Fast determination of betaine in Lycii Fructus by ultra-performance convergence chromatography- tandem mass spectrometry
ZHANG Xianfei, YANG Junli, CHEN Juan*, SHI Yanping*
(1. CAS Key Laboratory of Chemistry of Northwestern Plant Resources and Key Laboratory for Natural Medicine ofGansu Province, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences (CAS), Lanzhou 730000, China; 2. University of Chinese Academy of Sciences, Beijing 100049, China)
LyciumbarbarumL., which belongs to the Solanaceae family, is widely distributed in the wild in most regions of China and is mainly cultivated in Northwestern China, including Ningxia, Gansu, Qinghai, Inner Mongolia, and Xinjiang [1,2]. Lycii fructus is widely used as a functional health food [3] or as a valuable tonic in Chinese herbal medicine for nourishing the liver and kidneys, moistening the lungs, and improving eyesight [4,5]. Nowadays, Lycii fructus is marketed as a dietary supplement and functional tea in many countries in Asia, Europe, and North America [6]. Betaine is one of the major functional components in Lycii fructus and is a zwitterionic quaternary ammonium compound. It has many beneficial effects on the human body, including anti-atherosclerosis, anti-osteoporosis, anti-tumor effects; further, it lowers the blood pressure, heals peptic ulcers, and protects the liver against chemical injury [7].
Both the Chinese Pharmacopoeia [8] and the Korean Pharmacopoeia [9] stipulate betaine as the index compound of Lycii Fructus. In the Chinese Pharmacopoeia, the method for determination of betaine in Lycii Fructus is stipulated as thin-layer chromatography (TLC) scanning. The precision of TLC is not sufficient, and it is typically used for qualitative analysis. Furthermore, tailing often occurs during chromatography. In recent years, some quantitative analysis methods have been developed. Shin et al. [10] determined betaine inLyciumchinensefruits by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) and observed high baseline noise. Huang et al. [11] analyzed the betaine content in Lycii Fructus by high-performance liquid chromatography (HPLC) using a Luna 5u SCX 100A column and an evaporative light scattering detector (ELSD), but the target peak of betaine was not well separated from the other peaks. Zhao et al. [12] used an HILIC column and ELSD detector to analyze betaine in Lycii Fructus. The obtained peak shape of betaine was acceptable, but the retention time was slightly long, about 20 min. Besides Lycii Fructus, the determination of betaine in other matrices such as plasma [13], feed premixes [14], and human urine [15] was also reported, and good results were obtained.
In 2012, Waters Company launched ultra-performance convergence chromatography (UPC2), which integrated the advantages of both supercritical fluid chromatography (SFC) and ultrahigh-performance liquid chromatography (UPLC). The mobile phase contained supercritical carbon dioxide, which is an inert fluid and a small amount of organic solvents [16]. In the supercritical state, the polarity of carbon dioxide is similar to that of hexane, so a small amount of modifier is commonly added to increase the separation efficiency [17]. UPC2has many advantages, such as short analysis time, simple pretreatment, high sensitivity, good reproducibility, minimal consumption of organic reagents, and environmental friendliness. Nowadays, UPC2is widely used in medicine [18-21], food [22,23], biology [24,25], chemistry, and other fields [26-28].
Until now, there are only a few notable reports on the determination of betaine in Lycii Fructus, but there is no detailed research based on UPC2. The main purpose of this work was to establish a new quantitative analysis method based on UPC2-MS.
1 Experimental
1.1 Reagents and materials
In 2016, 11 batches of dried fruits ofL.barbarumwere collected from different regions of Ningxia and Gansu provinces in China and identified by Prof. Lin Jin, Gansu University of Traditional Chinese Medicine, Gansu, China. The betaine standard (purity>98.0%) was purchased from the National Institutes for Food and Drug Control (Beijing, China). Chromatographic-grade methanol was obtained from Merck Co. Ltd. (Darmstadt, Germany), and all other chemicals of analytical grade were provided by Tianjin Chemical Reagent Co. (Tianjin, China). Water was purified using an OKP-TS purification system (Exceed-AC-16, Shanghai Laikie Instrument Co. Ltd., China).
