Retinoid receptor-related orphan receptor alpha: a key gene setting brain circuits
Tania Vitalis , Jean Mariani
1 PROTECT, Institut National de la Santé et de la Recherche Médicale, Université Paris Diderot, Paris, France
2 Université Pierre et Marie Curie, Sorbonne Université, Paris, France
3 Centre National de la Recherche Scientifique, Unité Mixte de Recherche 8256, Institut de Biologie de Paris Seine (IBPS), Biological adaptation and ageing (B2A), Team Brain Development, Repair and Ageing, Paris, France
4 Assistance Publique ‐ Hôpitaux de Paris, Départements Hospitalo‐Universitaires FAST, Institut de la Longévité, Ivry‐Sur‐Seine, France
General Molecular Mechanisms and Functions of the Retinoid Receptor-Related Orphan Receptor Alpha (RORα)
The transcription factor, RORα belongs to the nuclear receptor family and is thought to act as a constitutive activator of transcription by binding to the ROR response element (RORE)of target genes. Through the ligand binding domain (LBD),co‐activators and sterol‐derived ligands (i.e., cholesterol,cholesterol metabolites and oxysterols) are able to modulate RORα activation. Binding to LBD leads to conformational changes of the LBD that facilitates the recruitment of tran‐scriptional co‐regulatory proteins (for review see Jetten, 2009;Figure 1). However, more needs to be done to understand how RORα expression is precisely regulated. At the physio‐logical level, RORα, on its own or in combination with other circadian related genes, participates in setting various physio‐logical functions, for instance, in setting the circadian rhythm,in regulating metabolism, immunity and neuroprotection and appears as a key gene regulating some aspects of brain development and aging (Jetten, 2009; Figure 1). At anatomical level RORα is widely expressed in various organs, including in specific regions of the central nervous system (Jetten, 2009). Here we will mainly focus on the roles of RORα in the development of the cerebellum and of the somatosensory system.
Roles of RORα in Cerebellar Development
The staggerer mutation “characterized by the vacillating lo‐comotion of the spontaneous mutant animal” was first identified by Richard Sidman during one of his visit to the Jack‐son laboratory in 1955. Staggerer mice were subsequently thoroughly analyzed with a focus on cerebellar development and motor functions. Subsequently, it was demonstrated that the staggerer mutation was a RORα deletion in the LDB that induced altered development, maturation, and maintenance of cerebellar Purkinje cells (PCs) and granular neurons resulting in the staggerer phenotype (i.e., Hamilton et al.,1996; reviewed in Jetten, 2009; the large array of work pro‐duced could not be acknowledged here due to space limitation). Recently, using elegant genetic models based on “cre‐lox inducible strategy” allowing cell type‐ and time‐specific ablation of RORα, the cell‐autonomous functions of RORα in PCs development and maturation have been clarified(Takeo et al., 2015). RORα has been shown to be necessary for the neurogenesis of PCs (E10-13), for their migration to the cerebellar cortex (E15-17) and the alignment of their cell bodies. At postnatal stage, by P4, RORα was shown to promote the retraction of the few primitive dendrites of PCs that will then enter the “stellate cell” stage. By P8, RORα was shown to be necessary for PCs to retract their perisomatic dendrites and then to grow spiny branchelets. RORα expres‐sion is maintained throughout life in PCs and is necessary for survival of PCs, for the maintenance of cell morphology, and to ensure it is correctly innervated by a single climbing fiber(Chen et al., 2013). Alterations in cerebellar granular cells alterations appeared to be secondary to the failure of PCs to produce the morphogen, sonic hedgehog among other factors.
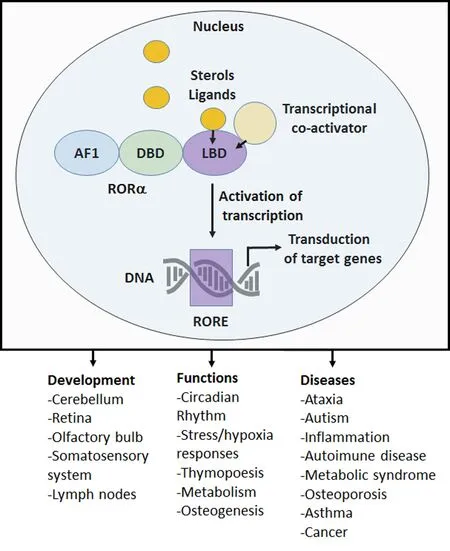
Figure 1 Schematic representation of the molecular mechanism of retinoid receptor-related orphan receptor alpha (RORα) action,physiological functions and roles in diseases.
