Evaluation of the effects of AlkylPhenolic compounds on kinetic coefficients and biomass activity in MBBR by means of respirometric techniques
Bijan Bina ,Farzaneh Mohammadi*,Mohammad Mehdi Amin ,Hamid Reza Pourzamani,Zeynab Yavari
1 Environment Research Center,Research Institute for Primordial Prevention of Non-communicable Disease,Isfahan University of Medical Sciences,Isfahan,Iran
2 Department of Environmental Health Engineering,School of Health,Isfahan University of Medical Sciences,Isfahan,Iran
3 Student Research Committee,Isfahan University of Medical Sciences,Isfahan,Iran
1.Introduction
AlkylPhenol Ethoxylates(APEs)are among the extensively used nonionic surfactants and widely used in consumer products such as detergents,lubricants,phenolic resins,rubber and plastic,polymers,antioxidants,heat stabilizers and other materials[1].
APs which are the metabolites of APEs are more stable,more toxic with stronger xenoestrogenic effects and more lipophilic properties than their parents.The most important and widely used APs are 4-NP and 4-t-OP[2]which have harmful effects on human health and environment.These compounds can lead to cancerous tumor,reproductive disorder,immune system disorder and obesity[3].
APs are regularly found in wastewater and commonly detected in water resources receiving wastewater effluents[4]therefore the fate and presence of APs in water resources,and wastewaters were recently reported in different papers[1,5].
According to conducted researches,there are about 0.25 to 193 μg·L-14-NP in domestic wastewater with a median of 16.7 μg·L-1,while 4-t-OP has been shown from an undetectable range to 23 μg·L-1with a median of 1 μg·L-1[6–8].
Among various processes during the past decade,MBBR has been successfully applied for the treatment of municipal and industrial wastewater.Attached growth of biofilm on the carriers is the fundamental of MBBR[9].Moving bed biofilm reactors are designed to offer the advantages of the attached biomass processes(compact,simplicity of operation,high and stable removal efficiency,high surface area for microbial growth)without its drawbacks(clogging,requirement for back washing,sludge recycling and bulking)[10].The MBBR process could eliminate 4-NP and 4-t-OP pollutants more than 90%[11,12].
In this process heterotrophs are faced with starvation status due to high biomass concentration and high cell retention time(SRT),and thus,physiological conditions are not appropriate for the growth of these bacteria.However,high SRT,common of MBBR process,and slower growth rate of autotrophic bacteria compared to heterotrophic bacteria are extremely advantageous for the growth of autotrophic bacteria[13,14].
Modeling is a reliable way to describe and predict the performance of biological treatment processes.Kinetic coefficients are required for developing a model with suitable capability for the design of biological processes.Types of substrate,microorganisms and environmental conditions in the bioreactor significantly affect the kinetic coefficients[15,16].The bacteria in the biological treatment processes comprise both heterotrophs and autotrophs so determining the growth kinetic coefficients of these bacteria in mathematical models appears to be bene ficial and essential[17].
Usually,models such as first and second order substrate removal models and the Stover–Kincannon model are used to determine the kinetic coefficients in suspended and attached growth bioreactors[10,16].Methods developed for activated sludge were strongly adapted to biofilm kinetics specifications.Also respirometric technique is an applicable tool for the determination of the kinetic coefficients of bacteria and biomass activity especially in attached growth processes[17].The oxygen uptake rate(OUR,mg O2·L-1·h-1)is the most important parameter in respirometric techniques in order to evaluate the biomass viability.In other words,in the case of domestic and industrial wastewaters,and in processes operated with various SRTs,respirometric methods have been used to assess the characteristics of wastewater and bacterial activities[14].But it should be noted that respirometric technique has not yet been used to estimate the kinetic coefficients and bacterial activities of wastewater containing priority pollutants especially APs.
This study aimed to investigate the effects of 4-NP and 4-t-OP on heterotrophic and autotrophic bacterial activity and kinetic coefficients on the sludge obtained from MBBR process.The kinetic coefficients and activity measurementwas evaluated through respirometric techniques.