1.2 Chromatographic system
UPC2analysis was performed on an ACQUITY UPC2system (Waters, Milford, MA, USA), which was equipped with a binary solvent delivery pump, a fixed-loop autosampler, a column thermostat, and an automated backpressure regulator (ABPR). The separation of betaine was successfully achieved on an ACQUITY UPC2BEH 2-EP column (150 mm×2.1 mm, 1.7 μm) by isocratic elution with a CO2-methanol (80∶20, v/v) mixture at a flow rate of 0.7 mL/min. The following were the conditions for this separation process: modifier, 0.1% formic acid in methanol; column temperature, 40 ℃; injection volume, 1 μL; and ABPR, 1.31×107Pa.
The system was coupled to an MS apparatus equipped with an ESI source, and the quantification of betaine was performed by MS analysis in selected ion recording (SIR) mode and positive ion mode. The MS analysis conditions were optimized as follows: source temperature, 120 ℃; desolvation temperature, 350 ℃; capillary voltage, 2.3 kV; cone voltage, 20 V; cone gas flow rate, 150 L/h; and desolation gas (nitrogen) flow rate, 600 L/h. Data acquisition and system control were performed using a MassLynx 4.1 workstation (Waters, USA).
1.3 Preparation of standard solutions
12.0 mg of betaine was accurately weighed and dissolved in methanol in a 25 mL volumetric flask to prepare the stock solution. In order to obtain the calibration curve, the stock solution was further diluted to 0.5, 1.0, 2.0, 5.0, 10.0, 20.0, and 50.0 μg/mL with methanol, filtered through a 0.22 μm millipore membrane, and stored at 4 ℃ before use.
1.4 Preparation of sample solutions
50.0 mg of the accurately weighed sample from each batch of Lycii Fructus was air-dried, crushed into powder, and extracted with 50 mL methanol in an ultrasonicator for 1 h. The extracts were separated in a centrifuge machine (TDL-5-A, Shanghai Anting Scientific Instrument Factory, China) at 4 000 r/min for 10 min. The obtained supernatants were concentrated to 5 mL using a vacuum rotavapor (EYELA N-1001, EYELA Co. Ltd., China). The resulting sample solutions were filtered through a 0.22 μm millipore membrane filter and injected into the UPC2-MS system for analysis. The betaine contents were calculated according to the calibration curve, and the yields were the mass ratios of the extracted betaine to the weighed medicinal herbs.
1.5 Method validation
The limit of detection (LOD) and limit of quantification (LOQ) were defined by the signal-to-noise ratios of 3 and 10, respectively. Precisions within one day (intraday) and within three consecutive days (interday) were analyzed by replicate injections of the same sample solution (n=6). Stability was tested at ~2 h intervals for three days. Repeatability was evaluated by the relative standard deviation of six different sample solutions, which were prepared in parallel with the same batch of sample S-01. Accuracy was investigated by spiking sample S-01 with three concentration levels of betaine standards, and the extraction and analysis were repeated three times for each level.
2 Results and discussion
2.1 Optimization of chromatographic conditions
To obtain good peak shapes and shorten the retention time of betaine, the optimal chromatographic conditions were investigated. For UPC2, the mobile phase, column type, injection volume (0.5-3.0 μL), column temperature (35-55 ℃), flow rate (0.5-0.9 mL/min), and backpressure (1.03×107-1.52×107Pa) were analyzed. For MS, the ionization mode, capillary voltage (2.3-3.0 kV), cone voltage (10-50 V), desolvation temperature (300-450 ℃), and desolvation gas (nitrogen) flow rate (500-1 000 L/h) were examined.