RORα is Cell-Autonomously Required for the Development of the Somatosensory System
Despite region and time specific expression of RORα in various brain regions only few studies have analyzed the roles of RORα in brain circuit formation outside the cerebellum. Na‐kagawa and O’Leary were the first to report that RORα was expressed from E12.5 in sensory thalamic nuclei and from E18.5 in the cortical layer IV (Nakagawa and O’Leary, 2003).The development of the somatosensory system is sequential in time and place (for review see Lokmane and Garel, 2014).Whiskers on the snout of the mouse send inputs to the tri‐geminal ganglion cells that in turn project to the brainstem where these projections are topographically organized and represent the whisker map. The brainstem nucleus principa‐lis (PrV) sends contralateral afferents to the ventrobasal tha‐lamic nucleus (VB) where the topography is reproduced. In VB, each barreloid send thalamocortical axons (TCs) toward the center of a single barrel in the layer IV of the primary so‐matosensory cortex (S1), where TCs arborize. In the cortex,this organization is clearly visible from P4 onwards. In layer IV, a large proportion of glutamatergic neurons display asymmetric “stellate‐like” morphologies with their soma located in the barrel walls and their dendrites preferentially oriented toward the barrel center forming synaptic contacts with TCs during the first postnatal weeks (Figure 2A–A’).
In our recent study, we have analyzed the roles of RORα in the early postnatal development of the somatosensory system in staggerer mutant (sg/sg) mice but also in mice in which RORα was selectively deleted in the sensory thalamic nuclei or in the cortex (Vitalis et al., 2017). Staggerer mutant mice showed both a defective arborization of TCs and an altered organization of layer IV neurons into barrels, but a spared organization of the barrelettes in the brainstem (Figure 2B–B’). Whether these alterations are cell‐autonomous or due to a combination of altered environmental and genetic signals remained to be clarified. To answer this question we performed genetic ablation of RORα in VB neurons from E15.5. Using the progeny of SERT:cre mice crossed with RORα floxed mice, we showed that RORα is cell‐au‐tonomously required for the growth and branching of TCs but not for their general topographical organization (Figure 2C–C’). Because the accurate topographical organization of TC neurons is necessary for normal layer IV organization,the roles of RORα on layer IV neurons could only be appreciated when specific ablation of RORα in layer IV neurons was achieved. Using the progeny of FoxG1:cre mice crossed with RORα floxed mice that ablates RORα from E18.5 in layer IV neurons, we showed that RORα is necessary for lay‐er IV neurons to segregate into barrels and to display com‐plex branched morphologies (Figure 2D–D’). To further confirm the cell autonomous requirement of RORα in TCs and barrels we obtained mutant mice lacking RORα in both the VB and the cortex. These animals, analyzed at early post‐natal stage, recapitulated the cerebral features displayed by staggerer mice. This suggested that physiological/peripheral alterations known to occur in staggerer mice may contribute only minimally to the barrelless phenotype. However, we cannot exclude that a defect in neuroprotective functions mediated by RORα in astrocytes (Jolly et al., 2011) would exacerbate the “staggerer phenotype” at later stages. Indeed,it has been shown that downregulation of RORα induces an excessive upregulation of the pro‐inflammatory cytokine IL6 and exerts an indirect repression of the nuclear factor kappa‐B (NF‐κB) pathway (Journiac et al., 2009).