2.Materials and Methods
2.1.MBBR reactor set-up
The experimental investigations were carried out on a laboratory scale plexiglass MBBR.In Fig.1,the reactor scheme is displayed.Total volume of the cylindrical reactor is 6.9 L and operating volume is 4.7 L.Air diffusers and in fluentpipes were installed on the floor ofthe reactor.The aeration rate(6.5 L·min-1)was constant at all stages of operation.40%of the reactor volume was filled with carriers.MBBR carrier media was made from high-density polyethylene(HDPE).The media characteristics are density(0.97 g·cm-3)and surface area(500 m2·m-3).The specifications of the synthetic wastewater during all periods of operation(180 d)are given in Table 1.
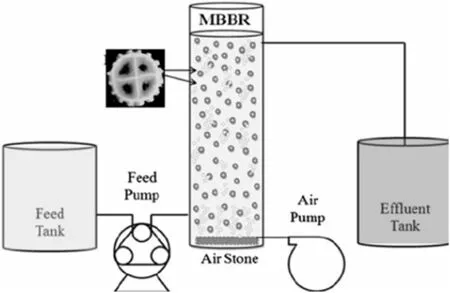
Fig.1.Schematic illustration of the MBBR reactor.
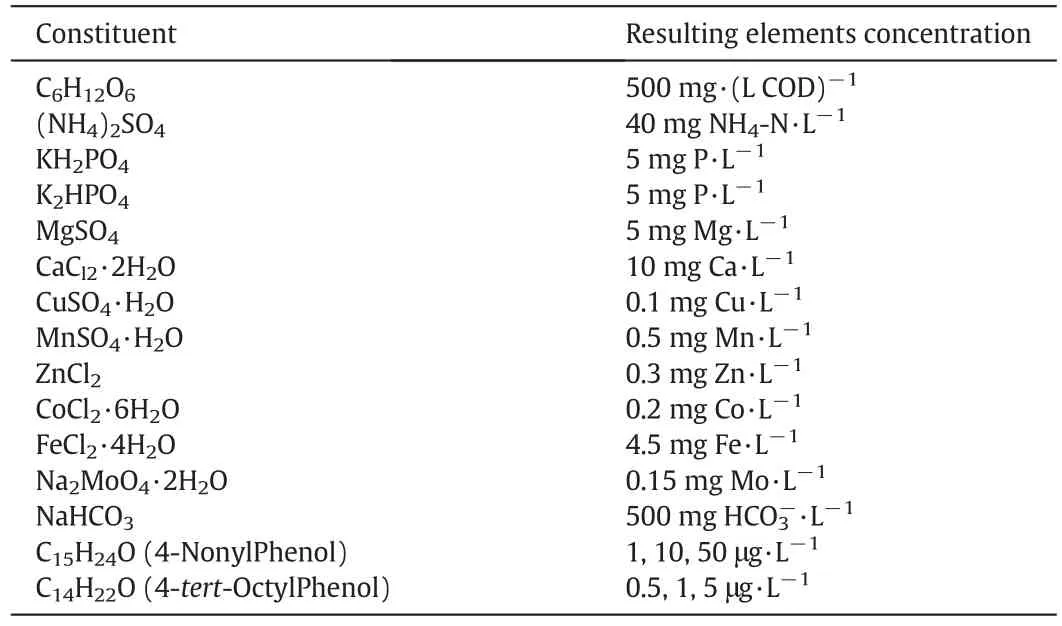
Table 1 Composition of synthetic wastewater injected into the MBBR reactor
At first,activated sludge from full scale municipal WWTP was used for reactor seeding.During the acclimation stage and biofilm growth on carriers(90 d),only glucose was used as a carbon source in the synthetic wastewater and the COD was about 500 mg·L-1during all periods of operation(180 d).4-NP and 4-t-OP were added to the in fluent wastewater with different concentrations when the COD removal efficiency exceeded 90%.Table 2 presents a summary of the operational modes in the MBBR reactor and in respirometric technique tests.The hydraulic retention times(HRT)of 4,8 and 16 h were evaluated in the MBBR reactor.

Table 2 Operational modes in the MBBR reactor and in respirometric techniques tests
2.2.Analytical methods
Parameters such as COD,soluble COD(sCOD),Mixed liquor suspended solids(MLSS),total suspended solids(TSS),volatile suspended solids(VSS)(three times per week),Total Kjeldahl Nitrogen(TKN),nitrate-nitrogen(NO3-N)(once a week)and Total Phosphorus(TP)(once in each operation period)were measured according to the standard methods[18].In order to determine the biofilm dry weight which was attached to the media,some carriers were selected and removed from the MBBR,then the biofilm on the carrier was scrapped completely,dried and then finally weighed[10,19].
