Forkhead box protein P1, a key player in neuronal development?
Forkhead box protein P1 (FOXP1) is a transcription factor belonging to the forkhead box (FOX) proteins, a family of transcriptional regulators sharing a highly conserved forkhead DNA‐binding domain (Bacon and Rappold, 2012). Previous reports have proposed a role for FOXP1 in functionally regulating the central nervous system (CNS), while mutations inFOXP1have been implicated in cognitive abnormalities (Bacon and Rappold, 2012). FOXP1 has been shown to promote differentiation of medium spiny neurons and to induce neuronal migration during embryonic neuronal development in the mouse cortex (Li et al., 2015; Precious et al., 2016). Mutations inFOXP1have been linked to various neurodevel‐opmental diseases, including autism, intellectual disabilities and speech defects (Hamdan et al., 2010; Horn et al., 2010). Furthermore, Bacon and colleagues demonstrated that Cre‐mediatedFoxp1deletion in Nestin‐pos‐itive cells induces autism‐like behavior in mice, and defects in morphol‐ogy of the striatum upon postnatal development (Bacon et al., 2015). In line with these findings, heterozygousFoxp1+/–mice have been reported to show deficits in vocal communication as well as a deregulation of autism‐associated genes in the hippocampus and striatum (Araujo et al.,2015). Taken together, this body of evidence indicates an important role of FOXP1 in regulating CNS development, however the molecular mechanisms underlying neuronal FOXP1 function have remained unclear.
Several recent studies have started to shed light on the role of FOXP1 during neural development (Braccioli et al., 2017; Rocca et al., 2017;Usui et al., 2017) The first study, authored by our group, defines a novel role for FOXP1 in promoting mouse embryonic neural stem cell (NSC)differentiation by repressing Jagged1 (JAG1) expression (Braccioli et al.,2017). In this study, RNA‐sequencing and chromatin‐immunoprecipi‐tation (ChIP)‐sequencing were utilized to show that FOXP1 binds to the promoter region of genes in NSCs that are associated with diseases of the CNS, as well as regulating the expression of genes associated with neuro‐genesis. Through gene‐set enrichment analysis (GSEA), it was observed that FOXP1 represses the expression of genes associated with Notch sig‐naling pathway in NSCs. These observations led us to investigate whether FOXP1 can regulate NSC differentiation. Short‐hairpin RNA (shR‐NA)‐mediated depletion and overexpression revealed that FOXP1 pro‐motes NSC differentiation in vitro towards both neurons and astrocytes,but not to oligodendrocytes. Consistently, FOXP1 is found to repress the maintenance of progenitor‐like characteristics. These observations were validatedin vivousing two different models. First,in uteroelectropora‐tion of FOXP1‐directed shRNAs demonstrated that FOXP1 is required for radial glia development by promoting both neuronal migration and intermediate progenitor differentiation during cortical development.Moreover, through intracranial transplantation of FOXP1‐depleted NSCs in a hypoxic‐ischemic (HI) brain damage model, FOXP1 was found to promote neuronal differentiation of transplanted NSCsin vivo. Addition‐ally, FOXP1 was found to be required for improvement of sensorimotor function mediated by NSC transplantation upon HI. FOXP1 was subse‐quently found to inhibit expression of the Notch ligand JAG1 by binding to its promoter, and this resulted in inhibition of the Notch pathway in NSCs bothin vitroand in the developing cortex. Treating FOXP1‐deplet‐ed NSCs with an anti‐JAG1 blocking antibody was found to rescue the reduction of neural differentiation caused by FOXP1 depletion. Taken together, these findings support a role for FOXP1 as a key inducer of embryonic NSC differentiation by repressing JAG1 expression. Confirming this hypothesis, FOXP1 depletion resulted in increased expression of hairy and enhancer of split‐1 (HES1), a downstream effector of the Notch pathway in NSCsin vitroand increased levels of the activated Notch intracellular domain in the developing cortex. FOXP1 was also found to regulate a subgroup of genes related to autism‐spectrum diseases (ASD)in NSCs, validating the notion that FOXP1 plays a fundamental role in autism (Hamdan et al., 2010; Araujo et al., 2015; Bacon et al., 2015).However, several issues underlying the mechanism by which FOXP1 regulates NSC differentiation remain unclear. For example, it remains unclear whether FOXP1 is promoting both migration and differentiation of NSCs separately during cortical development, or whether defects in neuronal differentiation are themselves responsible for the reduced migratory capacity of NSCs. While there are distinct FOXP1 isoforms in NSCs (FOXP1A and FOXP1C), it was not possible to discriminate the individual role of these isoforms in promoting NSC differentiation since the shRNAs utilized to target FOXP1 target both FOXP1A and FOXP1C which are translated from the same mRNA, but from differential starting codons. To investigate this issue, it would be necessary to generate mice carrying a mutation in the alternative start codon preventing FOXP1C translation. It would be also interesting to determine which co‐factors in‐teract with or activate FOXP1 in NSCs in order to repress JAG1 and pro‐mote NSC differentiation. In our study, we observed that overexpression of FOXP1 leads to increased neuronal differentiation. Therefore, ectopic expression of FOXP1 in NSCs improves the capacity of NSCs to generate neurons, potentially increasing the regenerative capacity of transplanted NSCs upon brain damage. For example, it would be relevant to investigate whether transplantation of FOXP1‐overexpressing NSCs upon HI could induce a more efficient generation of neurons when compared to regular NSCs, and if this could lead to increased improvements in func‐tional and anatomical impairments after HI, compared to regular NSC treatment.
