Inhibition of retinal ganglion cell apoptosis:regulation of mitochondrial function by PACAP
Huan-Huan Cheng , Hui Ye , Rui-Ping Peng Juan Deng , Yong Ding
1 Department of Ophthalmology, the Third Affiliated Hospital of Sun Yat‐sen University, Guangzhou, Guangdong Province, China
2 Department of Ophthalmology, The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong Province, China
Introduction
Glaucoma can cause permanent loss of vision and is char‐acterized by initial changes to retinal ganglion cell (RGC)axons and secondary death of RGC cell bodies (Calkins et al., 2012). Glaucoma is typically associated with intraocular pressure (IOP). However, even with treatment to reduce IOP, the symptoms of a substantial fraction of glaucoma patients worsen with time. Furthermore, the IOP of some glau‐coma patients never rises beyond the normal range (Pascale et al., 2012). Although the etiology of glaucoma is complex and enigmatic, RGC apoptosis is recognized as the ultimate reason for loss of vision (Tian et al., 2015). Neuroprotective treatments that directly target the injured RGCs are still in early stages of development. Therefore, there is an urgent need to develop therapeutic agents for glaucoma neuropathy and neurotrophic factors are promising candidates.
Pituitary adenylate cyclase‐activating polypeptide (PA‐CAP) exists in two forms (namely 27‐ or 38‐amino acid neuropeptides) and belongs to the vasoactive intestinal poly‐peptide/glucagon/secretin family (Bourgault et al., 2009).PACAP and its receptors are expressed in various neural tissues and it exerts potent neuroprotective effects both exogenously and endogenously (Endo et al., 2011). Most of PACAP’s effects are mediated through the activation of its specific receptor, PAC1 (Zhou et al., 2002). PACAP is a pleiotropic molecule exerting effects on a wide array of phys‐iological processes, including cell survival in neurodegener‐ative conditions, the stress response and cell division (Castorina et al., 2010; Giunta et al., 2012; D’Amico et al., 2013,2015; Maugeri, et al., 2016). It also acts as a neurotransmitter and/or a neuromodulator in both the peripheral and central nervous systems (Jówiak‐Bębenista et al., 2015; Yang et al.,2015; Shioda et al., 2016). The protective effects of PACAP in the retina have been investigated by numerous studies,including diabetic retinopathy induced by streptozotocin(Szabad fiet al., 2012), transient ischemia following high IOP(Seki et al., 2011) and ultraviolet‐light‐induced retinal dam‐age (Atlasz et al., 2010). We have previously demonstrated that a PACAP derivative attenuates the apoptosis of RGC‐5 cells induced by ultraviolet B irradiation and retinal excito‐toxicity induced by N‐methyl‐D‐aspartic acid (Cheng et al.,2014). However, thein vitroeffects of PACAP and PAC1 on the apoptosis of RGCs and the underlying mechanism re‐main largely unknown. In the current study, the neuropro‐tective effects of PACAP against serum deprivation (SD)‐in‐duced apoptosis of RGC‐5 cells were further explored.
Materials and Methods
RGC-5 cell culture
The RGC‐5 cell line (Li et al., 2011) was provided by Dr.Neeraj Agarwal from the Department of Cell Biology and Genetics, UNT Health Science Center, Fort Worth, TX,USA. The RGC‐5 cell line is a transformed RGC line that has been widely used, expresses RGC markers, and exhibits ganglion cell‐like behavior in culture (Wood et al., 2010).Cells were cultured in Dulbecco’s Modi fied Eagle’s Medium(DMEM) containing 10% (v/v) heat‐inactivated fetal bovine serum (FBS) in a humidified incubator at 37°C for 24 hours.The culture medium was then exchanged for DMEM containing normal 10% FBS or FBS‐free DMEM with or without PACAP1–38 at different concentrations (1 nM, 10 nM, 100 nM, 1 μM, 10 μM PACAP). Cells were further incubated for 48 hours only because a previous study (Fuma et al., 2016)demonstrated an approximately 50% loss of cell viability after serum withdrawal for 48 hours. After determining the optimal concentration of PACAP, cells were divided into three groups: control, serum deprivation (SD) and SD + PA‐CAP groups. In the SD + PACAP group, cells were exposed to SD and 100 nM PACAP for 48 hours, while SD group was exposed to SD without PACAP.
