Fatigability during volitional walking in incomplete spinal cord injury: cardiorespiratory and motor performance considerations
Jared M. Gollie
Fatigue, Fatigability, and Spinal Cord Injuries(SCI)
Fatigue is a symptom describing the lack of physical or mental energy interfering with usual or desired activities and assessed using self‐report questionnaires (Fawkes‐Kirby et al., 2008; Hammell et al., 2009; Craig et al., 2012; Wijesuriya et al., 2012; Nooijen et al., 2015; Smith et al., 2016; Anton et al., 2017). Fatigue is common among those with SCI and negatively impacts health‐related quality of life (Wijesuriya et al., 2012). Increased fatigue following SCI is related to pain,depression and hopelessness, side effects of medications, poor sleep quality, spasticity, poor posture, diet, anxiety, and poor self‐efficacy (Hammell et al., 2009; Craig et al., 2012). The time course of fatigue remains high throughout rehabilitation and into community living (Anton et al., 2017). Fatigue has also been shown to be negatively associated with participation even after controlling for pain, depressive mood, comorbidi‐ties, and level of injury (Smith et al., 2016). Some studies have demonstrated that individuals with incomplete injuries are at greater risk of fatigue compared to complete injuries (Noo‐ijen et al., 2015; Anton et al., 2017). These findings suggest a multitude of factors contributing to the complexity of fatigue experienced after SCI and underscore the importance of con‐trolling for fatigue status across SCI recovery.
Performance fatigability refers to a decline in objective measures of performance over a specified period of time(Enoka and Duchateau, 2016). Experiments performed on paralyzed skeletal muscle have demonstrated greater performance fatigability in SCI compared to non‐injured persons due to alterations in morphological, contractile, and metabolic processes (Shields, 1995; Pelletier and Hicks, 2011;Papaiordanidou et al., 2014). In a seminal study, Shields(1995) observed reduced relaxation time, increased type II fiber characteristics, and reduced oxidative capacity in chronically paralyzed human soleus muscle. More recently,mRNA expression of regulatory genes associated with mus‐cle atrophy and type II muscle fibers were shown to be up‐regulated in response to low force stimulation (3 Hz) fatigue protocol while genes associated with oxidative function and type I muscle fibers were repressed in chronic SCI (Petrie et al., 2014). These data posit skeletal muscle atrophy, shifts in muscle fiber‐type characteristics from type I to type II, and reduced oxidative skeletal muscle capacity as factors con‐tributing to increased performance fatigability in paralyzed skeletal muscle following SCI.
Fatigability associated with whole‐body activity reflects the relationship between performance fatigability and changes in sensations associated with performance regulation (i.e., perceived fatigability) (Dobkin, 2008; Eldadah,2010; Schnelle et al., 2012; Enoka and Duchateau, 2016).Time‐to‐exhaustion during constant‐load arm cranking exercise is significantly reduced in those with paraplegia compared to able‐bodied individuals (Al‐Rahamneh and Eston, 2011). Ratings of perceived exertion in response to constant‐load arm cycling was shown to have a strong lin‐ear relationship with time‐to‐exhaustion and develop more rapidly in those with SCI at the same relative intensities(Al‐Rahamneh and Eston, 2011). In adults with chronic mo‐tor‐incomplete SCI, elevated feelings of tiredness following prolonged self‐selected volitional treadmill walking were re‐ported whereas the able‐bodied group reported no changes in feelings of tiredness and in some instances reported feel‐ing more energetic (Figure 1) (Gollie et al., 2017b). This oc‐curred despite the incomplete SCI group walking for significantly less time at slower self‐selected walking speeds. This was observed despite the incomplete SCI group walking for significantly less time at slower self‐selected walking speeds.The greater sense of effort required to perform a given level of activity may prevent individuals with chronic motor‐in‐complete SCI from engaging in activities such as walking despite the potential for regaining ambulatory function after injury (Burns et al., 1997; Yang and Musselman, 2012).