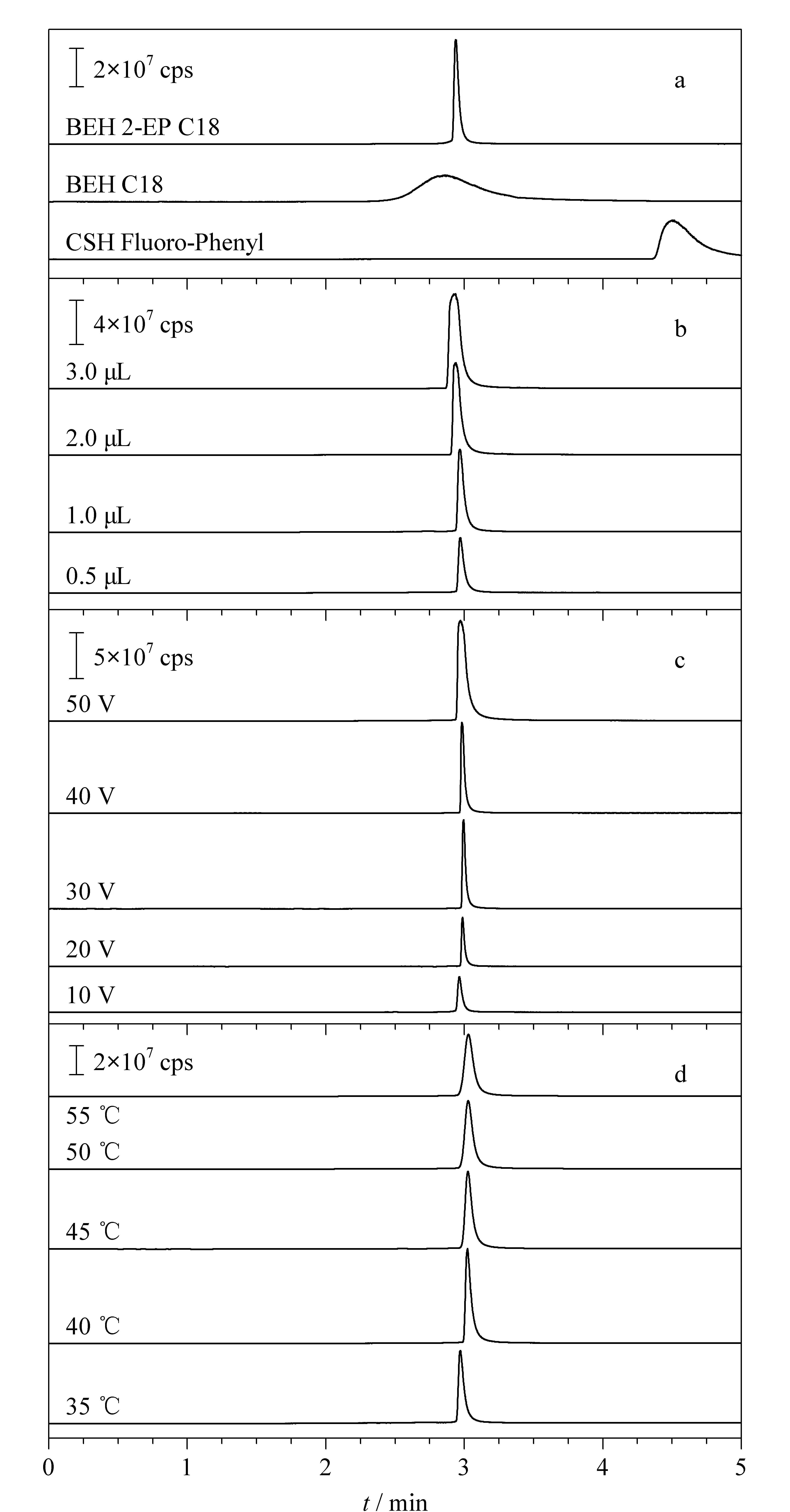
Fig. 1 Effects of (a) column type, (b) injection volume,(c) cone voltage, and (d) column temperature on the peak shape of betaine
A suitable chromatographic column plays an important role in the acceptable separation of components. As shown in Fig. 1a, three columns, Waters ACQUITY UPC2BEH 2-EP (150 mm×2.1 mm, 1.7 μm), Waters ACQUITY UPC2BEH C18 (100 mm×3.0 mm, 1.7 μm), and Waters ACQUITY UPC2CSH Fluoro-Phenyl (150 mm×2.1 mm, 1.7 μm), were screened. The Waters ACQUITY UPC2BEH 2-EP column is filled using hybrid silica with 2-ethylpyridine bonding. The hydrophilic interactions in the silica column are conducive for the elution of alkaloids [29]. As indicated in Fig. 1a, this column was proved to be the best since it possessed a basic character at its surface, and was chosen for subsequent analyses. Different modifiers (methanol, methanol: isopropanol, and methanol: acetonitrile) and additives (formic acid, ammonium formate, and ammonium acetate) were screened, and 0.1% (v/v) formic acid in methanol was found to be the best. Changing the injection volume or the cone voltage hardly influenced the retention time but significantly changed the peak shape. As shown in Figs. 1b and c, the peak height and peak width increased with an increase in the injection volume or cone voltage, and the peak shape worsened. Given that larger areas are required for quantitative analysis, the injection volume and cone voltage were selected as 1 μL and 20 V, respectively.