Cell viability assay
Forty‐eight hours after treatment with SD or SD + PACAP,cell viability was assessed with Cell Counting Kit‐8 (Dojindo,Japan). Brie fly, cells were stained with 10 μL Cell Counting Kit‐8 solution for 3 hours. Optical density (OD) of each well was measured with a microplate reader (Tecan Sa fire2,Männedorf, Switzerland) at 450 nm. Wells with only culture medium were used as the blank control. Cell viability was equal to (ODSDgrouporSD+PACAPgroup— ODblankcontrol)/(ODcontrolgroup— ODblankcontrol) × 100%.
Cell cycle analysis
RGC‐5 cells were deprived of serum with or without 100 nM PACAP for 12 or 24 hours. The phase distribution of DNA content in the cells was then detected with propidium iodide (PI) staining and flow cytometry. Following 100 nM PACAP treatment for 12 or 24 hours, RGC‐5 cells were collected, fixed in 70% ethanol and stored overnight at −20°C.The next morning, cells were washed and stained with PI staining solution (50 μg/mL PI and 10 μg/mL RNase) for 30 minutes in the dark. The cell cycle was then analyzed by flow cytometry using Cell‐Quest software (FACSAriaTM, BD, San Jose, CA, USA). The percentages of cells in S, G0/G1 and G2/M phases were analyzed by pairwise comparisons.
Annexin V/PI staining and JC-1 assays
RGC‐5 cells (5 × 105) were collected following treatment for 48 hours and suspended in 200 μL binding buffer. Then cells were stained with 10 μL Annexin V‐FITC and 10 μL PI for 15 minutes. The apoptosis of cells was subsequently detected by flow cytometry (FACSAriaTM, BD).
After SD treatment with or without PACAP for 48 hours,cells were incubated with 200 μL JC‐1 solution for 15 minutes. Then cells were washed with phosphate buffered saline(PBS), pelleted by centrifugation, resuspended in 500 μL PBS and analyzed with a flow cytometer (FACSAriaTM,BD). The percentage of apoptotic cells with mitochondrial depolarization was analyzed.
Hoechst 33342 staining
Brie fly, RGC‐5 cells were seeded on 6‐well plates at a density of 5 × 104cells/mL. After treatment, cells were washed with PBS and fixed with 4% paraformaldehyde for 20 minutes.After removing paraformaldehyde, cells were stained with 10 μM Hoechst 33342 solution (Sigma, Shanghai, China) for 20 minutes and observed under a fluorescence microscope(BZ X700, Keyence, Osaka, Japan).
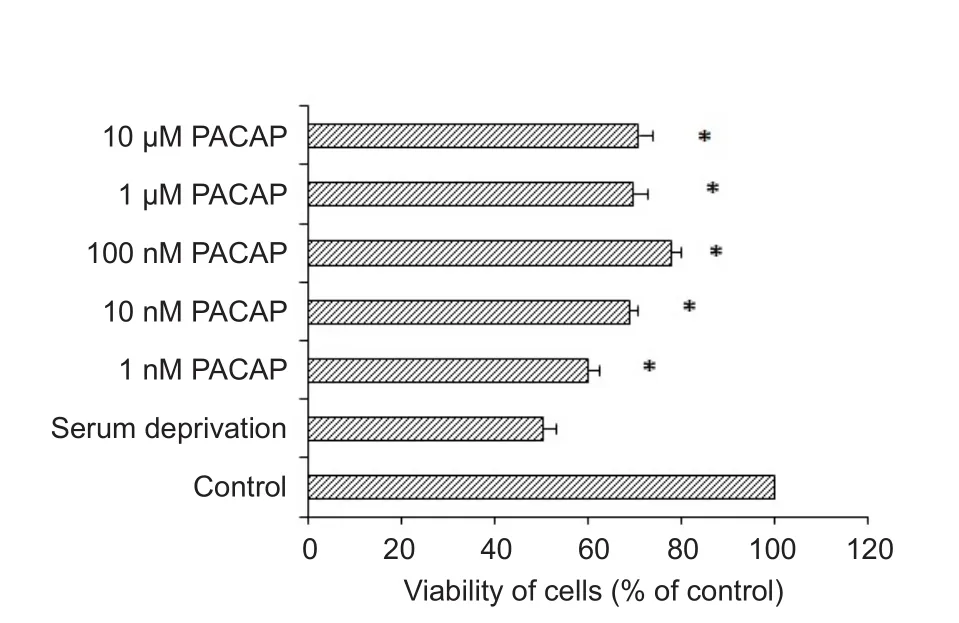
Figure 1 The effect of PACAP on the viability of RGC-5 cells subjected to serum deprivation for 48 hours.