Cardiorespiratory Limitations and Fatigability
The ability to overcome disturbances to metabolic homeostasis is an essential characteristic of fatigue resistance. Efficient and rapid cardiorespiratory responses to activity‐induced metabolic stress prevents the accumulation of metabolic by‐products implicated in muscular fatigue (Keyser, 2010; Grassi et al., 2011). The challenge imposed on the cardiorespiratory and skeletal muscle bioenergetic systems is dependent upon the task and the intensity at which the task is performed. In non‐pathological conditions, skeletal muscle oxidative capacity is a primary contributor to exercise tolerance during pro‐longed submaximal whole‐body activity (Davies et al., 1981;Holloszy and Coyle, 1984; Bassett and Howley, 2000; Poole et al., 2008; Grassi et al., 2011). In the presence of SCI, the com‐bination of compromised skeletal muscle oxidative capacity and limitations in oxygen delivery due to impaired cardiac output may contribute to elevated fatigability (Dearwater et al., 1986; Hjeltnes, 1986; West et al., 2013b).
Energetic cost of activity and exercise capacity after SCI is shown to be related to the completeness and level of injury(Hjeltnes, 1986; Davis, 1993; Collins et al., 2010). Those with lower‐level incomplete tetraplegia are reporter to have great‐er energy expenditure during wheeling outside compared to those with complete SCI (Collins et al., 2010). In paraplegics performing arm cranking at 80 watts, those with higher‐lev‐el injuries experience elevated energy expenditure com‐pared to lower‐level injuries (Collins et al., 2010). During prolonged constant work‐rate submaximal arm exercise paraplegics with injuries below T6 were able to compensate for lower stroke volume by increasing heart rate to maintain cardiac output and thus oxygen delivery (Hopman et al.,1993). Conversely, cardiac output is compromised in individuals with higher level injuries due to reduced stroke vol‐ume and the inability to increase heart rate during maximal arm‐crank and wheelchair activity (Hopman et al., 1993;Hostettler et al., 2012). Furthermore, decreased reactivity and vascular atrophy are also observed after SCI impeding hemodynamic responses to contracting muscle (Olive et al.,2002, 2003; West et al., 2013a).
The rate of oxygen uptake at the rest‐to‐work transition(i.e., oxygen uptake kinetics (VO2on‐kinetics)) is used to gain information into the dynamic processes involved in the delivery and utilization of oxygen (Poole and Jones, 2012).The primary component (i.e., phase II; oxygen uptake time constant (τVO2)) of the VO2on‐kinetic response is suggest‐ed to reflect oxidative metabolic processes associated with active skeletal muscle in healthy individuals (Grassi et al.,2011). The on‐kinetic response of VO2is prolonged in in‐dividuals with SCI during electrically stimulated unloaded leg cycling with lower than expected heart rates (Barstow et al., 1995). When comparing trained individuals with SCI to healthy controls, VO2on‐kinetics were reported to be similar during upper extremity exercise while cardiac output was greater in the control group and arterio‐venous oxygen oxygen difference (a‐vO2difference) was greater in the SCI group (Fukuoka et al., 2002). During self‐selected volitional treadmill walking τVO2was 55.4% slower in individuals with chronic motor‐incomplete SCI compared to able‐bodied controls (Gollie et al., 2017b). The delayed τVO2results in a greater oxygen deficit at the onset of muscular activity and therefore accelerating the accumulation of metabolic byproducts associated with activity termination due to an increased reliance on anaerobic energy metabolism (Figure 2). The evidence from VO2on‐kinetic responses following SCI posit both reductions in skeletal muscle oxidative properties and oxygen delivery as potential mechanisms contributing to compromised oxidative metabolism (Barstow et al., 1995; Fu‐kuoka et al., 2002; Gollie et al., 2017b).