4-NP (CAS:84852-15-3),4-t-OP (97%,CAS:140-66-9),derivatization reagent(BSTFA+TMCS(N,O-Bistri fluoroacetamide+trimethylchlorosilane),99:1,CAS:25561-30-2)and internal standard n-OP(99%,CAS:1806-26-4,liner compound)were purchased from Sigma-Aldrich.Chloroform,GC-grade methanol and n-Hexane were obtained from Merck.The stock solutions of 4-NP,4-t-OP and n-OP at concentrations of about 1000 mg·L-1were prepared in methanol.
Dispersive liquid–liquid microextraction(DLLME)method was applied for the extraction of APs.n-OP(internal standard),methanol(dispersion solvent)and chloroform(extraction solvent)were used in the DLLME technique.The DLLME extracts were dried under nitrogen stream in room temperature.
A mixture of BSTFA+TMCS and n-Hexane were applied as derivatization reagent.Afterwards 3 μl of the derivatized sample was injected into a gas chromatograph(GC)–mass spectrometer(MS).The GC–MS device was run with helium as the carrier gas.The oven temperature program was started at 60°C,and it remained constant for 1 min and then rose at the rate of 15 °C·min-1and continued till 170 °C,and then it again rose at the rate of 10 °C·min-1until it reached 300 °C as the final oven temperature and this was maintained for 2 min.The injector and the detector temperature were set to 280°C.Measurements were performed in SIM mode and the mass to charge(m/z)values of fragment ions are 207,179 and 193 for 4-NP,207 for 4-t-OP and 179 and 278 for n-OP.All the samples were analyzed in triplicate.
2.3.Respirometric technique
Respirometric batch experiments were carried out according to the static gas–static liquid approach.At first,0.5 L of mixed liquor(wastewater,suspended solids and biofilm carriers)containing 10%carriers was removed from the MBBR and poured into the test jar.The biofilm on the carriers was scrapped completely and then ground into the size comparable to suspended flocs using a 0.45 mm sieve to eliminate diffusion effects on kinetic parameters[17].The obtained samples were transferred into the batch respirometric beaker and aerated to achieve endogenous respiration conditions.Aeration was stopped after reaching to about 7–8 mg·L-1dissolved oxygen concentration.The samples were kept in lab temperature at 20°C.
Stock solutions of glucose,Ammonium sulfate,4-NP and 4-t-OP were prepared;known volumes of stock solutions were added to the sample after the aeration stoppage.The resulting initial concentrations after addition were 500 mg·L-1readily biodegradable substrate expressed as COD for the characterization of active heterotrophic bacteria and 40 mg·L-1total nitrogen for the characterization of autotrophic bacteria.Afterward the batch respirometric beaker was placed on a magnetic stirrer.The agitation speed was fixed at 200 r·min-1.The kinetic coefficients of heterotrophic and autotrophic bacteria were determined by a respirometric batch test in the presence of 4-NP and 4-t-OP compounds at mentioned concentrations in operational modes(Table 2).At the start of respirometric tests,the sludge concentration was measured in the range of 250–350 mg·L-1.Experimental set-up of the respirometric test is shown in Fig.2.
The Hach sc100™Controller device was used to measure and record the OUR.The dissolved oxygen probe was placed inside the beaker.To prevent air from getting into the respirometric beaker,the probe has a rubber O-ring fitted in the beaker door and the dissolved oxygen changes were read and recorded with Datacom software,Ver.1.4.3.0 installed on a computer connected to the device.DO data were recorded until a concentration close to 1 mg·L-1was reached.Each experiment was repeated three times and lasted about 24–65 h.During the test period,15 ml samples were taken every 2 h and COD,NH4_N and TSS were measured in these samples.Instead of removed volume the synthetic wastewater added to respirometric beaker.

Fig.2.OUR measurement system in the laboratory.