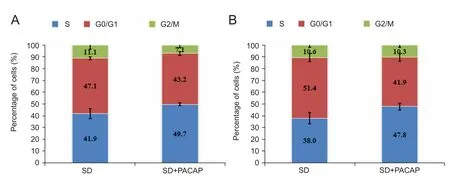
Figure 2 Effects of 100 nM PACAP on the cell cycle of RGC-5 cells at various time points using flow cytometric analysis.

Figure 3 Representative dot plots determined by flow cytometry following Annexin V/PI staining.

Figure 4 Representative micrographs of RGC-5 cells stained with Hoechst 33342.
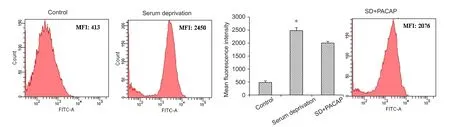
Figure 5 Effect of PACAP on ROS levels in RGC-5 cells subjected to SD for 48 hours.
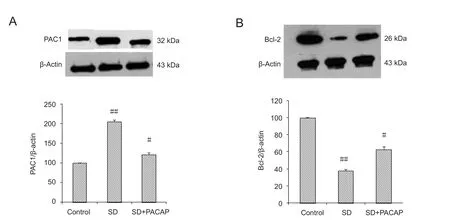
Figure 6 Densitometric analysis of PAC1 and Bcl-2 levels in RGC-5 cells exposed to SD with or without 100 nM PACAP.
ROS quantification
Following treatment, RGC‐5 cells were stained with 10 μM DCFH‐DA for 15 minutes in the dark at 37°C. Cells were then washed with PBS and analyzed within 30 minutes by flow cytometry (FACSAriaTM, BD, equipped with an air‐cooled argon laser at 488 nm). The intensity of green fluorescence, which indicates the level of intracellular ROS accumulation, was detected and compared between groups.
Western blot assay
To control cell number, RGC‐5 cells were seeded at a density of 5 × 104cells/mL in 6‐well plates and then subjected to var‐ious treatments. Forty‐eight hours after treatment with SD in the presence or absence of 100 nM PACAP, cells were collected, lysed with RIPA buffer sup‐plied with protease and phos‐phatase inhibitor cocktail and sonicated on ice. The sonicated cell samples were then centrifuged for 20 minutes at 15,000 ×gat 4°C. After centrifugation, the supernatant was collected.Proteins (10 μg) were then separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Membranes were incubated at 4°C with primary antibodies as follows: mouse monoclonal anti‐Bcl‐2 antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti‐PAC1 antibody (1:1000; Santa Cruz Biotechnology), and mouse monoclonal anti‐β‐actin antibody (1:3000; Cell Signaling,Danvers, MA, USA). After over‐night incubation with primary antibodies, secondary an‐tibodies conjugated with horse‐radish peroxidase (1:3000; Santa Cruz Biotechnology) were applied for 1 hour at room temperature. The signals were detected with enhanced chemiluminescence (BeyoECL Plus).Protein levels were evaluated by measuring the optical value ratios of Bcl‐2 and PAC1 to β‐actin, and then normalized to the control group. The immunoblot band of each protein was quantified and normalized to that of β‐actin.
Statistical analysis
Data were analyzed with SPSS 17.0 software (SPSS, Chica‐go, IL, USA) and are presented as the mean ± SD. One‐way analysis of variance and the Student‐Newman‐Keuls test were used to determine statistical comparison.P< 0.05 was considered statistically significant.
Results
PACAP increased the viability of RGC-5 cells
The cell viability of RGC‐5 cells deprived of FBS was significantly increased by 1 nM to 10 μM PACAP38 (Figure 1).The optimum protection was achieved with 100 nM PACAP.DNA content analysis showed that 100 nM PACAP treatment resulted in remarkably more cells at S phase compared to cells subjected to SD only. As shown in Figure 2, PACAP increased the percentage of cells in S phase and reduced the percentage of cells in G0/G1phase compared to RGC‐5 cells subjected to SD only. The proportion of cells in S‐phase was significantly higher in the SD + PACAP group than in the SD group at both 12 hours (49.65 ± 1.17%,vs. 41.85 ± 4.13%)and 24 hours (47.80 ± 2.78%,vs. 38.00 ± 4.94%).
