Semaphorin 3A: from growth cone repellent to promoter of neuronal regeneration
During embryonic development, the axonal guidance cues facilitate the navigation of axonal growth cones towards the targets of innervation. These guidance cues could, broadly speaking, be either growth cone attractive or repulsive. The Semaphorin family of proteins are of fundamental important in neural circuit development (Pasterkamp, 2012) as well as a wide range of morphogenic functions. Signaling through a receptor complex of neuropilins and plexins, semaphorin 3A (Sema 3A), or collapsin‐1, is a prototypi‐cal chemorepellent of axonal growth which induces growth cone turning and collapse (Figure 1A). Abundant in the developing embryo, Sema 3A has a pleomorphic function in tissue remodeling processes, and in most cases its activity is perceived to be dispersive or disruptive of cell/tissue structures. In the adult central nervous system (CNS), Sema 3A became less abundant and more con fined to particular regions. However, its expression could be induced and upregulated by injury (De Winter et al., 2002) and this re‐expression could hinder neuronal regeneration upon injury. In this regard, inhibition of Sema 3A with a specific small compound in‐hibitor has indeed been shown to effectively enhance regenerative responses of the CNS (Kaneko et al., 2006).
Despite the general perception for Sema 3A being a retardant for neuronal regeneration, several findings over the years suggest that Sema 3A’s activity may not be solely repulsive. During cortical development, the radial migration of rat cortical layer II/III neurons uses Sema 3A as a guidance cue, where the latter apparently functioned as a chemoattractive signal (Chen et al., 2008). Further‐more, the local effect of Sema 3A on dendrites may differ markedly from axons, and in a few cases it has been shown that contrasting to axons, growing dendrites were attracted towards Sema 3A. For example, the growth of apical dendrites of cortical pyramidal neurons towards the pial surface appears to be positively regulated by a Sema 3A gradient (Polleux et al., 2000), likewise dendritic development in adult newborn neurons at the hippocampal dentate gyrus(Ng et al., 2013) (Figure 1A).
Two recent reports have now demonstrated that beyond its at‐tractive or repulsive activities towards axons or dendrites, Sema 3A could actually play a role in morphological regeneration of damaged adult cornea peripheral nerve (Zhang et al., 2018), as well as cortical tissues in a CNS injury model (Xu et al., 2018). These findings and their implications are discussed in the following paragraphs.
Sema 3A promoted nerve regeneration in the adult cornea:The cornea is one place where Sema 3A levels remain high in adult.Zhang et al. (2018) noted significant levels of Sema 3A expression in the corneal epithelium and the trigeminal ganglion (TG) of adult mice. Isolated dorsal root ganglion (DRG) neurons in culture could be induced to sprout neurites with the addition of nerve growth factor (NGF). Interestingly, while Sema 3A induced axonal retraction and growth cone collapse in NGF‐induced neurites of embryonic DRG neurons, it is ineffective in this regard with adult DRG neurons. In fact, not only did Sema 3A not antagonize NGF‐in‐duced neurite growth, it could on its own induce neurite outgrowth from both cultured adult DRG and TG neurons in a dose‐depen‐dent manner, and to an extent that is equivalent to that by NGF. In further exploring this observation in anin vivomodel, the authors showed that Sema 3A could indeed be a potent inducer of cornea nerve regeneration. The central corneal epithelium and the super ficial nerve plexus were surgically removed from mice while leaving the corneal stroma intact. Sema 3A was then introduced as a pellet inserted into the stromal and the extent of regeneration of the corneal nerves was assessed using β‐III tubulin staining. Mice treated with Sema 3A showed increased nerve growth extension and high‐er nerve density compared to control, which received instead pellet of phosphate‐buffered saline.
The findings of Zhang et al. (2018) mirrored the findings made earlier with another Semaphorin family member, Sema 7A (Nam‐avari et al., 2012), which unlike Sema 3A, is a lipid‐anchored pro‐tein. Like Sema 3A, however, Sema 7A is also abundantly expressed in the corneal epithelium and its level is increased significantly in the cornea after lamellar corneal surgery and found localized to stromal cells near the regenerating nerves. Exposure of TG neurons in culture to Sema 7A markedly increased neurite length, while an implanted Sema 7A‐containing pellet also significantly increased corneal nerve lengthin vivo. The above findings, taken together,indicate that some members of the Semaphorin family, particularly Sema 3A, which generally repels growth cones of embryonic peripheral nervous system (PNS) neurons, instead promotes neurite outgrowth from adult PNS neurons.