Density is the main factor contributing to retention and selectivity, and it depends on three parameters: mobile phase composition, column temperature, and pressure [30,31]. Temperature is known to affect the distribution constant, and thereby, the retention and separation factors of the analytes. The density of CO2decreases with an increase in temperature, so the elution capacity decreases and the retention time increases [32]. As presented in Fig. 1d, the retention time increased slightly when the temperature was increased to 55 ℃; further, the peak became wider as the temperature increased. This was because temperature variations would modify the chemical characteristics of the analytes and the stationary phase, leading to a change in selectivity. In this work, the column temperature was set at 40 ℃.
The backpressure hardly impacted the peak shape but had a notable influence on the retention time in this work. A higher backpressure would increase the density of and solvation power of the mobile phase solvation power, thereby shortening the retention time [33]. As seen in Fig. 2a, the retention time decreased with an increase in backpressure, so the backpressure was selected as 1.31×107Pa. Under operation at high temperature and low backpressure, the fluid will behave like a gas, thus causing problems with repeatability and sensitivity [34]. Hence, intermediate values of temperature and backpressure may be more suitable. In Ref. [34], 40 ℃ and 1.50×107Pa were suggested as the temperature and backpressure, respectively, which were consistent with the results of our investigation.
In this study, the flow rate, too, had no clear influence on the peak shape. However, as seen from Fig. 2b, the retention time would decrease with an increase in the flow rate. It is well known that the inlet pressure increases with increasing flow rate, and this leads to an increased density of the mobile phase in the column. Thus, the retention time decreases [33]. Given that increasing the flow rate by 0.1 mL/min would result in an increase of about 3.45×106Pa in the system pressure and very high pressure would hinder system maintenance, the flow rate was selected as 0.7 mL/min. Other parameters such as desolvation gas flow rate and desolvation temperature were also investigated and demonstrated to have minor effects on the sensitivity.
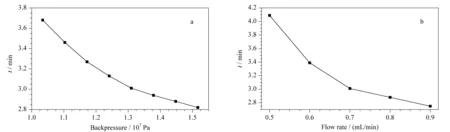
Fig. 2 Effects of (a) backpressure and (b) flow rate on the retention time of betaine

Fig. 3 UPC2-MS total ion chromatograms obtained in (a) positive ion mode and (b) negative ion mode
The detection of betaine was performed with the ESI source and in SIR mode; the obtained UPC2-MS total ion chromatograms are presented in Fig. 3. As shown in Fig. 3a, the retention times of the standard and the sample solutions in positive ion mode agreed well with one another, and good peak shapes were observed. These results indicated that this mode was suitable for betaine detection. As presented in Fig. 3b, UPC2-MS analysis in negative ion mode was also attempted, but this did not give acceptable results. The mechanism for the fragmentation pathways of betaine in the mass spectrum is shown in Fig. 4. In the positive ion mode, the mass-to-charge ratios (m/z) were detected based on the formation of [M+Na]+ions.
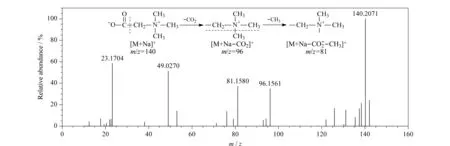
Fig. 4 Mass scan spectrum in positive ion mode and fragmentation pathways of betaine
2.2 Optimization of sample preparation conditions
Using a mono-factorial experimental design, four sample preparation conditions (solvent, extraction time, sample amount, and extraction method) were optimized. Methanol and ethanol at six volume percentages (50%, 60%, 70%, 80%, 90%, and 100%) were investigated in sample assays using an ultrasonicator, and 100% methanol was proved to be the best. Five extraction times (30, 45, 60, 75, and 90 min) were examined with 100% methanol as the solvent, and the extraction was saturated after 60 min. Samples of six different amounts (30, 40, 50, 60, 70, and 80 mg) were extracted with 50 mL methanol for 60 min, and the extraction efficiency for 50 mg was proved to be the best. Finally, two extraction methods (reflux and ultrasonication) were attempted, and the extraction efficiency with the ultrasonicator was found to be slightly better.