PACAP inhibited SD-induced apoptosis in RGC-5 cells
PACAP treatment significantly decreased the rate of apoptosis, which was 10.50 ± 1.23% in the SD + PACAP group and 25.14 ± 1.84% in the SD group. With JC‐1 staining, we discovered that PACAP treatment remarkably decreased the proportion of cells with mitochondrial depolarization to 22.57 ± 2.24%, while the percentage of depolarized cells in the SD group was 64.17 ± 1.70% (Figure 3). Hoechst 33342 staining showed that the nuclei of serum‐deprived cells were shrunken and condensed, while PACAP treatment attenuated nuclei change (Figure 4).
ROS, PAC1 and Bcl-2 levels
The mean intensity of ROS fluorescence was elevated over 5‐fold in RGC‐5 cells subjected to SD but was considerably decreased with 100 nM PACAP treatment (Figure 5).
To further investigate the protective mechanism of PA‐CAP, we analyzed the expression levels of PAC1 and Bcl‐2. Forty‐eight hours after SD, a significant increase in PAC1 and a simultaneous decrease in Bcl‐2 levels were seen.Treatment with PACAP dramatically inhibited such changes(Figure 6).
Discussion
PACAP is an important growth factor capable of preventing apoptosis in neuronal cells (Maino et al., 2015). Protection against RGC apoptosis by PACAP has been investigated using ultraviolet B irradiation (Ding et al., 2012), N‐meth‐yl‐D‐aspartic acid excitotoxicity (Cheng et al., 2014), ischemic retinal degeneration (Szabadfi, 2012) and experimental diabetic retinopathy (Szabadfiet al., 2014, 2016). On the ba‐sis of our previousin vivoandin vitrostudies (Cheng et al.,2014), the question emerged as to whether PACAP can at‐tenuate RGC apoptosis induced by other unexplored insults.Protective effects of PACAP on serum deprived neuronal cells have been reported for cerebellar granule cells (Maino et al., 2015), schwannoma cells (Castorina et al., 2008) and rat cortical neurons (Frechilla et al., 2001). SD is a well‐es‐tablished model to investigate apoptosis in RGCs (Sun et al.,2012; Majid et al., 2013; Miki et al., 2013). However, to our knowledge, no previous study has assessed the protection of PACAP on RGC apoptosis induced by SD. The present study demonstrated that PACAP attenuates SD‐induced apoptosis in RGC‐5 cells.
PACAP is neuroprotective in the retina and 100 nM was the optimal concentration (Seki et al., 2008). In our study,the anti‐apoptotic effect of PACAP was not dose‐dependent and peaked at a concentration of 100 nM. The SD + PACAP group exhibited a higher percentage of cells in S‐phase com‐pared with the SD group, indicating that PACAP promoted cell viability partly by accelerating the cell cycle. Neuronal damage in glaucoma has been associated with increased free radical production and to a low level of endogenous antioxidant defense (Izzotti et al., 2006). In the present re‐search, SD led to ROS over‐generation. The over generation of ROS is indicative of compromised antioxidant capacity and makes cells vulnerable to injury (Kang et al., 2010). Nu‐merous stressors capable of causing excessive ROS production are involved in glaucoma (Pinazo‐Durán et al., 2012).Mitochondria are the major site for superoxide production,and are vulnerable to direct attack by ROS (Orrenius, 2007).Mitochondrial dysfunction leads to increased production of ROS, which in turn aggravates oxidative stress (Jezek et al., 2005). PACAP prevents the decrease of mitochondrial activity in astroglial cells (Masmoudi‐Kouki et al., 2011) and inhibits the excessive generation of ROS in ultraviolet B ir‐radiated RGC‐5 cells (Cheng et al., 2014). However, whether PACAP reduces ROS production in serum‐deprived RGC‐5 cells has not been elucidated. Our study revealed that the rise in ROS levels in response to SD was markedly quenched by 100 nM PACAP treatment.