The autonomic nervous system plays a key role in regulation of fatigability in response to metabolic disturbances.Feedback from group III/IV muscle afferents stimulates cir‐culatory and ventilatory responses to increase muscle blood flow and oxygen delivery to meet the energetic demands of activity (Hjeltnes, 1986; Amann et al., 2015). During exhaustive activity group III/IV muscle afferents have been shown to limit output of spinal motoneurons through inhibition of the central nervous system (CNS) (Amann et al., 2015).After SCI, peak oxygen consumption (VO2peak) has been shown to be correlated with minute ventilation regardless of the level of injury (Hjeltnes, 1986). West et al. (2015)demonstrated greater endurance performance in paracy‐cling athletes with autonomic incomplete cervical SCI com‐pared to those with autonomic complete SCI. Athletes with autonomic incomplete cervical SCI also had higher maximal and average heart rates during competition than athletes with autonomic complete cervical SCI (West et al., 2015).In addition, thermoregulatory mechanisms are potentially impaired below the level of injury increasing the possibility of hyperthermia (Theisen, 2012). This highlights the importance of the autonomic nervous system in the regulation of oxygen delivery and utilization and thermoregulation in response to activity and thus fatigability severity after SCI.However, disassociations between changes in perceived ratings of effort and physiological responses have been ob‐served during activity suggesting perceived fatigability may not be solely reflective of physiological processes (Chaudhuri and Behan, 2004; Lewis et al., 2007; Au et al., 2017).
Implications of Fatigability on Walking Performance and Recovery
The redundancy of the motor system allows a given motor task to be accomplished through a variety of different motor solutions. In the presence of fatigue, interjoint and inter‐muscular coordination patterns adapt to compensate forlocal effects of fatigue and to maintain essential movement characteristics necessary for successful task execution (Cote et al., 2008). The actions selected as part of the motor repertoire are influenced, in part, by the mechanical and energetic constraints of the person. Sparrow and Newell (1998)hypothesized metabolic energy regulation as a fundamental principle underlying the control and learning of motor skills(Sparrow and Newell, 1998). Thus, elevated fatigability due to cardiorespiratory limitations may contribute to motor control strategies and walking performance following SCI(Figure 3).
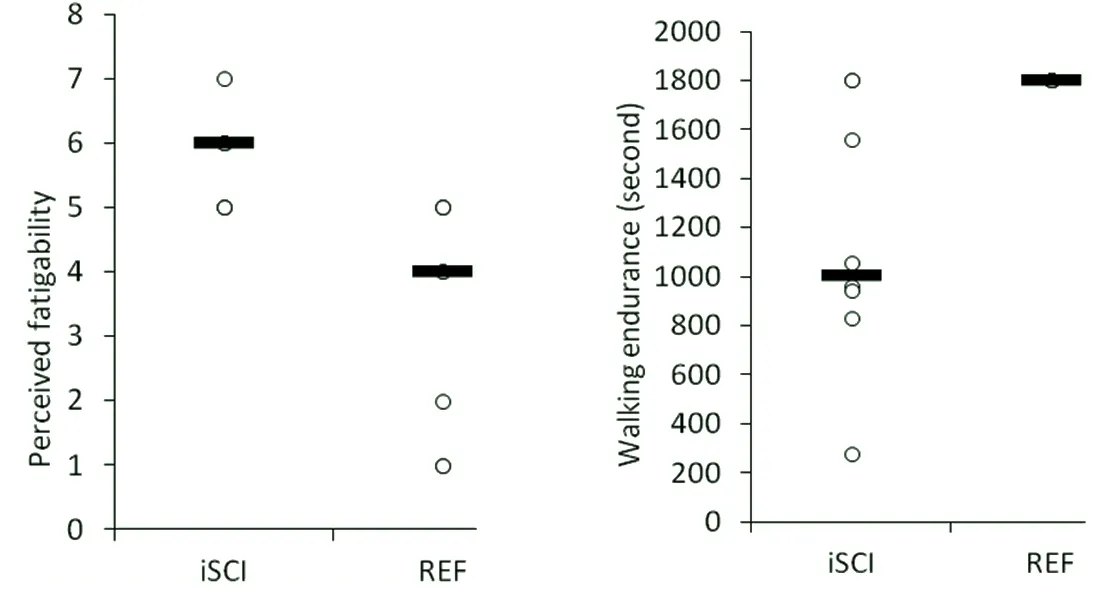
Figure 1 Perceived (A) and performance (B) fatigability for chronic motor-incomplete spinal cord injured (iSCI) and able-bodied (REF)adults during self-selected volitional treadmill walking for 30 minutes or until exhaustion.