A second study by Konopka and colleagues has tackled the question as to whether FOXP1 plays a role in the neocortex during postnatal develop‐ment in mice, as this stage is relevant for ASD (Usui et al., 2017). To this end, they generated a conditional FOXP1 knockout by crossing Emx1‐Cre mice, expressing the Cre‐recombinase specifically in radial glia, withFoxp1flox/floxmice. This led to deletion ofFoxp1in both the cortex and the hippocampus and caused abnormalities in vocal communication during postnatal development. Additionally, it was observed that FOXP1 depletion caused alterations in brain structure and architecture of the postnatal neocortex, as indicated by reduction in neocortical size and altered lo‐calization of neurons in the deep cortical layers. Furthermore, analysis of gene expression changes in the postnatal cortex upon FOXP1 depletion revealed that FOXP1 regulates the expression of genes involved in syn‐apse formation and neuronal development. A subset of genes identified was associated with ASD and regulated by FOXP1, an observation that provides additional weight to the idea thatFOXP1is a relevant gene in‐volved in the pathogenesis of ASD. In exploring the mechanisms regulating FOXP1 activity during neuronal development, FOXP1 was found to be sumoylated, and levels of sumoylated‐FOXP1 decreased from embryonic to postnatal development. By generation of a sumoylation‐deficient mutant of FOXP1 (K636R), it was demonstrated that FOXP1 sumoyla‐tion is necessary to promote neurite outgrowth of mouse and human cortical neuronsin vitro, and neuronal migration in mouse embryonic cortexin vivo. Sumoylation of FOXP1 was also found to prevent the interaction of FOXP1 with members of the nucleosome remodeling deacetylase(NuRD) chromatin remodeling complex, including histone deacetylases(HDAC) 1/2 and metastasis associated proteins (MTA) 1/2. However,while sumoylated‐FOXP1 was shown to promote neurite outgrowth and neuronal migration, it remains unclear whether either this sumoylation as such, or the association of FOXP1 with the NuRD complex, is directly required for FOXP1‐mediated transcriptional regulation. Rocca et al.(2017) also provide evidence that FOXP1 can be sumoylated in rat cells(in this case the sumoylated residue is K670) and that this modification promotes dendritic outgrowth in embryonic rat cortical neurons. The authors suggest that sumoylation of FOXP1 enhances binding of FOXP1 to the transcriptional co‐repressor C‐terminal‐binding protein 1 (CTBP1).In this study, it was also shown that sumoylation at K670 is required for FOXP1‐mediated transcriptional repression of the simian virus (SV)40 promoter in human embryonic kidney cells (HEK)293T, implicating that this modification could be important in regulating the transcriptional targets of FOXP1 (Rocca et al., 2017) (Figure 1). Taken together, these observations indicate that FOXP1 requires sumoylation to interact with CTBP1, thereby repressing transcription of its target genes. However, it would be relevant to investigate whether the expression of relevant target genes is affected in sumoylation‐deficient FOXP1 mutants during em‐bryonic and postnatal cortical development. Furthermore, two different FOXP1 isoforms were identified in the postnatal cortex, FOXP1A and FOXP1D. It would be interesting to investigate whether both isoforms(together with FOXP1C) become sumoylated, and how this modification affects the transcriptional output.