Regarding to respirometric batch tests were conducted on heterotrophic kinetics and activity,Allylthiourea(ATU,10–20 mg·L-1)was poured into the sample in order to inhibit autotrophic bacteria[14,17,20].
The kinetic coefficients of heterotrophic and autotrophic bacteria were calculated by equations provided by Grady et al.[21].These equations have been also used in articles by Chandran and Smets[22],Torretta et al.[20],and Trojanowicz et al.[17].Thus,we avoided repeating all the equations in this article.After determination of the kinetic coefficients in different concentrations of APs,the effect of their presence on bacterial activity and kinetic coefficients of heterotrophic and autotrophic bacteria was investigated.
3.Results and Discussion
3.1.MBBR reactor performance
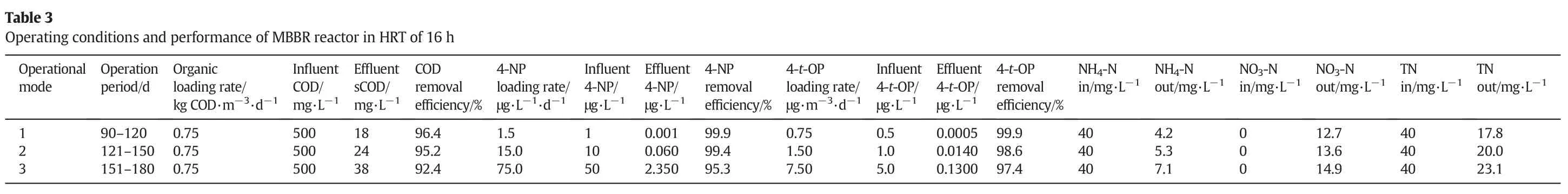
Different hydraulic,organic and AlkylPhenol loading rates have been investigated in the MBBR system.The results indicated that the efficiency of COD removal was appropriate,though the COD loading rates were several times higher as compared to common biological WWTPs.The best performance of the reactor was achieved at a hydraulic retention time(HRT)of16 h,SRT ofabout47 d,food to microorganism(F/M)ratio equal to 0.1 per day and organic loading rates(OLR)of 0.53 kg COD·m-3·d-1.The results in HRT of 16 h for COD,4-NP,4-t-OP,NH4-N,NO3-N and total nitrogen(TN)are illustrated in Table 3.
Based on the results presented in Table 3,increasing the concentration of4-NP and 4-t-OPhas shown an adverse effecton the performance of MBBR.An increase in the concentration of 4-NP and 4-t-OP based on operational modes,will lead to a decrease in the removal of COD and total nitrogen(TN)by about 4%and 13%,respectively.
By increasing the concentration of APs,the nitrification rate in the reactor was decreased and less ammonia nitrogen was converted into nitrate.However,in effluent,nitrate has been observed at higher concentration.Therefor it is obvious that denitrification rate was decreased too.This expressed the adverse impactofAPs on the nitrification and denitrification rate occurred in the MBBR process.
3.2.Characteristics of heterotrophic bacteria in the presence of APs
The biomass activity and kinetic coefficients of heterotrophic bacteria could be measured using respirometric batch tests in the suspended and attached biomass of the MBBR.Accordingly,the respirometric tests were performed at three operational modes(Table 2)in lab temperature at about 20°C.Fig.3 represents an OUR profile and Michaelis Menten model curve fitting for heterotroph bacteria.
Based on Fig.3a,by increasing AP concentration,the OURH(heterotrophic oxygen uptake rate)was determined for operational modes 1:3,respectively,in the ranges of(6.39–3.46 mg O2·L-1·h-1),(5.21–2.99 mg O2·L-1·h-1)and(4.35–2.07 mg O2·L-1·h-1).
The obtained results showed that the biomass activity in substrate utilization decreased by increasing AP concentration,so that the rate of substrate utilization by heterotrophic bacteria has declined from 22 to 11 mg COD·L-1·h-1.The heterotrophic activity was also calculated in the ranges of(2.64–1.14 g COD·(g VSS)-1·d-1),(2.00–0.96 g COD·(g VSS)-1·d-1)and(1.13–0.73 g COD·(g VSS)-1·d-1)in different AP concentrations.It is worth noting that the F/M ratio has decreased from 1.5 d-1at the beginning of the tests to about 0.05 d-1at the end of the respirometric tests.Therefore,it is clear that by increasing AP concentration,despite identical substrates and environmental conditions,the heterotrophic activity has decreased obviously.The consumption rate of rapidly biodegradable substrates by heterotrophic bacteria has been declared in the range of 2.38 to 1.67 g COD·(g VSS)-1·d-1[23].