The Lack of RORα in the Ventrobasal Thalamus and Somatosensory Cortex Induces the Downregulation of Key Genes Some of Which are Regulated by the Triiodothyronine (T3)
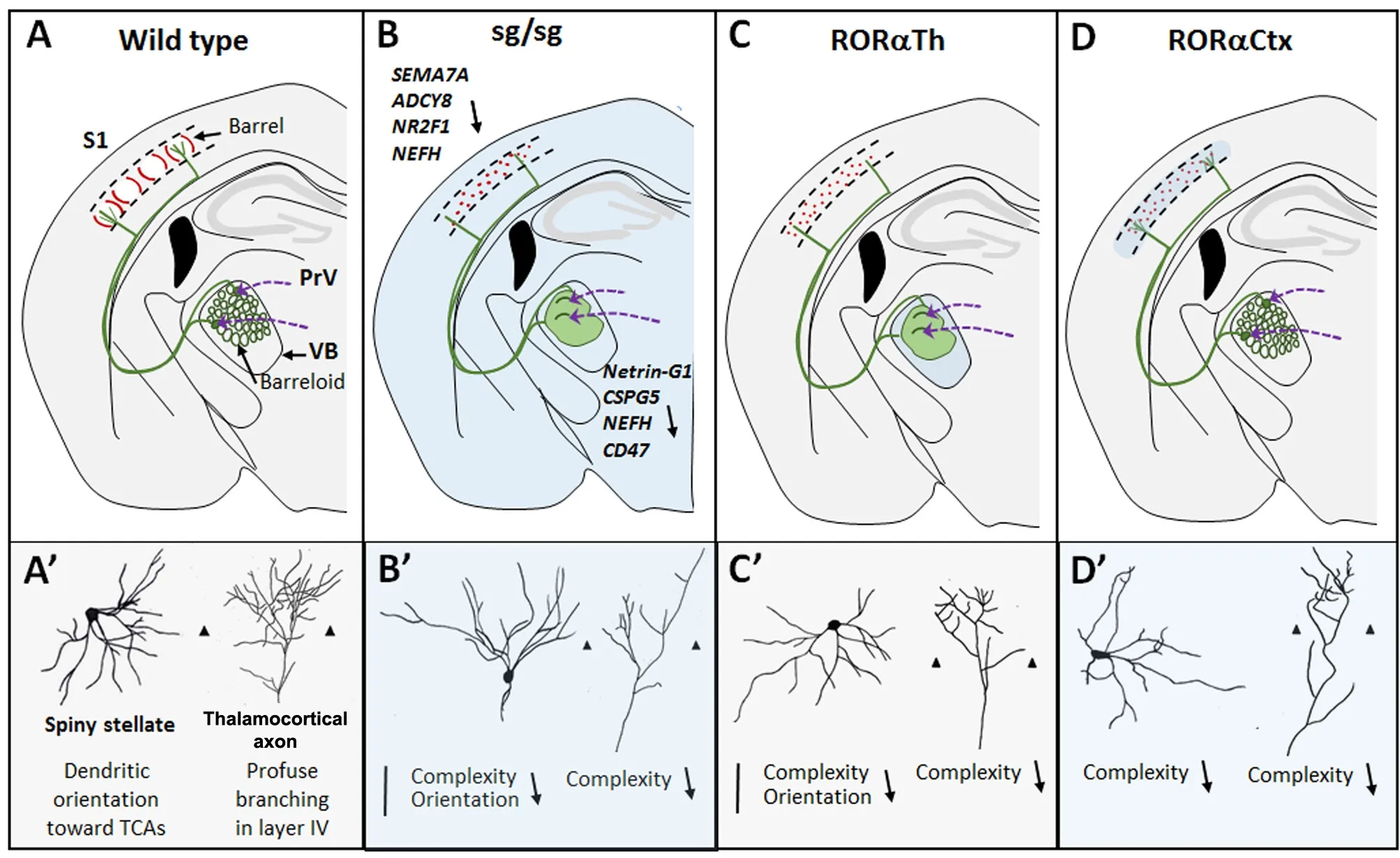
Figure 2 Schematic drawings showing the alterations found in the somatosensory system in various mouse models lacking retinoid receptorrelated orphan receptor alpha (RORα) totally or partially.
Our microarray analysis performed on the staggerer mice revealed that specific genes could be implicated in VB or layer IV staggerer phenotypes (see Lokmane and Garel,2014; Vitalis et al., 2017) that review the role of some relevant molecules for barrel field development). Since RORα is considered to act as a positive activator of transcription, we focused our attention on genes downregulated in VB (131)and in S1 cortex (126) in staggerer mice. Among the genes potentially important for thalamic outgrowth we foundNetrin-G1 ligand, theCSG5(chondroitin sulfate proteoglycan 5) andCD47(coding the transmembrane protein CD47)that were shown to promote the neuritogenesis and the maturation of cerebellar neurons. Similarly, several genes downregulated in the cortex and necessary for cortical development retained our attention:NephandNepm(coding neurofilament high and medium molecular weight),Adenylate Cyclase 8, the transcription factorNR2F1andSemaphorin7A. Downregulation of the neurofilaments protein H and Semaphorin 7A proteins were confirmed in staggerer mice. NEFM and NEFH are expressed in neuritic processes and participates in neuritic elongation and neuritic stability by phosphorylating cytoskeletal proteins including neurofilaments. The observed downregulation of heavy chain neurofilament in thalamic axons could induce a defective stability,or a delayed maturation of neurites. The lower expression of the GPI‐linked Semaphorin 7A in staggerer mice could participate in the staggerer phenotype since its downregulation was shown to decrease the preferred orientation of spiny stellate neurons toward barrel centers and Semaphorin 7A is known to act as a positive signal for TCs outgrowth and branching (please, see our discussion in Vitalis et al., 2017).