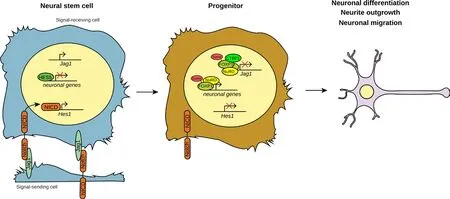
Figure 1 Schematic model illustrating the role of forkhead box protein P1(FOXP1) in neurogenesis.
Both our study and study of Usui and colleagues described above in‐dicate that FOXP1 regulates ASD‐related genes. It is now important to evaluate whether these ASD‐related FOXP1 target genes are conserved between embryonic and postnatal development. To this end, we com‐pared the results of our study with the results obtained by Konopka’s group: we overlapped the genes associated with ASD and those differentially regulated by FOXP1 found by Usui et al. (2017) and targeted by FOXP1 from our study. Interestingly, we identified a significant overlap of three ASD‐associated genes (Cttnbp2,Pcdh15andCdh10), besidesFoxp1itself, that were downregulated both in NSCs upon FOXP1 knockdown in our dataset and in the cortex at postnatal day(P)0 uponFoxp1deletion in the study (Usui et al., 2017) (Figure 2A). Conversely,while we found only 1 gene (Sparcl1) that was upregulated both in NSCs upon FOXP1 knockdown and in the cortex at P0 uponFoxp1deletion(Figure 2B). It would be interesting to address the function of these genes in relation to FOXP1 activity as they could underlie a conserved mechanism of regulation in both embryonic and postnatal neurogenesis.During adulthood, neurogenesis continues to occur in specialized niches in the CNS such as the subventricular zone (SVZ) and the subgranular zone (SGZ) of the dentate gyrus of the hippocampus (Kriegstein and Alvarez‐Buylla, 2009). However, it is not known whether FOXP1 plays a role in this process. Conditional deletion of FOXP1 in the adult neural progenitor compartment would help resolve the question whether FOXP1 promotes adult NSC differentiation and neuronal migration. In this adult setting, it would be interesting to investigate whether FOXP1 sumoylation is also required to promote neural development as well as to inhibit Notch pathway by repressing JAG1 expression.
Moreover, it would be relevant to investigate the interactome of FOXP1, in order to identify neurodevelopmental proteins that might cohoperate with FOXP1 in regulating neurogenesis. To this end, a recent study published by Estruch et al. (2018) indicates that FOXP1 interacts with SOX5, SATB2, SATB1, NR2F1 and NR2F2, which have been linked to neurodevelopment.
In conclusion, these studies show that FOXP1 is necessary to promote neuronal migration and differentiation during embryonic and postnatal brain development. Additionally, sumoylation of FOXP1 promotes neuronal migration and neurite outgrowth, as well as inhibits FOXP1 inter‐action with the NuRD complex (Figure 1). Since the evidence presented by Usui et al. (2017) and Rocca et al. (2017) indicates that sumoylation regulates FOXP1 transcriptional activity and its function in promoting neurite outgrowth and neuronal migration, it would be relevant to study whether inducing or inhibiting FOXP1 sumoylation in NSCs could alter the regenerative capacity of NSC transplantation upon HI brain damage.Vice versa, increasing the amount of sumoylated FOXP1 in NSCs might be a strategy to promote neurogenesis and neuronal maturation. Finally,FOXP1 promotes embryonic neural differentiation by inhibiting the Notch pathway, at least in part by repressing JAG1 expression (Figure 1).Moreover, in both embryonic and postnatal brain development, FOXP1 regulates a subset of ASD‐associated genes. Taken together, these observations indicate a relevant role for FOXP1 in promoting neural develop‐ment both during embryogenesis and postnatally, and confirm FOXP1 as a key gene involved in the etiology of ASD.
Luca Braccioli, Cora H. Nijboer, Paul J. Coffer*
Center for Molecular Medicine and Division of Pediatrics, University Medical Center Utrecht, Utrecht University, The Netherlands (Coffer PJ)Laboratory of Neuroimmunology and Developmental Origins of Disease(NIDOD), University Medical Center, Utrecht University, Utrecht, The Netherlands (Braccioli L, Nijboer CH)
Regenerative Medicine Center, University Medical Center Utrecht,Utrecht University, The Netherlands (Braccioli L, Coffer PJ)
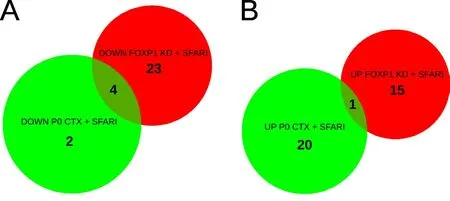
Figure 2 Overlap between autism-spectrum diseases (ASD)-associated genes and genes regulated by forkhead box protein P1 (FOXP1).