?
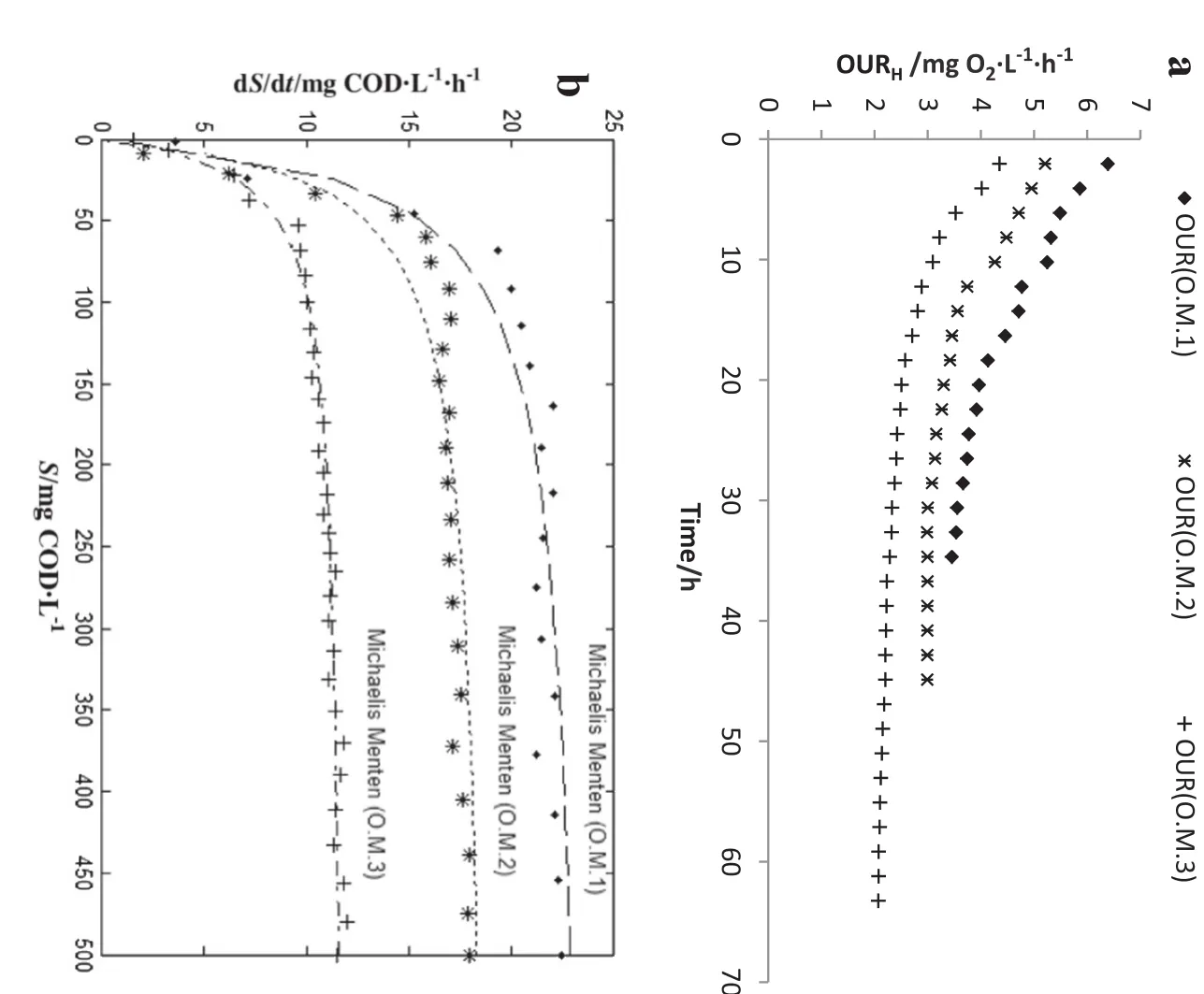
Fig.3.a)OUR profile;b)Michaelis Menten model curve fitting for heterotrophic bacteria in various concentrations of APs.