An implanted molecular gradient of Sema 3A promoted cortical regeneration:Regeneration of CNS neurons after injury is notoriously difficult, and this could be compounded by injury‐induced re‐expression of repellent molecules. However, contrasting to previous findings in spinal cord (De Winter et al., 2002; Majed et al., 2006), a recent report has now provided some evidence indicating that Sema 3A acting in a certain context could improve CNS regeneration (Xu et al., 2018). The authors noted the earlier finding that Sema 3A forms a descending gradient across the cortical layers during the development of mouse cortex, with highest Sema 3A concentration at the pial surface, which appeared to attract cortical neuron dendrites (Polleux et al., 2000). To see if a Sema 3A gradient that mimics the situationin vivocould promote neural progenitor cell migration towards a site of cortical injury and promote regeneration, the authors designed a gradient‐sustaining implant with Sema 3A seeded on top of a hydrogel sheathed by a glass cylinder. Cortical injury was induced by surgically creating a small cylindrical cavity at a site near the neurogenic subgranular zone (SGZ) of the hippocam‐pal dentate gyrus (which is closed to the cortex), with the implant then inserted at the injury site. Tissue harvesting and immune‐histo‐logical examinations were made at day 12 and day 30.
The authors observed a tissue extension into the injury site from the surrounding and regeneration of the lesioned region could be morphometrically quantified. Interestingly, injury sites receiving an implant with a Sema 3A gradient demonstrated a regeneration volume that was better than control, and for the more extended period of 30 days, also better than another implant with a chemoat‐tractive Netrin‐1 gradient. At day 12 after injury, confocal imaging showed that the numbers of Nestin‐positive neural progenitor cells(NPC), doublecortin (DCX)‐positive migrating neuroblasts or glialfibrillary acidic protein (GFAP)‐positive glia cells at the Sema 3A implanted site were significantly above that of control. There was also significantly more β‐III tubulin‐positive young neurons. At day 30 DCX‐positive cells were reduced, but with a concomitant increase in neuronal nuclei (NeuN)‐positive neuronal cells. Cells at the bottom of the Sema 3A hydrogel position have a substantial number of Nestin and DCX labeled cells, and these together with those within the Sema 3A implant site are also positive for the mitotic marker 5‐bromo‐2′‐deoxyuridine (BrdU), which implies that these are newborn cells that have most probably migrated to‐wards the lesion site from the SGZ. Transcriptome analyses of the regenerated tissues with Sema 3A implant indicated upregulation of genes associated with neuronal migration and differentiation, as well those associated with Sema 3A signaling. Interestingly, Wnt signaling pathway genes were also upregulated. On the whole, the artificially generated Sema 3A gradient in adult cortical lesions that mimic that during embryonic corticogenesis appears able to pro‐mote neural progenitor cell migration and neuronal differentiation at an adult CNS site (Figure 1B).
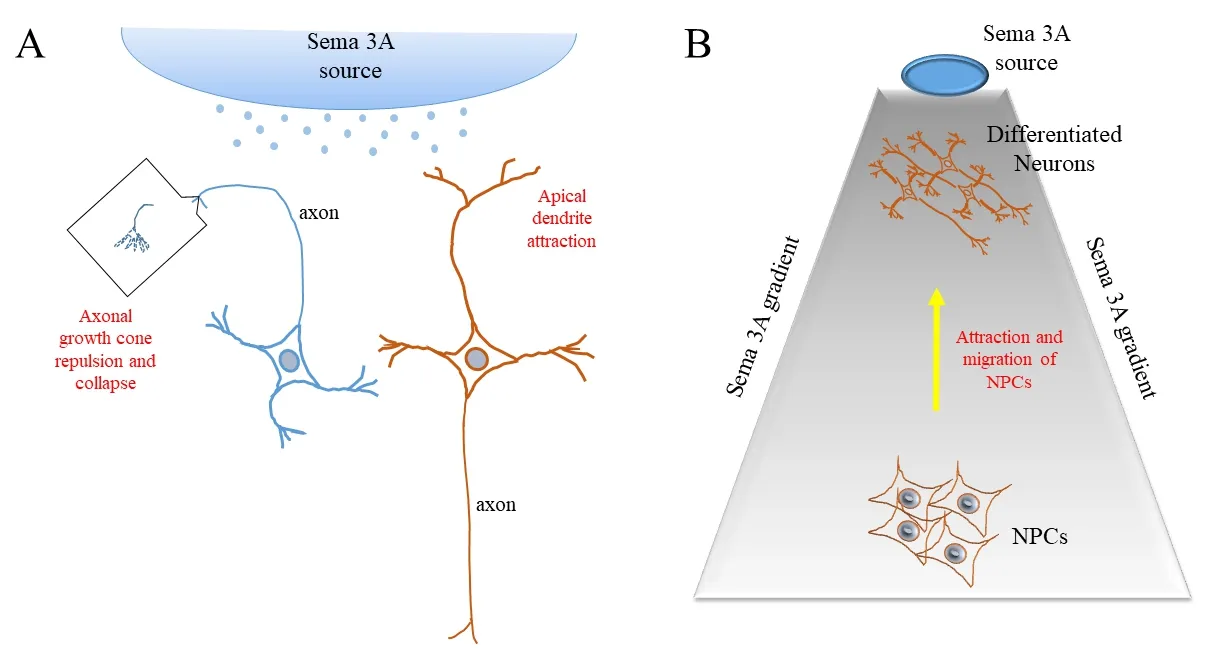