*Correspondence to:Paul J. Coffer, P.J.Coffer@umcutrecht.nl.
Accepted:2018-03-28
doi:10.4103/1673-5374.232467
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Rui Silva, Universidade de Lisboa Faculdade de Farmacia,Portugal.
Araujo DJ, Anderson AG, Berto S, Runnels W, Harper M, Ammanuel S, Rieger MA, Huang HC, Rajkovich K, Loerwald KW, Dekker JD, Tucker HO, Dougherty JD, Gibson JR, Konopka G (2015) FoxP1 orchestration of ASD‐relevant signal‐ing pathways in the striatum. Genes Dev 29:2081‐2096.
Bacon C, Rappold GA (2012) The distinct and overlapping phenotypic spectra of FOXP1 and FOXP2 in cognitive disorders. Hum Genet 131:1687‐1698.
Bacon C, Schneider M, Le Magueresse C, Froehlich H, Sticht C, Gluch C, Monyer H, Rappold GA (2015) Brain‐specific Foxp1 deletion impairs neuronal development and causes autistic‐like behaviour. Mol Psychiatry 20:632‐639.
Braccioli L, Vervoort SJ, Adolfs Y, Heijnen CJ, Basak O, Pasterkamp RJ, Nijboer CH, Coffer PJ (2017) FOXP1 promotes embryonic neural stem cell differentiation by repressing Jagged1 expression. Stem Cell Reports 9:1530‐1545.
Estruch SB, Graham SA, Quevedo M, Vino A, Dekkers DHW, Deriziotis P, Sollis E, Demmers J, Poot RA, Fisher SE (2018) Proteomic analysis of FOXP proteins reveals interactions between cortical transcription factors associated with neu‐rodevelopmental disorders. Hum Mol Genet 27:1212‐1227.
Hamdan FF, Daoud H, Rochefort D, Piton A, Gauthier J, Langlois M, Foomani G, Dobrzeniecka S, Krebs MO, Joober R, Lafrenire RG, Lacaille JC, Mottron L,Drapeau P, Beauchamp MH, Phillips MS, Fombonne E, Rouleau GA, Michaud JL (2010) De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am J Hum Genet 87:671‐678.
Horn D, Kapeller J, Rivera‐Brugués N, Moog U, Lorenz‐Depiereux B, Eck S,Hempel M, Wagenstaller J, Gawthrope A, Monaco AP, Bonin M, Riess O, Wohl‐leber E, Illig T, Bezzina CR, Franke A, Spranger S, Villavicencio‐Lorini P, Seifert W, Rosenfeld J, et al. (2010) Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits.Hum Mutat 31:E1851‐E1860.
Kriegstein A, Alvarez‐Buylla A (2009) The glial nature of embryonic and adult neu‐ral stem cells. Annu Rev Neurosci 32:149‐184.
Li X, Xiao J, Fröhlich H, Tu X, Li L, Xu Y, Cao H, Qu J, Rappold GA, Chen JG (2015)FOXP1 regulates cortical radial migration and neuronal morphogenesis in developing cerebral cortex. PLoS One 10:e0127671.
Precious SV, Kelly CM, Reddington AE, Vinh NN, Stickland RC, Pekarik V, Scherf C, Jeyasingham R, Glasbey J, Holeiter M, Jones L, Taylor MV, Rosser AE (2016)FoxP1 marks medium spiny neurons from precursors to maturity and is re‐quired for their differentiation. Exp Neurol 282:9‐18.
Rocca DL, Wilkinson KA, Henley JM (2017) SUMOylation of FOXP1 regulates transcriptional repression via CtBP1 to drive dendritic morphogenesis. Sci Rep 7:877.
Usui N, Araujo DJ, Kulkarni A, Co M, Ellegood J, Harper M, Toriumi K, Lerch JP,Konopka G (2017) Foxp1 regulation of neonatal vocalizations via cortical development. Genes Dev 31:2039‐2055.
- 中国神经再生研究(英文版)的其它文章
- Novel function of the chemorepellent draxin as a regulator for hippocampal neurogenesis
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Semaphorin 3A: from growth cone repellent to promoter of neuronal regeneration
- The role of undifferentiated adipose-derived stem cells in peripheral nerve repair
- Nerve conduction models in myelinated and unmyelinated nerves based on three-dimensional electrostatic interaction
- Fatigability during volitional walking in incomplete spinal cord injury: cardiorespiratory and motor performance considerations