SD induces apoptotic cell death of transformed rat RGCsviamitochondrial signaling pathways (Charles et al., 2005).The number and morphology of mitochondria vary widely among cell types and are regulated by intrinsic and extrinsic mechanisms (Davis et al., 2014). RGCs probably have more mitochondria than any other neuronal cell type and efficient intraocular axon mitochondrial function is essential to maintain overall function of RGCs (Osborne et al., 2014).Mitochondrial stress in individual RGCs has been proposed as a major trigger of glaucoma and pharmacological agents that maintain mitochondrial functions might, therefore,provide a novel way of delaying RGC death (Osborne et al.,2013). As indicated by JC‐1 assays, PACAP compensated the loss of mitochondrial membrane potential caused by SD in RGC‐5 cells. PAC1 is abundant in the retina (Dénes et al., 2014) and we have previously reported the expression of PAC1 in RGC‐5 cells, which was increased in ultravi‐olet B‐induced apoptotic cells (Ding et al., 2012). In our present study, there is a direct involvement of PAC1 in the anti‐apoptotic function because PAC1 expression is significantly increased in RGC5 cells exposed to SD. Similarly,expression of the gene encoding PAC1 increases markedly during in flammation and disease to alleviate inflammation,oxidative stress and apoptosis (Xu et al., 2016). We suggest that the protective effect of PACAP is mediatedviaPAC1 and an exogenous PACAP supply counteracts the compensational increase of PAC1 signaling. The activation of PAC1 by PACAP modulates cell death in the retina through the intracellular cAMP/cAMP‐dependent protein kinase pathway(Silveira et al., 2002) and the protective effect of PACAP in‐volves complex kinase signaling pathways related to cAMP/ERK/CREB activation (Racz et al., 2006). PACAP, through activation of its receptor, PAC1, and the protein kinase A,protein kinase C, and MAP‐kinases signaling pathways, prevents accumulation of ROS, which allows the preservation of mitochondrial membrane integrity (Han et al., 2014; Douiri et al., 2016). Further studies are needed to gain deeper in‐sights into the mechanism underlying the protective effect of PACAP against SD‐induced apoptosis in RGC‐5 cells.
Bcl‐2 levels in RGC‐5 cells subjected to SD were significantly lower than those in RGC‐5 cells. The expression of anti‐apoptotic Bcl‐2 increases greatly in apoptotic RGC‐5 cells in response to other insults like H2O2. Members of the Bcl‐2 family participate in the initiation of the mitochondrial signal pathway, thus regulating cell apoptosis (Wang et al., 2013). Therefore, PACAP may inhibit the apoptosis of RGC‐5 cellsviathe mitochondrial pathway by reducing ROS levels and, at the same time, by increasing Bcl‐2 levels.Although there is controversy over the validity of RGC‐5 cells, they are widely accepted as retinal neuronal precursor cells (Van Bergen et al., 2009). Furthermore, in our previous study, the RGC‐5 cells that we use have been proven to express specific markers for RGCs. Our results suggest that PACAP may protect RGCs from apoptotic death by inhibiting the mitochondrial apoptosis pathway. However, as intracellular signaling pathways are very complex, the involvement of other pathways cannot be excluded. Furthermore,the protective mechanism of PACAP mediated by PAC1 is complicated and varies among cell types. The protective effect of PACAP and its underlying mechanism should be further investigated in animal models.
In conclusion, PACAP attenuates SD‐induced apoptosis in RGCs and might play an important role in the neuroprotection of RGCs. Perturbations in the PACAP/PAC1 path‐way are involved in abnormal stress responses underlying post‐traumatic stress disorder (Ressler et al., 2011). We can‐not help but wonder whether the PACAP/PAC1 pathway is associated with glaucomatous neuropathy, which will be the direction of our future research.
Acknowledgments:We are very grateful to Pei-Zhen Zhao from Dermatology Hospital of Southern Medical University, Guangzhou, China who offered advice on data processing and provided useful critical comments.
Author contributions:HHC and YD designed the study. HHC and RPP performed experiments. JD and HY analyzed data. HHC wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:The authors declare no competing financial interests.
Financial support:This study was supported by grants from the Medical Scientific Research Foundation of Guangdong Province of Chian,No. A2016271; the Natural Science Foundation of Guangdong Province of China, No. 2016A030313208; and the Science and Technology Planning Project of Guangdong Province of China, No. 2014A020212393.The funding bodies played no role in the study design, in the collection,analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Javier Francisco-Morcillo, University of Extremadura, Spain; Arne M. Nystuen, University of Nebraska Medical Center, USA.
Additional file:Open peer review reports 1 and 2.
Atlasz T, Szabadfi K, Kiss P, Racz B , Gallyas F, Tamas A, Gaal V, Mar‐ton Z, Gabriel R, Reglodi D (2010) Review of pituitary adenylate cyclase activating polypeptide in the retina: focus on the retinoprotective effects. Ann N Y Acad Sci 1200:128‐139.