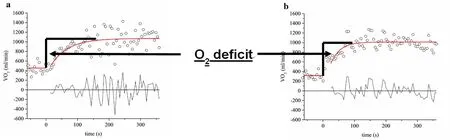
Figure 2 Oxygen uptake kinetic (VO2 on-kinetics) response comparing the oxygen deficit (O2 deficit) for a subject with chronic motor-incomplete spinal cord injury (iSCI) (A) and an able-bodied reference subject (B) during 6-minute constant work-rate treadmill walking at a self-selected walking speed.
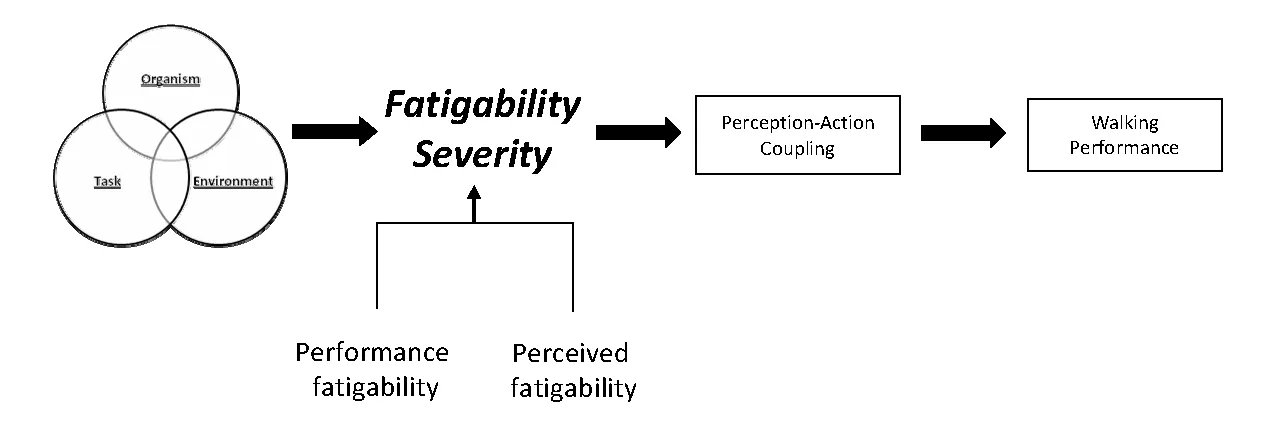
Figure 3 Cardiorespiratory limitations viewed within the context of constraint interactions contributing to fatigability severity impacting perception-action coupling and walking performance following chronic motor-incomplete spinal cord injury.
Locomotor training aims to enhance walking performance through the promotion of physiological and behavioral adaptations (Behrman and Harkema, 2007; Harkema et al.,2012; Gollie and Guccione, 2017; Gollie et al., 2017a). From a performance outcomes standpoint, locomotor training has been shown to improve both walking speed and endurance in individuals with incomplete SCI (Alexeeva et al.,2011; Mehrholz et al., 2012; Morawietz and Moffat, 2013).Despite numerous studies demonstrating increased walking endurance only modest improvements cardiorespiratory fitness have been reported (Alexeeva et al., 2011). Interest‐ingly, the demand placed on the cardiorespiratory system seems to play a key role in walking recovery (Yang et al.,2014; Leech et al., 2016; Leech and Hornby, 2017). While enhanced metabolic responses to volitional activity may underlie these findings, moderate‐to‐high intensity training has also been shown to be associated with elevated levels of serum brain‐derived neurotrophic factor (BDNF) (Leech and Hornby, 2017). From the perspective of mechanical and metabolic constraints, intralimb coordination variability is shown to decrease (i.e., coordination becomes more stable)with no changes in intralimb coordination pattern in those with incomplete SCI (Awai and Curt, 2016) in response to rehabilitation. Furthermore, walking becomes more economical following periods locomotor training (Kressler et al., 2013; Gollie et al., 2017a).
