy in post‐menopausal woman. Eur J Pharm Sci 65:167‐173.
Prado N, Cañamero M, Villalba M, Rodriguez R, Batanero E (2010) Bystander suppression to unrelated allergen sensitization through intranasal administration of tolerogenic exosomes in mouse. Mol Immunol 47:2148‐2151.
Pusic AD, Pusic KM, Clayton BL, Kraig RP (2014) IFNgamma‐stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neu‐roimmunol 266:12‐23.
Qin J, Xu Q (2014) Functions and application of exosomes. Acta Pol Pharm 71:537‐543.
Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K(2006) Alzheimer’s disease beta‐amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103:11172‐11177.
Rajendran L, Bali J, Barr MM, Court FA, Kramer‐Albers EM, Picou F, Raposo G, van der Vos KE, van Niel G, Wang J, Breake field XO (2014) Emerging roles of extracellular vesicles in the nervous system. J Neurosci 34:15482‐15489.
Record M (2014) Intercellular communication by exosomes in placenta: a possible role in cell fusion? Placenta 35:297‐302.
Russo I, Bubacco L, Greggio E (2012) Exosomes‐associated neurodegeneration and progression of Parkinson’s disease. Am J Neurodegener Dis 1:217‐225.
Salido‐Guadarrama I, Romero‐Cordoba S, Peralta‐Zaragoza O, Hidalgo‐Mi‐randa A, Rodríguez‐Dorantes M (2014) MicroRNAs transported by exo‐somes in body fluids as mediators of intercellular communication in cancer.Onco Targets Ther 7:1327‐1338.
Saman S, Lee NC, Inoyo I, Jin J, Li Z, Doyle T, McKee AC, Hall GF (2014) Proteins recruited to exosomes by tau overexpression implicate novel cellular mechanisms linking tau secretion with Alzheimer’s disease. J Alzheimers Dis 40 Suppl 1:S47‐70.
Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC,Alvarez VE, Lee NC, Hall GF (2012) Exosome‐associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287:3842‐3849.
Santos JR, Gois AM, Mendonca DM, Freire MA (2014) Nutritional status,oxidative stress and dementia: the role of selenium in Alzheimer’s disease.Front Aging Neurosci 6:206.
Schneider A, Simons M (2013) Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res 352:33‐47.
Seifert HA, Collier LA, Chapman CB, Benkovic SA, Willing AE, Pennypacker KR (2014) Pro‐inflammatory interferon gamma signaling is directly associated with stroke induced neurodegeneration. J Neuroimmune Pharmacol 9:679‐689.
Sharples RA, Vella LJ, Nisbet RM, Naylor R, Perez K, Barnham KJ, Masters CL, Hill AF (2008) Inhibition of gamma‐secretase causes increased secre‐tion of amyloid precursor protein C‐terminal fragments in association with exosomes. FASEB J 22:1469‐1478.
Shi M, Kovac A, Korff A, Cook TJ, Ginghina C, Bullock KM, Yang L, Stewart T,Zheng D, Aro P, Atik A, Kerr KF, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Montine TJ, Banks WA, Zhang J (2016) CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement 12:1125‐1131.
Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA (2014)Microvesicles and exosomes for intracardiac communication. Cardiovasc Res 102:302‐311.
Soderberg A, Barral AM, Söderström M, Sander B, Rosén A (2007) Redox‐signaling transmitted in trans to neighboring cells by melanoma‐derived TNF‐containing exosomes. Free Radic Biol Med 43:90‐99.
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the an‐ti‐inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606‐1614.
Tam JH, Seah C, Pasternak SH (2014) The Amyloid Precursor Protein is rap‐idly transported from the Golgi apparatus to the lysosome and where it is processed into beta‐amyloid. Mol Brain 7:54.
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569‐579.
Tonekaboni SH, Mollamohammadi M (2014) Neurodegeneration with brain iron accumulation: an overview. Iran J Child Neurol 8:1‐8.
Tsanova B, Spatrick P, Jacobson A, van Hoof A (2014) The RNA exosome affects iron response and sensitivity to oxidative stress. RNA 20:1057‐1067.
Tsunemi T, Hamada K, Krainc D (2014) ATP13A2/PARK9 regulates secretion of exosomes and alpha‐synuclein. J Neurosci 34:15281‐15287.
Vingtdeux V, Sergeant N, Buee L (2012) Potential contribution of exosomes to the prion‐like propagation of lesions in Alzheimer’s disease. Front Physiol 3:229.
Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R,Manogue K, Cerami A (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci U S A 91:4766‐4770.
Vlassov AV, Magdaleno S, Setterquist R, Conrad R (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 1820:940‐948.
Walberer M, Jantzen SU, Backes H, Rueger MA, Keuters MH, Neumaier B,Hoehn M, Fink GR, Graf R, Schroeter M (2014) In‐vivo detection of inflammation and neurodegeneration in the chronic phase after permanent embolic stroke in rats. Brain Res 1581:80‐88.
Xin H, Li Y, Chopp M (2014) Exosomes/miRNAs as mediating cell‐based therapy of stroke. Front Cell Neurosci 8:377.
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013a) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33:1711‐1715.
Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M (2013b)MiR‐133b promotes neural plasticity and functional recovery after treat‐ment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome‐enriched extracellular particles. Stem Cells 31:2737‐2746.
Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, Tanaka M,Osada‐Oka M, Shimada K, Miura K, Yoshiyama M, Iwao H (2015) Repeat‐ed remote ischemic conditioning attenuates left ventricular remodeling via exosome‐mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol 178:239‐246.
Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y (2014) Decreased amyloid‐beta pathologies by intracerebral loading of glycosphingolipid‐enriched exo‐somes in Alzheimer model mice. J Biol Chem 289:24488‐24498.
Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, Kimura N,Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y(2015) A potential function for neuronal exosomes: sequestering intra‐cerebral amyloid‐beta peptide. FEBS Lett 589:84‐88.
Zhang F, Jiang L (2015) Neuroinflammation in Alzheimer’s disease. Neuropsychiatr Dis Treat 11:243‐256.
Zhang HG, Grizzle WE (2014) Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and me‐tastasis of neoplastic lesions. Am J Pathol 184:28‐41.
Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y(2015) Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg 122:856‐867.
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H (2013) Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatininduced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell ResTher 4:34.
- 中国神经再生研究(英文版)的其它文章
- Novel function of the chemorepellent draxin as a regulator for hippocampal neurogenesis
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Semaphorin 3A: from growth cone repellent to promoter of neuronal regeneration
- The role of undifferentiated adipose-derived stem cells in peripheral nerve repair
- Nerve conduction models in myelinated and unmyelinated nerves based on three-dimensional electrostatic interaction
- Fatigability during volitional walking in incomplete spinal cord injury: cardiorespiratory and motor performance considerations