Figure 1 Schematic illustrations of the regeneration promoting effect of Semaphorin 3A (Sema 3A).
What underlies the pro-regeneration effect of Sema 3A towards adult nerves?Two important summarizing observations could be made from the findings discussed above. Firstly, despite being growth cone repulsive for embryonic PNS neurons, Sema 3A could instead promote neurite outgrowth in certain adult PNS neurons,as demonstrated for those found at the cornea. One should bear in mind that the findings contradicted previous findings showing that Sema 3A repels DRG and TG sensory afferents (Tanelian et al., 1997). It also remains to be seen whether any neurite growth enhancing effect could be generalized to PNS neurons at other sites.The underlying Sema 3A‐mediated signaling process that may differ between embryonic neurons and adult neurons was not investigated and is largely unclear. Sema 3A’s effect on axonal growth cone turning and growth cone collapse goes through signaling from the neu‐ropilin‐plexin complex, which downstream engagement of members of collapsin‐response‐mediator protein (CRMP) family and the small GTPases Rac1 and Rho, resulting in the changes in actin dynamics, endocytosis of the growth cone cell membrane as well as possible changes in the microtubules (as CRMP binds tubulin). How this pathway is suppressed or quantitatively altered in adult PNS neurons is unclear. Notably, neurite outgrowth measured from DRG or TG explants are likely a mixture of axons and dendrites.How Sema 3A signaling may differ between axons and dendrites so as to elicit different growth responses is not clear.
As regeneration of CNS neurons is generally much more difficult than PNS, the demonstration that a strategically placed artificial Sema 3A gradient could promote NPC migration and differentiation in an injured CNS environment is intriguing. The signaling process engaged by Sema 3A with NPCs and newborn neurons may differ from the canonical growth cone based signaling, or those that occur with regenerating spinal cord axons. In this re‐gard, NPCs or newborn neurons may not yet have a morpholog‐ically and functionally defined axonal growth cone that could be repel or collapse by Sema 3A. It has also been shown by another report on NPCs from the adult mouse dentate gyrus that Sema 3A could affect other signaling pathways, such as the Focal adhesion kinase through activation of cyclin‐dependent kinase 5 (Ng et al.,2013). Xu and colleagues also showed that the Sema 3A gradient appear to result in enhanced Wnt signaling in the migrating neu‐roprogenitors (Xu et al., 2018). It is notable that Wnt signaling is known to be involved in Sema 3A function in other systems, such as osteoblast differentiation and osteoprotection. Wnt signaling is also known to be critical for adult neurogenesis and axonal regen‐eration (Wu and Murashov, 2013). If this is elicited by the Sema 3A gradient in NPCs and newborn neurons, it could at least partially,account for the regenerative effect seen. As the model of Xu et al.(2018) have a low resolution in terms of cellular and molecular lev‐el events, the question of how exactly does a dentate gyrus NPC or newborn neuron respond to Sema 3A would require further investigations.