Recently a framework has been proposed which attempts to account for both physiological adaptation and motor learning in incomplete SCI following overground locomo‐tor training (Gollie and Guccione, 2017). According to this framework, movement after SCI is viewed as an emergent phenomenon produced from the interactions of the organ‐ism, task, and environment (Davids et al., 2003; Gollie and Guccione, 2017). Modifications of constraints (i.e., organismic, task, environment), either independently or in combination with one another, alters the affordances available to the individual for successful locomotion (Vaz et al., 2017).Similarly, the practice of the activity in its entirety allows for exploration of the most optimal movement solutions (Gol‐lie and Guccione, 2017). Given the potential constraints of the cardiorespiratory system during activity following SCI,interventions may aim to increase cardiorespiratory fitness prior to engaging in locomotor training. This would enable patients to engage in exercises of greater intensities and vol‐ume accelerating the motor learning process (Schmidt and Lee, 2014). On the other hand, interventions may emphasize simultaneous improvements in cardiorespiratory fitness and motor function through appropriate structuring and design of locomotor programs. At present, it is not clear how to most appropriately design interventions to address both cardiore‐spiratory adaptations and motor performance concurrently.
Conclusion
Fatigability presents a challenge to activity performance in those with SCI. Compromised cardiorespiratory adjustments to whole‐body volitional activity may contribute to fatigability severity after SCI. The potential for altered autonomic nervous system function increases the risk for greater susceptibility to fatigability in responses to activity. The increased energy expenditure and injury to the nervous system may impact the motor solutions available to accomplish walking. Additionally, reductions in training intensities and volume as a result of increased fatigability may delay the motor learning process. Locomotor training approaches designed to reduce fatigability and enhance aerobic capacity in combination with motor learning may to be advantageous for promoting functional recovery after SCI. Future research is required to advance the understanding of the relationship between fatigability, cardiorespiratory function and motor performance following SCI.
Acknowledgments:The author would like to thank Dr. Andrew A. Guccione and the Department of Rehabilitation Science at George Mason University for their feedback and suggestions throughout the development of the ideas presented in this review.
Author contributions:JMG contributed to the development of the conceptual framework, the drafting of the initial manuscript, and completion of the revision process.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Conflicts of interest:None declared.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review report:
Reviewer:Daniel P. Credeur, University of Southern Mississippi, USA.
Comments to author:The authors present a unique perspective regarding barriers associated with both performance related and perceived fatigueability in response to volitional walking training in motor incomplete SCI. While fatigue in SCI has been investigated, this piece presents a novel framework by which not only task and environmental barriers are considered, but other physiological barriers, i.e., reduced skeletal muscle oxidative capacity and altered cardiorespiratory adjustments to physical stress, when designing future rehabilitation programs.
Alexeeva N, Sames C, Jacobs PL, Hobday L, Distasio MM, Mitchell SA,Calancie B (2011) Comparison of training methods to improve walking in persons with chronic spinal cord injury: a randomized clinical trial. J Spinal Cord Med 34:362‐379.
Al‐Rahamneh H, Eston R (2011) Rating of perceived exertion during two different constant‐load exercise intensities during arm cranking in paraplegic and able‐bodied participants. Eur J Appl Physiol 111:1055‐1062.
Amann M, Sidhu SK, Weavil JC, Mangum TS, Venturelli M (2015) Autonomic responses to exercise: Group III/IV muscle afferents and fatigue.Auton Neurosci 188:19‐23.