Bourgault S, Vaudry D, Dejda A, Doan ND, Vaudry H, Fournier A(2009) Pituitary adenylate cyclase‐activating polypeptide: focus on structure‐activity relationships of a neuroprotective peptide. Curr Med Chem 16:4462‐4480.
Calkins DJ (2012) Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res 31:702‐719.
Castorina A, Giunta S, Mazzone V, Cardile V, D’Agata V (2010) Effects of PACAP and VIP on hyperglycemia‐induced proliferation in murine microvascular endothelial cells. Peptides 31:2276‐2283.
Castorina A, Tiralongo A, Giunta S, Carnazza M, Rasi G, D’Agata V(2008) PACAP and VIP prevent apoptosis in schwannoma cells.Brain Res 1241:29‐35.
Charles I, Khalyfa A, Kumar DM, Krishnamoorthy RR, Roque RS,Cooper N, Agarwal N (2005) Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci 46:1330‐1338.
Cheng H, Ding Y, Yu R, Chen J,Wu C (2014) Neuroprotection of a novel cyclopeptide C★HSDGIC★ from the cyclization of PACAP (1‐5) in cellular and rodent models of retinal ganglion cell apoptosis.PLoS One 9:e108090.
D’Amico AG, Maugeri G, Reitano R, Bucolo C, Saccone S, Drago F,D’Agata V (2015) PACAP modulates expression of hypoxia‐in‐ducible factors in streptozotocin‐induced diabetic rat retina. J Mol Neurosci 57:501‐509.
D’Amico AG, Scuderi S, Saccone S, Castorina A, Drago F, D’Agata V(2013) Antiproliferative effects of PACAP and VIP in serum‐starved glioma cells. J Mol Neurosci 51:503‐513.
Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebu‐chi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, Marsh‐Armstrong N (2014) Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci USA 111:9633‐9638.
Dénes V, Czotter N, Lakk M (2014) PAC1‐expressing structures of neural retina alter their PAC1 isoform splicing during postnatal development. Cell Tissue Res 355:279‐288.
Ding Y, Cheng H, Yu R, Tang C, Liu X, Chen J (2012) Effects of cyclo‐peptide C★HSDGIC★ from the cyclization of PACAP (1—5) on the proliferation and UVB‐induced apoptosis of the retinal ganglion cell line RGC‐5. Peptides 36:280‐285.
Douiri S, Bahdoudi S, Hamdi Y, Cubì R, Basille M, Fournier A, Vau‐dry H, Tonon MC, Amri M, Vaudry D, Masmoudi‐Kouki O (2016)Involvement of endogenous antioxidant systems in the protective activity of pituitary adenylate cyclase‐activating polypeptide against hydrogen peroxide‐induced oxidative damages in cultured rat as‐trocytes. J Neurochem 137:913‐930.
Endo K, Nakamachi T, Seki T, Kagami N , Wada Y , Nakamura K,Kishimoto K, Hori M, Tsuchikawa D, Shinntani N, Hashimoto H,Baba A, Koide R, Shioda S (2011) Neuroprotective effect of PACAP against NMDA‐induced retinal damage in the mouse. J Mol Neurosci 43:22‐29.
Frechilla D, García‐Osta A, Palacios S, Cenarruzabeitia E, Del R (2001)BDNF mediates the neuroprotective effect of PACAP‐38 on rat cor‐tical neurons. Neuroreport 12:919‐923.
Fuma S, Shimazawa M, Imamura T, Kanno Y, Takano N, Tsuruma K,Hara H (2016) Neuroprotective effect of ocular hypotensive drugs:latanoprost/timolol in combination are more effective than each as monotherapy in RGC‐5. Biol Pharm Bull 39:192‐198.
Giunta S, Castorina A, Bucolo C, Magro G, Drago F, D’Agata V (2012)Early changes in pituitary adenylate cyclase‐activating peptide, va‐soactive intestinal peptide and related receptors expression in retina of streptozotocin‐induced diabetic rats. Peptides 37:32‐39.
Han P, Tang Z, Yin J, Maalouf M, Beach TG, Reiman EM, Shi J (2014)Pituitary adenylate cyclase‐activating polypeptide protects against β‐amyloid toxicity. Neurobiol Aging 35:2064‐2071.
Jezek P, Hlavatá L (2005) Mitochondria in homeostasis of reactive ox‐ygen species in cell, tissues, and organism. Int J Biochem Cell Biol 37:2478‐2503.