3.2.1.Heterotrophic maximum specific growth rate(μH,max)and the halfsaturation constant(KS)
The μH,maxand KSwere determined according to the respirometric technique provided by Grady et al.[21].The Specific Oxygen Uptake Rate(SOUR)and heterotrophic yield coefficient(YH)were used to calculate μH,maxand KS.SOUR can be obtained bydividingOUR by active heterotroph concentration.The specific growth rate of heterotrophic bacteria(μH)is obtained from Eq.(1).

The values of μH,maxand KSat various concentrations of APs were calculated through plotting the μHagainst the remained concentration of rapidly biodegradable substrate during the time intervals and by fitting the non-linear Monod curve(given in Eq.(2)),which are shown in Fig.4 and Table 4.

The μH,maxand KSvalues strongly depend on the types of microorganisms and substrate biodegradability.The rapidly biodegradable substrate has larger μH,maxand smaller KS,while the slowly biodegradable substrate has smaller μH,maxand larger KS[21].Also,the results lie in the typical range reported in the technical literature:μH,max=0.6–13.2 d-1and KS=5–225 mg·L-1[24].An outline summary of calculations is presented in Table 4.According to Table 4,the μH,maxwas decreased and the KSwas increased by increasing AP concentration.
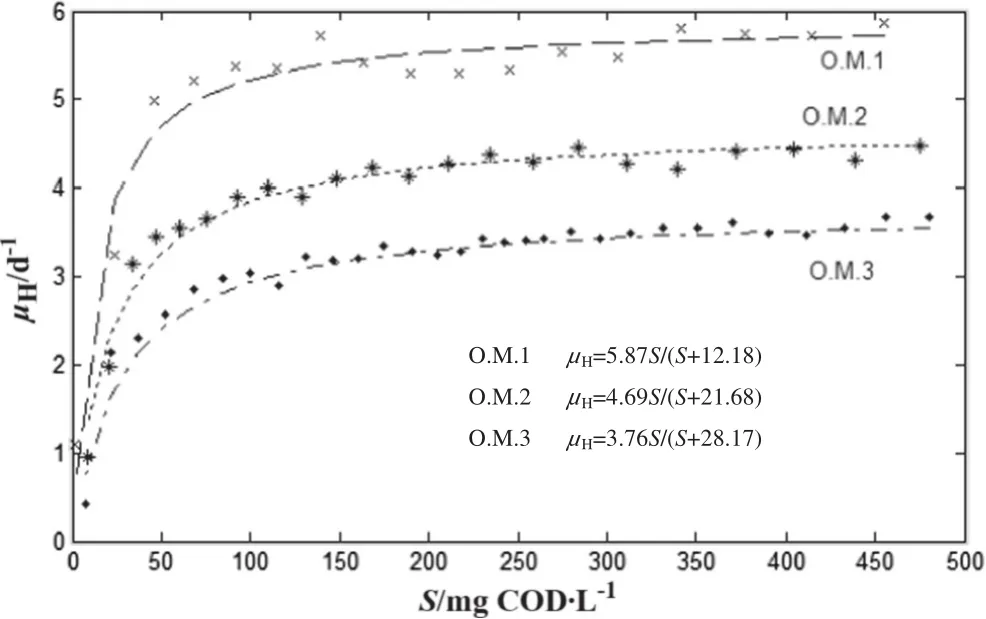
Fig.4.Finding the Monod parameters K S and μH,max by fitting the curve to experimental data in various concentrations of APs.
3.2.2.Heterotrophic decay coefficient(bH)and active fraction of heterotrophic bacteria in the total suspended solids(fA,H)
By plotting the ln OUR curve versus time,the bHand fA,Hcoefficients have been calculated as the slope and intercept of the straightinterpolation line according to Eq.(3)[14,17,21].The calculated values are given in Fig.5 and Table 4.