Anton HA, Miller WC, Townson AF, Imam B, Silverberg N, Forwell S (2017)The course of fatigue after acute spinal cord injury. Spinal Cord 55:94‐97.
Au JS, Totosy De Zepetnek JO, Macdonald MJ (2017) Modeling perceived exertion during graded arm cycling exercise in spinal cord injury. Med Sci Sports Exerc 49:1190‐1196.
Awai L, Curt A (2014) Intralimb coordination as a sensitive indicator of motor‐control impairment after spinal cord injury. Front Hum Neurosci 8:148.
Awai L, Curt A (2016) Locomotor recovery in spinal cord injury: insights beyond walking speed and distance. J Neurotrauma 33:1428‐1435.
Barstow TJ, Scremin AM, Mutton DL, Kunkel CF, Cagle TG, Whipp BJ (1995)Gas exchange kinetics during functional electrical stimulation in subjects with spinal cord injury. Med Sci Sports Exerc 27:1284‐1291.
Bassett DR, Howley ET (2000) Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 32:70‐84.
Behrman AL, Harkema SJ (2007) Physical rehabilitation as an agent for re‐covery after spinal cord injury. Phys Med Rehabil Clin N Am 18:183‐202, v.
Burns SP, Golding DG, Rolle WA, Graziani V, Ditunno JF (1997) Recovery of ambulation in motor‐incomplete tetraplegia. Arch Phys Med Rehabil 78:1169‐1172.
Chaudhuri A, Behan PO (2004) Fatigue in neurological disorders. Lancet Lond Engl 363:978‐988.
Collins EG, Gater D, Kiratli J, Butler J, Hanson K, Langbein WE (2010) En‐ergy cost of physical activities in persons with spinal cord injury. Med Sci Sports Exerc 42:691‐700.
Cote JN, Feldman AG, Mathieu PA, Levin MF (2008) Effects of fatigue on intermuscular coordination during repetitive hammering. Motor Control 12:79‐92.
Craig A, Tran Y, Wijesuriya N, Middleton J (2012) Fatigue and tiredness in people with spinal cord injury. J Psychosom Res 73:205‐210.
Davids K, Glazier P, Araújo D, Bartlett R (2003) Movement systems as dy‐namical systems: the functional role of variability and its implications for sports medicine. Sports Med Auckl NZ 33:245‐260.
Davies KJ, Packer L, Brooks GA (1981) Biochemical adaptation of mitochon‐dria, muscle, and whole‐animal respiration to endurance training. Arch Biochem Biophys 209:539‐554.
Davis GM (1993) Exercise capacity of individuals with paraplegia. Med Sci Sports Exerc 25:423‐432.
Dearwater SR, LaPorte RE, Robertson RJ, Brenes G, Adams LL, Becker D(1986) Activity in the spinal cord‐injured patient: an epidemiologic analy‐sis of metabolic parameters. Med Sci Sports Exerc 18:541‐544.
Dobkin BH (2008) Fatigue versus activity‐dependent fatigability in patients with central or peripheral motor impairments. Neurorehabil Neural Re‐pair 22:105‐110.
Eldadah BA (2010) Fatigue and fatigability in older adults. PM R 2:406‐413.
Enoka RM, Duchateau J (2016) Translating fatigue to human performance.Med Sci Sports Exerc 48:2228‐2238.
Fawkes‐Kirby TM, Wheeler MA, Anton HA, Miller WC, Townson AF,Weeks CAO (2008) Clinical correlates of fatigue in spinal cord injury. Spi‐nal Cord 46:21‐25.
Fukuoka Y, Endo M, Kagawa H, Itoh M, Nakanishi R (2002) Kinetics and steady‐state of VO2 responses to arm exercise in trained spinal cord injury humans. Spinal Cord 40:631‐638.
Gollie JM, Guccione AA (2017) Overground locomotor training in spinal cord injury: a performance‐based framework. Top Spinal Cord Inj Rehabil 23:226‐233.