2.3 Method validation
The calibration curve was established by linear regression analysis, and the calibration range was 0.5-50.0 μg/mL. The regression equation was expressed asy=563 544.9 957x-17 781.6 200, whereyis the peak area andxis the concentration (μg/mL) of betaine. The correlation coefficient (r2) of 0.999 2 implied that the linear regression of the calibration curve was good within the calibration range; the low LOD (0.013 μg/mL) and LOQ (0.045 μg/mL) meant that overload and pollution of the chromatographic columns could be avoided. In Ref. [12], betaine in Lycii Fructus was analyzed using HPLC-ELSD, and the LOD was 2.31 μg/mL. In Ref. [15], betaine was determinated using UPLC-MS/MS, and the LODs for plasma and urine were 0.03 and 3.00 μg/mL, respectively. In the present work, the LOD was 0.013 μg/mL, which was much lower than the values obtained with HPLC and UPLC.
With the present method, the relative standard deviations (RSDs) of the intraday and interday (three days) precisions were found to be 0.9% and 1.1% (n=6), respectively, which indicated high precision of the instrument. The stability of the sample was reasonable over the tested period (RSD, 1.6%), and the repeatability of the method was acceptable (RSD, 1.4%). The accuracy was validated by the recovery (R), which was calculated using the following formula:R=[(found amount-original amount)/spiked amount]×100%. As shown in Table 1, the average recovery of 96.3% with RSD of 0.5% demonstrated the high accuracy of the method.

Table 1 Study of recovery for validation of accuracy
Recovery: mean±standard deviation (SD);n=3.
2.4 Sample analysis
The developed UPC2-MS method was applied to analyze the betaine contents in 11 batches of Lycii Fructus samples, which were collected from eleven different counties of Ningxia and Gansu provinces in China. In Table 2, the obtained betaine contents are listed. It could be seen that the betaine contents in the Lycii Fructus samples from different counties were slightly different. The betaine content of sample S-01 was the best (1.39 μg/mg), while that of S-09 was the worst (1.20 μg/mg). This was because the difference in the growing conditions, such as growing regions, growing years, and harvest seasons, would result in variations in quality [2].
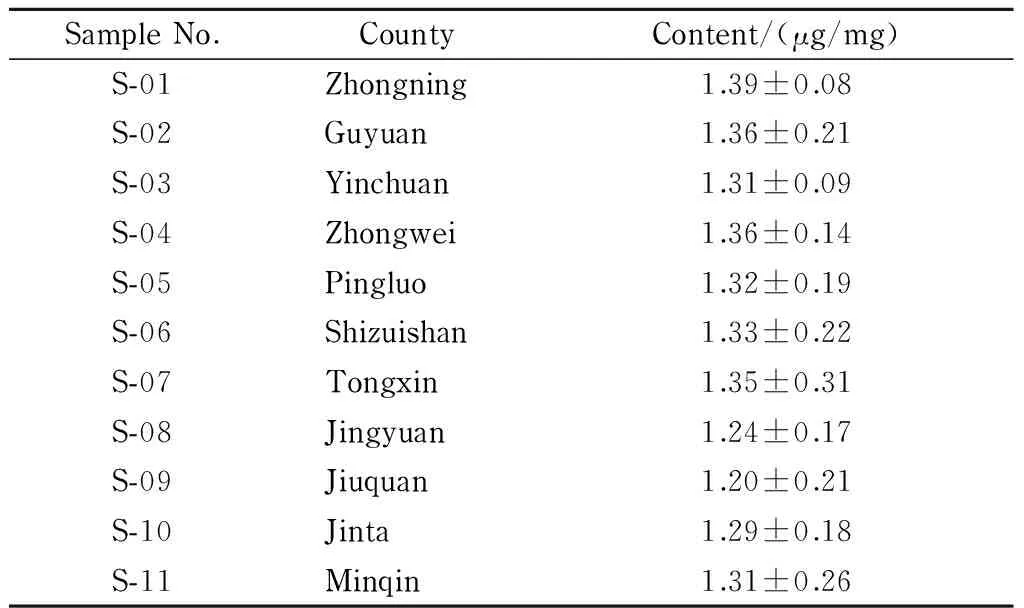
Table 2 Betaine contents of 11 batches of Lycii Fructus samples
Content: mean±SD;n=3.