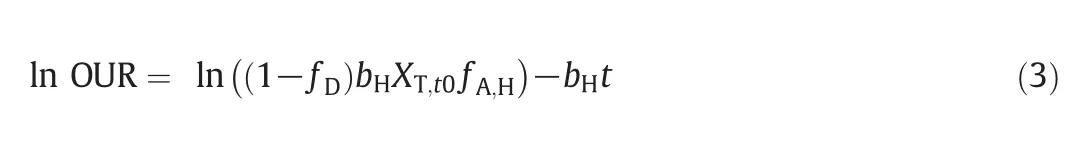
According to Table 4,by increasing AP concentration based on operational modes,bHwas increased and the μH,maxwas decreased.Given that the F/M ratio has been the same at the all of respirometric tests,therefore μH,maxand bHcoefficients are not affected by changes in F/M ratio.So it was understood that APs had inhibitory effects on the activity of heterotrophic bacteria.This result was confirmed by reduction of oxygen consumption rate in Fig.3.The values of bHcoefficient have been reported in the range of 0.05–1.6 d-1(typically 0.6 d-1@20°C)for activated sludge process[24].The results of this study are consistent with previous studies.
By knowing the initial total suspending solid concentration XT,t0(250–350 mg·L-1)and initial fraction of biomass debris fD(0.20),it is possible to calculate the active fraction of heterotrophic bacteria in the total suspended solids.The values of fA,Hare shown in Table 4.With increasing APs,the active fraction of heterotrophic bacteria was decreased by 15%.Trojanowicz et al.reported that the fA,Hcoefficient in biofilm systems which seeding with petrochemical wastewater was measured about 46%[17].
Fig.5.The plot of ln OUR versus time during determination of the b H and f A,H of heterotrophic bacteria at different AP concentrations.
3.2.3.Heterotrophic yield coefficient(YH)
Heterotrophic yield coefficient was calculated according to the methodology described by Trojanowicz et al.in 2009.For determining the YHby respirometric technique,it's essential to measure the actual growth of heterotrophic bacteria with taking the decaying rate coefficient into consideration during the time intervals with relation to the amount of soluble COD utilized at the time period[17].
The obtained YHvalues are given in Table 4.By increasing APs,the YHvalue has decreased from 0.516 to 0.396 mg CODbiomass·(mg COD)-1.The yield coefficient depends on the type of substrate[21,25].For aerobic heterotroph bacteria degrading rapidly biodegradable substrates such as carbohydrates,YHvalues were commonly in the range of 0.48–0.72 mg CODbiomass·(mg COD)-1.Under the same condition,YHvalues for heterotrophic growth on some xenobiotic compounds,including phenols,benzenes and phthalate esters were in the range of 0.2–0.6 mg CODbiomass·(mg COD)-1[21].
The bacteria oxidize a portion of biodegradable COD to provide energy and use another portion of biodegradable COD for biomass growth.When the biodegraded COD remained constant but the yield coefficient reduced,it represents that higher amount of carbon has been used for energy production[26].According to the abovementioned data,by increasing AP concentration,heterotrophic bacteria will require more energy for substrate utilization;therefore the greater portion of carbon source convert to energy and smaller portion of that convert to biomass compared with lower AP concentration.