Gollie JM, Guccione AA, Panza GS, Jo PY, Herrick JE (2017a) Effects of overground locomotor training on walking performance in chronic cervical motor incomplete spinal cord injury: a pilot study. Arch Phys Med Rehabil 98:1119‐1125.
Gollie JM, Herrick JE, Keyser RE, Chin LMK, Collins JP, Shields RK, Panza GS, Guccione AA (2017b) Fatigability, oxygen uptake kinetics and muscle deoxygenation in incomplete spinal cord injury during treadmill walking.Eur J Appl Physiol 117:1989‐2000.
Grassi B, Porcelli S, Salvadego D, Zoladz JA (2011) Slow VO2 kinetics during moderate‐intensity exercise as markers of lower metabolic stability and lower exercise tolerance. Eur J Appl Physiol 111:345‐355.
Hammell KW, Miller WC, Forwell SJ, Forman BE, Jacobsen BA (2009) Fa‐tigue and spinal cord injury: a qualitative analysis. Spinal Cord 47:44‐49.
Harkema SJ, Hillyer J, Schmidt‐Read M, Ardolino E, Sisto SA, Behrman AL(2012) Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil 93:1588‐1597.
Hjeltnes N (1986) Cardiorespiratory capacity in tetra‐ and paraplegia shortly after injury. Scand J Rehabil Med 18:65‐70.
Holloszy JO, Coyle EF (1984) Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56:831‐838.
Hopman MT, Oeseburg B, Binkhorst RA (1993) Cardiovascular responses in persons with paraplegia to prolonged arm exercise and thermal stress.Med Sci Sports Exerc 25:577‐583.
Hostettler S, Leuthold L, Brechbühl J, Mueller G, Illi S, Spengler C (2012)Maximal cardiac output during arm exercise in the sitting position after cervical spinal cord injury. J Rehabil Med 44:131‐136.
Keyser RE (2010) Peripheral fatigue: high‐energy phosphates and hydrogen ions. PM R 2:347‐358.
Kressler J, Nash MS, Burns PA, Field‐Fote EC (2013) Metabolic responses to 4 different body weight‐supported locomotor training approaches in per‐sons with incomplete spinal cord injury. Arch Phys Med Rehabil 94:1436‐1442.
Leech KA, Hornby TG (2017) High‐intensity locomotor exercise increases brain‐derived neurotrophic factor in individuals with incomplete spinal cord injury. J Neurotrauma 34:1240‐1248.
Leech KA, Kinnaird CR, Holleran CL, Kahn J, Hornby TG (2016) Effects of locomotor exercise intensity on gait performance in individuals with in‐complete spinal cord injury. Phys Ther 96:1919‐1929.
Lewis JE, Nash MS, Hamm LF, Martins SC, Groah SL (2007) The relationship between perceived exertion and physiologic indicators of stress during graded arm exercise in persons with spinal cord injuries. Arch Phys Med Rehabil 88:1205‐1211.
Mehrholz J, Kugler J, Pohl M (2012) Locomotor training for walking after spinal cord injury. In: Cochrane Database of Systematic Reviews (The Co‐chrane Collaboration, ed.). Chichester, UK: John Wiley & Sons, Ltd.
Morawietz C, Moffat F (2013) Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil 94:2297‐2308.
Nooijen CFJ, Vogels S, Bongers‐Janssen HMH, Bergen MP, Stam HJ, van den Berg‐Emons HJG (2015) Fatigue in persons with subacute spinal cord injury who are dependent on a manual wheelchair. Spinal Cord 53:758‐762.
Olive JL, Dudley GA, Mccully KK (2003) Vascular remodeling after spinal cord injury. Med Sci Sports Exerc 35:901‐907.
Olive JL, McCully KK, Dudley GA (2002) Blood flow response in individuals with incomplete spinal cord injuries. Spinal Cord 40:639‐645.