Jówiak‐Bębenista M, Kowalczyk E, Nowak JZ (2015) The cyclic AMP effects and neuroprotective activities of PACAP and VIP in cultured astrocytes and neurons exposed to oxygen‐glucose deprivation.Pharmacol Rep 67:332‐338.
Kang KD, Majid AS, Kim KA, Kang K, Hong R, Chu W, Sang H (2010)Sulbutiamine counteracts trophic factor deprivation induced apop‐totic cell death in transformed retinal ganglion cells. Neurochem Res 35:1828‐1839.
Li J, Dong Z, Liu B, Zhuo Y ,Sun X ,Yang Z , Ge J , Tan Z (2011) Hy‐poxia induces beta‐amyloid in association with death of RGC‐5 cells in culture. Biochem Biophys Res Commun 410:40‐44.
Lzzotti A, Bagnis A, Saccà SC (2006) The role of oxidative stress in glaucoma. Mutat Res 612:105‐114.
Maino B, D’Agata V, Severini C, Ciotti M, Calissano P, Copani A,Chang Y, DeLisi C (2015) Igf1 and Pacap rescue cerebellar granule neurons from apoptosis via a common transcriptional program.Cell Death Discov 1:15029.
Maino B, D’Agata V, Severini C, Ciotti MT, Calissano P, Copani A,Chang YC, DeLisi C, Cavallaro S (2015) Igf1 and Pacap rescue cerebellar granule neurons from apoptosis via a common transcription‐al program. Cell Death Discov 1:15029.
Majid AS, Yin ZQ, Ji D (2013) Sulphur antioxidants inhibit oxidative stress induced retinal ganglion cell death by scavenging reactive ox‐ygen species but influence nuclear factor (erythroid‐derived 2)‐like 2 signalling pathway differently. Biol Pharm Bull 36:1095‐1110.
Masmoudi‐Kouki O, Douiri S, Hamdi Y, Kaddour H, Bahdoudi S,Vaudry D, Basille M, Leprince J, Fournier A, Vaudry H, Tonon MC, Amri M (2011) Pituitary adenylate cyclase‐activating polypep‐tide protects astroglial cells against oxidative stress‐induced apopto‐sis. J Neurochem 117:403‐411.
Maugeri G, D’Amico AG, Reitano R, Magro G, Cavallaro S, Salomone S, D’Agata V (2016) Parkin modulates expression of HIF‐1 alpha and HIF‐3 alpha during hypoxia in gliobastoma‐derived cell lines in vitro. Cell Tissue Res 364:465‐474.
Miki A, Kanamori A, Negi A, Naka M, Nakamura M (2013) Loss of aquaporin 9 expression adversely affects the survival of retinal gan‐glion cells. Am J Pathol 182:1727‐1739.
Orrenius S (2007) Reactive oxygen species in mitochondria‐mediated cell death. Drug Metab Rev 39:443‐455.
Osborne NN, del Olmo‐Aguado S (2013) Maintenance of retinal ganglion cell mitochondrial functions as a neuroprotective strategy in glaucoma. Curr Opin Pharmacol 13:16‐22.
Osborne NN, Núñez‐Álvarez C, Del Olmo‐Aguado S (2014) The effect of visual blue light on mitochondrial function associated with retinal ganglions cells. Exp Eye Res 128:8‐14.
Pascale A, Drago F, Govoni S (2012) Protecting the retinal neurons from glaucoma: lowering ocular pressure is not enough. Pharmacol Res 66:19‐23.
Pinazo‐Durán MD, Zanón‐Moreno V, García‐Medina JJ, Galle‐gopinazo R (2012) Evaluation of presumptive biomarkers of oxidative stress, immune response and apoptosis in primary open‐angle glaucoma. Curr Opin Pharmacol 13:98‐107.
Racz B, Tamas A, Kiss P et al (2006) Involvement of ERK and CREB signaling pathways in the protective effect of PACAP in monosodi‐um glutamate‐induced retinal lesion. Ann NY Acad Sci 1070:507‐511.
Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm S, Kilaru V, Smith A, Myers A, Ramirez M, Engel A, Hammack S, Toufexis D, Braas K, Binder E, May V (2011)Post‐traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470:492‐497.
Seki T, Itoh H, Nakamachi T, Endo K, Wada Y, Nakamura K, Shioda S (2011) Suppression of rat retinal ganglion cell death by PACAP following transient ischemia induced by high intraocular pressure. J Mol Neurochem 43:30‐34.