That Sema 3A may be regeneration promoting rather than inhibitory has important implications in terms adult neuronal re‐generation promoting strategies. The studies discuss herein should prompt further attempts to better characterize the respective Sema 3A‐induced regenerative responses and the understanding of its underlying molecular and cellular basis.
Bor Luen Tang*
Department of Biochemistry, Yong Loo Lin School of Medicine,National University Health System, Medical Drive, Singapore,Singapore; Graduate School for Integrative Sciences and Engineering,National University of Singapore, Medical Drive, Singapore,Singapore
*Correspondence to:Bor Luen Tang, Ph.D., bchtbl@nus.edu.sg.
orcid:0000-0002-1925-636X (Bor Luen Tang)
Accepted:2018-04-17
doi:10.4103/1673-5374.232463
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer review reports:
Reviewer 1:Ozgur Boyraz, Gulhane Military Medical Academy, Turkey.
Reviewer 2:Angel Gato, Universidad de Valladolid Facultad de Medicina,Spain.
Comments to author:The manuscript address an interesting subject as is the complex effect of Semaphorin 3A on neuronal regeneration. It puts together different and, in some cases, contradictory research about the Semaphorin 3A repulsive effect on axonal growth cone with an recently proposed property,the influence in neurorregeneration. The manuscript is well-written, is actual and the subject is relevant leaving open questions and new research lines. It follows the structure and extension proposed for a perspective article.
Chen G, Sima J, Jin M, Wang KY, Xue XJ, Zheng W, Ding YQ, Yuan XB(2008) Semaphorin‐3A guides radial migration of cortical neurons during development. Nat Neurosci 11:36‐44.
De Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ,Pasterkamp RJ, Gispen WH, Verhaagen J (2002) Injury‐induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol 175:61‐75.
Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S,Okano HJ, Ikegami T, Moriya A, Konishi O, Nakayama C, Kumagai K,Kimura T, Sato Y, Goshima Y, Taniguchi M, Ito M, He Z, Toyama Y,Okano H (2006) A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med 12:1380‐1389.
Majed HH, Chandran S, Niclou SP, Nicholas RS, Wilkins A, Wing MG,Rhodes KE, Spillantini MG, Compston A (2006) A novel role for Se‐ma3A in neuroprotection from injury mediated by activated microglia. J Neurosci 26:1730‐1738.
Namavari A, Chaudhary S, Ozturk O, Chang JH, Yco L, Sonawane S, Kat‐am N, Khanolkar V, Hallak J, Sarkar J, Jain S (2012) Semaphorin 7a links nerve regeneration and inflammation in the cornea. Invest Ophthalmol Vis Sci 53:4575‐4585.
Ng T, Ryu JR, Sohn JH, Tan T, Song H, Ming GL, Goh EL (2013) Class 3 semaphorin mediates dendrite growth in adult newborn neurons through Cdk5/FAK pathway. PLoS One 8:e65572.
Pasterkamp RJ (2012) Getting neural circuits into shape with semaphorins.Nat Rev Neurosci 13:605‐618.
Polleux F, Morrow T, Ghosh A (2000) Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 404:567‐573.
Tanelian DL, Barry MA, Johnston SA, Le T, Smith GM (1997) Semaphorin III can repulse and inhibit adult sensory afferents in vivo. Nat Med 3:1398‐1401.
Wu D, Murashov AK (2013) MicroRNA‐431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1.Front Mol Neurosci 6:35.
Xu Z, Wang W, Ren Y, Zhang W, Fang P, Huang L, Wang X, Shi P (2018)Regeneration of cortical tissue from brain injury by implantation of defined molecular gradient of semaphorin 3A. Biomaterials 157:125‐135.
Zhang M, Zhou Q, Luo Y, Nguyen T, Rosenblatt MI, Guaiquil VH (2018)Semaphorin3A induces nerve regeneration in the adult cornea‐a switch from its repulsive role in development. PLoS One 13:e0191962.
- 中国神经再生研究(英文版)的其它文章
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Exosomes: a novel therapeutic target for Alzheimer’s disease?
- Inhibition of retinal ganglion cell apoptosis:regulation of mitochondrial function by PACAP
- Trillium tschonoskii maxim extract attenuates abnormal Tau phosphorylation
- Association between Alzheimer’s disease pathogenesis and early demyelination and oligodendrocyte dysfunction
- Nogo receptor expression in microglia/macrophages during experimental autoimmune encephalomyelitis progression