Papaiordanidou M, Varray A, Fattal C, Guiraud D (2014) Neural and mus‐cular mechanisms of electrically induced fatigue in patients with spinal cord injury. Spinal Cord 52:246‐250.
Pelletier CA, Hicks AL (2011) Muscle fatigue characteristics in paralyzed muscle after spinal cord injury. Spinal Cord 49:125‐130.
Petrie MA, Suneja M, Faidley E, Shields RK (2014) Low force contractions induce fatigue consistent with muscle mRNA expression in people with spinal cord injury. Physiol Rep 2:e00248.
Poole DC, Barstow TJ, Mcdonough P, Jones AM (2008) Control of oxygen uptake during exercise. Med Sci Sports Exerc 40:462‐474.
Poole DC, Jones AM (2012) Oxygen Uptake Kinetics. In: Comprehensive Physiology (Terjung R, ed.). Hoboken, NJ, USA: John Wiley & Sons, Inc.
Schmidt RA, Lee TD (2014) Motor Learning and Performance: from Principles to Application. 5th ed. Champaign, IL: Human Kinetics.
Schnelle JF, Buchowski MS, Ikizler TA, Durkin DW, Beuscher L, Simmons SF (2012) Evaluation of two fatigability severity measures in elderly adults.J Am Geriatr Soc 60:1527‐1533.
Shields RK (1995) Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol 73:2195‐2206.
Smith EM, Imam B, Miller WC, Silverberg ND, Anton HA, Forwell SJ,Townson AF (2016) The relationship between fatigue and participation in spinal cord injury. Spinal Cord 54:457‐462.
Sparrow WA, Newell KM (1998) Metabolic energy expenditure and the reg‐ulation of movement economy. Psychon Bull Rev 5:173‐196.
Theisen D (2012) Cardiovascular determinants of exercise capacity in the Paralympic athlete with spinal cord injury. Exp Physiol 97:319‐324.
Vaz DV, Silva PL, Mancini MC, Carello C, Kinsella‐Shaw J (2017) Towards an ecologically grounded functional practice in rehabilitation. Hum Mov Sci 52:117‐132.
Waters RL, Lunsford BR (1985) Energy cost of paraplegic locomotion. J Bone Joint Surg Am 67:1245‐1250.
Waters RL, Mulroy S (1999) The energy expenditure of normal and patho‐logic gait. Gait Posture 9:207‐231.
West CR, AlYahya A, Laher I, Krassioukov A (2013a) Peripheral vascular function in spinal cord injury: a systematic review. Spinal Cord 51:10‐19.
West CR, Bellantoni A, Krassioukov AV (2013b) Cardiovascular function in individuals with incomplete spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil 19:267‐278.
West CR, Gee CM, Voss C, Hubli M, Currie KD, Schmid J, Krassioukov AV(2015) Cardiovascular control, autonomic function, and elite endurance performance in spinal cord injury: Endurance performance in SCI. Scand J Med Sci Sports 25:476‐485.
Wijesuriya N, Tran Y, Middleton J, Craig A (2012) Impact of fatigue on the health‐related quality of life in persons with spinal cord injury. Arch Phys Med Rehabil 93:319‐324.
Yang JF, Musselman KE (2012) Training to achieve over ground walking after spinal cord injury: a review of who, what, when, and how. J Spinal Cord Med 35:293—304.
Yang JF, Musselman KE, Livingstone D, Brunton K, Hendricks G, Hill D,Gorassini M (2014) Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? a pilot random‐ized clinical trial. Neurorehabil Neural Repair 28:314‐324.
- 中国神经再生研究(英文版)的其它文章
- Forkhead box protein P1, a key player in neuronal development?
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Novel function of the chemorepellent draxin as a regulator for hippocampal neurogenesis
- The role of undifferentiated adipose-derived stem cells in peripheral nerve repair
- Nerve conduction models in myelinated and unmyelinated nerves based on three-dimensional electrostatic interaction
- Retinoid receptor-related orphan receptor alpha: a key gene setting brain circuits