Seki T, Itoh H, Nakamachi T, Shioda S (2008) Suppression of ganglion cell death by PACAP following optic nerve transection in the rat.Mol Neurochem 36:57‐60.
Shioda S, Takenoya F, Wada N, Hirabayashi T, Seki T, Nakamachi T(2016) Pleiotropic and retinoprotective functions of PACAP. Anat Sci Int 91:313‐324.
Silveira MS, Costa MR, Bossa M, Linden R (2002) Pituitary adenylyl cyclase‐activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP‐dependent protein kinase. J Biol Chem 277:16075‐16080.
Sun J, Shao Z, Yang Y, Wu D, Zhou X, Yuan H (2012) Annexin 1 protects against apoptosis induced by serum deprivation in trans‐formed rat retinal ganglion cells. Mol Biol Rep 39:5543‐5551.
Szabadfi K, Atlasz T, Kiss P, Reglodi D , Szabo A , Kovacs K , Szalontai B , Setalo G Jr , Banki E , Csanaky K , Tamas A , Gabriel R (2012)Protective effects of the neuropeptide PACAP in diabetic retinopathy. Cell Tissue Res 348:37‐46.
Szabadfi K, Danyadi B, Kiss P, Tamas A, Fabian E, Gabriel R, Reglodi D (2012) Protective effects of vasoactive intestinal peptide (VIP) in ischemic retinal degeneration. J Mol Neurosci 48:501‐517.
Szabadfi K, Reglodi D, Szabo AB, Szalontai A. Valasek, Setalo G Jr,Kiss P, Tamas A, Wilhelm M, Gabriel R (2016) Pituitary adenylate cyclase activating polypeptide, a potential therapeutic agent for diabetic retinopathy in rats: focus on the vertical information processing pathway. Neurotox Res 29:432‐446.
Szabadfi K, Szabo A, Kiss P, Reglodi D, Setalo G Jr, Kovacs K, Tamas A,Toth G, Gabriel R (2014) PACAP promotes neuron survival in early experimental diabetic retinopathy. Neurochem Int 64:84‐91.
Tian K, Shibata‐Germanos S, Pahlitzsch M, Cordeiro MF (2015) Cur‐rent perspective of neuroprotection and glaucoma. Clin Ophthal‐mol 9:2109‐2118.
Van Bergen NJ, Wood JP, Chidlow G, Trounce IA, Casson RJ, Ju WK,Weinreb RN, Crowston JG (2009) Recharacterization of the RGC‐5 retinal ganglion cell line. Invest Ophthalmol Vis Sci 50:4267‐4272.
Wang H, Zhang C, Lu D, Shu X, Zhu L, Qi R, So K, Lu D, Xu Ying(2013) Oligomeric proanthocyanidin protects retinal ganglion cells against oxidative stress‐induced apoptosis. Neural Regen Res 8:2317‐2326.
Wood JP, Chidlow G, Tran T, Crowston JG, Casson RJ (2010) A com‐parison of differentiation protocols for RGC‐5 cells. Invest Oph‐thalmol Vis Sci 51:3774‐3783.
Xu Z, Ohtaki H, Watanabe J, Miyamoto K, Murai N, Sasaki S, Mat‐sumoto M, Hashimoto H, Hiraizumi Y, Numazawa S, Shioda S(2016) Pituitary adenylate cyclase‐activating polypeptide (PACAP)contributes to the proliferation of hematopoietic progenitor cells in murine bone marrow via PACAP‐specific receptor. Sci Rep 6:22373.
Yang R, Jiang X, Ji R, Meng L, Liu F, Chen X, Xin Y (2015) Therapeu‐tic potential of PACAP for neurodegenerative diseases. Cell Mol Biol Lett 20:265‐278.
Zhou CJ, Shioda S, Yada T, Inagaki N, Pleasure SJ, Kikuyama S (2002)PACAP and its receptors exert pleiotropic effects in the nervous system by activating multiple signaling pathways. Curr Protein Pept Sci 3:423‐439.
- 中国神经再生研究(英文版)的其它文章
- Novel function of the chemorepellent draxin as a regulator for hippocampal neurogenesis
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Semaphorin 3A: from growth cone repellent to promoter of neuronal regeneration
- The role of undifferentiated adipose-derived stem cells in peripheral nerve repair
- Nerve conduction models in myelinated and unmyelinated nerves based on three-dimensional electrostatic interaction
- Fatigability during volitional walking in incomplete spinal cord injury: cardiorespiratory and motor performance considerations