Interestingly, several genes downregulated in the staggerer VB or cortex were previously shown to be regulated by T3(Berbel et al., 2014; see also the work of the group of Beatriz Morte:i.e., Gil‐Ibañez et al., 2017). Hypothyroid rats display characteristic features reminiscent to those displayed by staggerer mice: a preserved targeting of TCs toward S1 associated with defective axonal outgrowth and branching and a reduced layer IV cortical thickness (Berbel et al., 2014). Alteration in T3 signaling also induces cerebellar atrophy with a reduced growth and branching of PCs, among other cerebellar phenotypes, a feature partially overlapping with what was described in RORα deficient mice (Hamilton et al., 1996; Jetten, 2009;Chen et al., 2013; Takeo et al., 2015).In vitro, it was shown that T3 was one of the molecules able to positively regulate the activity of the RORα promoter and this regulation is lost in staggerer mice. This suggests that the defective T3/RORα pathway may account for some of the barrel field alterations we observed in staggerer mice. However, the complex inter‐play between T3/thyroid hormone receptors and RORα re‐mains to be deciphered further. In addition, T3‐independent pathways regulated by RORα and necessary for somatosensory system development remain to be clearly identified.
In our study, we were not able to detect significant modification of other circadian related genes and we believe that this may be due to technical limitations since these genes tend to regulate each other (see Jetten, 2009). In this respect, it needs to be mentioned that RORβ has been reported as a key regulator of barrel formation. RORβ is expressed at the same time and place than RORα (Nakagawa and O’Leary, 2003) and its early cortical upregulation (prior to the normal emergence of barrel formation) leads to anticipated layer IV clustering and to TC attraction in RORβ+regions (Jabaudon et al., 2012).Interestingly, REV‐ERBα that represses transcription through RORE (opposite function of RORα; Jetten, 2009) is also ex‐pressed in VB and in S1 (see the expression pattern at www.alleninstitute.org) during the first postnatal week and might also play critical roles in somatosensory formation. Further investigations on the role of these circadian related genes on barrel formation will be of great value in the field.
Deregulation of RORα in Psychiatric Disorders
The RORα gene is, as nicely shown by the work of Valerie Hu’s group, at the crossroad of many biological processes and pathways which, when altered could lead to the emergence of various disorders (Sarachana and Hu, 2013). In human,polymorphism in the RORα gene has been associated with the susceptibility to develop several mental illnesses such as autistic‐like syndrome (ASD), anxiety, depression and bipolar disorders. Several studies have shown that ASD patients displayed lower levels of RORα in the cerebellum and pre‐frontal cortex. In addition, in human and in mouse, RORα appears to be linked to the male bias of ASD since reduction of RORα regulates the aromatase enzyme and thus decrease RORα expression, downregulates androgens at the expense of oestrogens and can in turn influence circulating sex steroids (Sarachana and Hu, 2013). Following this work, other studies have shown that RORα expression, in males, was also associated with the emergence of anxiety following childhood maltreatment. In addition, the defective neuroprotection oc‐curring when RORα is downregulated might exacerbate these pathologies (Journiac et al., 2009; Jolly et al., 2011). The data presented in this perspective strongly suggest a role for RORα in modeling and maintaining brain circuits and functions that could be affected in various psychiatric disorders.
Conclusion
RORα, like other members of the circadian related genes,appears to play key roles in various physiological processes.RORα acts initially in various aspects of brain construction and later on in neuroprotection. Moreover, RORα, displays expression and regulation modified in various pathological conditions including psychiatric and degenerative disorders. Determining the appropriate amount of RORα activity could be crucial in detecting and preventing the emergence of specific diseases. Interestingly, natural or synthetic agonists, antagonists and inverse agonists are now available and could serve as potential diagnostic and therapeutic tools(Kojetin and Burris, 2014).
Acknowledgments:We warmly thank Patricia Gaspar (Institut National de la Santé et de la Recherche Médicale, Unité Mixte de Recherche S839, Institut du Fer à Moulin, 75005 Paris, France), Marie-Claude Potier (Centre National de la Recherche Scientifique, Unité Mixte de Recherche 7225 and Institut National de la Santé et de la Recherche Médicale Unité 1127, Institut du Cerveau et de la Moelle, 75013 Paris, France) and Pierre Gressens (PROTECT, Institut National de la Santé et de la Recherche Médicale, Université Paris Diderot, Sorbonne Paris Cité, 75019 Paris, France) that helped to analyze the data and to write the original paper that is central in this perspective (Vitalis et al., 2017).We also warmly thank Shryamala Mani (PROTECT, Institut National de la Santé et de la Recherche Médicale, Université Paris Diderot, Sorbonne Paris Cité, 75019 Paris, France) for carefully reading and correcting our manuscript.