3 Conclusions
In the present study, a novel UPC2-MS method operating in positive ion mode was developed to analyze the betaine contents of Lycii Fructus samples. The results showed that the separation of betaine was successful on an ACQUITY UPC2BEH 2-EP column, and the UPC2-MS total ion chromatograms showed good baseline resolution and peak shapes within a short time (3 min). The calibration curve was established, and the method validation indicated that the precision, repeatability, stability, and accuracy of this method were excellent. In particular, the LOD was much lower than that in previous reports. The developed UPC2-MS method was further applied to analyze the quality of eleven batches of Lycii Fructus samples. The betaine contents in samples from different areas were slightly different. The present method is thus expected to be very useful for evaluating the quality of Lycii Fructus.
[1] Wu D T, Cheong K L, Deng Y, et al. Carbohyd Polym, 2015, 134: 12
[2] Lu W, Jiang Q, Shi H, et al. J Agric Food Chem, 2014, 62: 9073
[3] Seeram N P. J Agric Food Chem, 2008, 56: 627
[4] Potterat O. Planta Med, 2010, 76: 7
[5] Amagase H, Farnsworth N R. Food Res Int, 2011, 44: 1702
[6] Duan H, Chen Y, Chen G. J Chromatogr A, 2010, 1217: 4511
[7] Craig S A S. Am J Clin Nutr, 2004, 80: 539
[8] The Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of People’s Republic of China, Part 1. Beijing: China Medical Science Press, 2015: 249
[9] Korea Food and Drug Administration. Korean Pharmacopoeia IX, Part II. Seoul: Shinil Books, 2007: 913
[10] Shin Y G, Cho K H, Kim J M, et al. J Chromatogr A, 1999, 857: 331
[11] Huang H, Chen X S, Liao Q. Chinese Journal of Experimental Traditional Medical Formulae, 2010, 16: 59
[12] Zhao B T, Jeong S Y, Hwangbo K, et al. Arch Pharm Res, 2013, 36: 1231
[13] Holm P I, Ueland P M, Kvalheim G, et al. Clin Chem, 2003, 49: 286
[14] Suo D, Li L, Zhang S, et al. Anal Methods, 2013, 5: 59
[15] Ocque A J, Stubbs J R, Nolin T D. J Pharm Biomed Anal, 2015, 109: 128
[16] Berger T A. J Chromatogr A, 2015, 1421: 171
[17] Zhu L L, Zhao Y, Xu Y W, et al. J Pharm Biomed Anal, 2016, 120: 72
[18] Breitenbach S, Rowe W F, McCord B, et al. J Chromatogr A, 2016, 1440: 201
[19] Hicks M B, Regalado E L, Tan F, et al. J Pharm Biomed Anal, 2016, 117: 316
[20] Ganipisetty V N R, Ravi B, Reddy C R, et al. Anal Methods, 2015, 7: 1092
[21] Berger T A, Berger B K. Chromatographia, 2013, 76: 1631
[22] Lin C, Xie X, Fan N, et al. Chinese Journal of Chromatography, 2015, 33: 397
[23] Said A B, Guinot C, Ruiz J C, et al. J Supercrit Fluid, 2016, 110: 22
[24] Perrenoud A G G, Guillarme D, Boccard J, et al. J Chromatogr A, 2016, 1450: 101
[25] Hegstad S, Havnen H, Helland A, et al. J Chromatogr B, 2017, 1061/1062: 103
[26] Lesellier E, Mith D, Dubrulle I. J Chromatogr A, 2015, 1423: 158
[27] Dai X, Wei B, Wang X, et al. Chinese Journal of Chromatography, 2015, 33: 1059
[28] Li W, Li X, Li G, et al. Chinese Journal of Chromatography, 2016, 34: 795
[29] West C, Lemasson E, Bertin S, et al. J Chromatogr A, 2016, 1440: 212
[30] West C, Bouet A, Routier S, et al. J Chromatogr A, 2012, 1269: 325
[31] Åsberg D, Enmark M, Samuelsson J, et al. J Chromatogr A, 2014, 1374: 254
[32] Li K, Fu Q, Xin H, et al. Analyst, 2014, 139: 3577
[33] Jumaah F, Larsson S, Essén S, et al. J Chromatogr A, 2016, 1440: 191
[34] Desfontaine V, Guillarme D, Francotte E, et al. J Pharm Biomed Anal, 2015, 113: 56