Author contributions:TV and JM wrote this manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:The work was supported by the INSERM and the CNRS.Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Berbel P, Navarro D, Román GC (2014) An evo‐devo approach to thy‐roid hormones in cerebral and cerebellar cortical development: etiolog‐ical implications for autism. Front Endocrinol (Lausanne) 5:146.
Chen XR, Heck N, Lohof AM, Rochefort C, Morel MP, Wehrle R,Doulazmi M, Marty S, Cannaya V, Avci HX, Mariani J, Rondi‐Reig L,Vodjdani G, Sherrard RM, Sotelo C, Dusart I (2013) Mature Purkinje cells require the retinoic acid‐related orphan receptor‐alpha (RORal‐pha) to maintain climbing fiber mono‐innervation and other adult characteristics. J Neurosci 33:9546‐9562.
Gil‐Ibañez P, García‐García F, Dopazo J, Bernal J, Morte B (2017) Global transcriptome analysis of primary cerebrocortical cells: identification of genes regulated by triiodothyronine in specific cell types. Cereb Cortex 27:706‐717.
Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W,Kusumi K, Russell LB, Mueller KL, van Berkel V, Birren BW, Kruglyak L, Lander ES (1996) Disruption of the nuclear hormone receptor RO‐Ralpha in staggerer mice. Nature 379:736‐739.
Jabaudon D, Shnider SJ, Tischfield DJ, Galazo MJ, Macklis JD (2012)RORbeta induces barrel‐like neuronal clusters in the developing neo‐cortex. Cereb Cortex 22:996‐1006.
Jetten AM (2009) Retinoid‐related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism.Nucl Recept Signal 7:e003.
Jolly S, Journiac N, Naudet F, Gautheron V, Mariani J, Vernet‐der Ga‐rabedian B (2011) Cell‐autonomous and non‐cell‐autonomous neu‐roprotective functions of RORalpha in neurons and astrocytes during hypoxia. J Neurosci 31:14314‐14323.
Journiac N, Jolly S, Jarvis C, Gautheron V, Rogard M, Trembleau A,Blondeau JP, Mariani J, Vernet‐der Garabedian B (2009) The nuclear receptor ROR(alpha) exerts a bi‐directional regulation of IL‐6 in rest‐ing and reactive astrocytes. Proc Natl Acad Sci U S A 106:21365‐21370.
Kojetin DJ, Burris TP (2014) REV‐ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov 13:197‐216.
Lokmane L, Garel S (2014) Map transfer from the thalamus to the neo‐cortex: inputs from the barrel field. Semin Cell Dev Biol 35:147‐155.
Nakagawa Y, O’Leary DD (2003) Dynamic patterned expression of or‐phan nuclear receptor genes RORalpha and RORbeta in developing mouse forebrain. Dev Neurosci 25:234‐244.
Sarachana T, Hu VW (2013) Genome‐wide identification of transcrip‐tional targets of RORA reveals direct regulation of multiple genes asso‐ciated with autism spectrum disorder. Mol Autism 4:14.
Takeo YH, Kakegawa W, Miura E, Yuzaki M (2015) RORalpha regulates multiple aspects of dendrite development in cerebellar purkinje cells in vivo. J Neurosci 35:12518‐12534.
Vitalis T, Dauphinot L, Gressens P, Potier MC, Mariani J, Gaspar P (2017)RORalpha coordinates thalamic and cortical maturation to instruct barrel cortex development. Cereb Cortex doi: 10.1093/cercor/bhx262.
- 中国神经再生研究(英文版)的其它文章
- Forkhead box protein P1, a key player in neuronal development?
- Weak phonation due to unknown injury of the corticobulbar tract in a patient with mild traumatic brain injury: a diffusion tensor tractography study
- Novel function of the chemorepellent draxin as a regulator for hippocampal neurogenesis
- The role of undifferentiated adipose-derived stem cells in peripheral nerve repair
- Nerve conduction models in myelinated and unmyelinated nerves based on three-dimensional electrostatic interaction
- Fatigability during volitional walking in incomplete spinal cord injury: cardiorespiratory and motor performance considerations