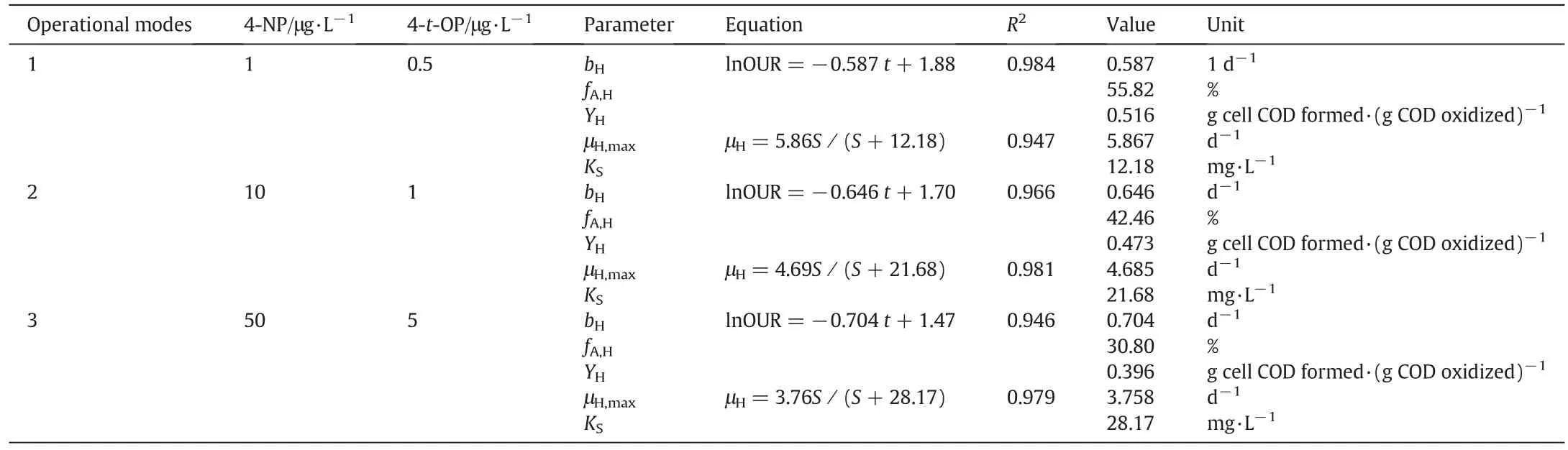
Table 4 Kinetic coefficients calculated in this study for heterotrophic bacteria

Fig.6.a)OUR profile;b)Michaelis Menten model curve fitting for autotrophic bacteria in various concentrations of APs.
3.3.Characteristics of autotrophic bacteria in the presence of APs
To estimate the autotrophic bacteria characteristics in the presence of APs,several respirometric batch tests were performed at different operational modes on biomass obtained from MBBR.The OUR profile and Michaelis Menten model curve fitting for autotrophic bacteria could be seen in Fig.6.
At low concentration of APs,the obtained results for ammonia utilization and nitrification rate are well in the range proposed for biofilm systems and above the proposed range for activated sludge systems[14,27].By increasing AP concentrations,ammonia and oxygen consumption rate decreased,and also the decrease of the activity for autotrophic bacteria,especially nitrifying bacteria happens.
The consumption rate of ammonia nitrogen by nitrifying bacteria was decreased from 3.5 to 2 mg NH4-N·L-1·h-1.Autotrophic activity was decreased from 0.480 to 0.274 g NH4-N·(g VSS)-1·d-1.The ammonia consumption rate by autotrophic bacteria was reported up to 0.74 g NH4-N·(g VSS)-1·d-1[28].
3.3.1.Autotrophic yield coefficient(YA)
Eq.(4)represents the basis of autotrophic yield coefficient calculation.Here,SNH(0)is the initial ammonia nitrogen concentration[20,29].The values obtained for the kinetic coefficients referring to autotrophic bacteria are represented in Table 5.
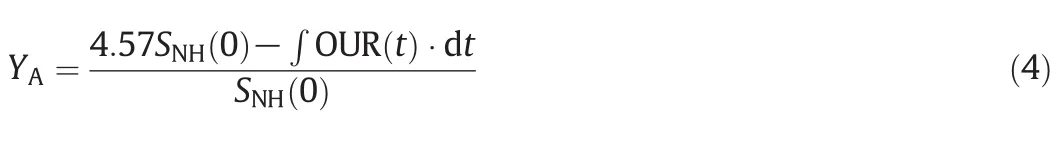
The obtained YAvalues in this paperwere wellin the range proposed by other references that vary between 0.06 and 0.35 g cell COD formed·(g NH4-N oxidized)-1,for a CAS process[21].Increased concentrations of APs have inhibitory effects on the biomass production rate of autotrophic bacteria.
3.3.2.Autotrophic decay coefficient(bA)and active fraction of autotrophic bacteria in the total suspended solids(fA,A)
Eq.(5)was used to calculate the coefficients of bAand fA,A[30].The ixband ixpare respectively the nitrogen mass in the biomass at the beginning and end of the test(0.086 g N·(g CODbiomass)-1and 0.06 g N·(g CODendogenousmass)-1are the defaults of ASM1 model)[24].The value proposed for bAis in the range of 0.20 to 0.05 d-1[23].As shown in Table 5,the calculated values in this study are in general agreement with those found in past studies.As heterotrophic bacteria,by increasing AP concentrations,the autotrophic decay coefficient was increased while the autotrophic growth rate was decreased.The active fraction of autotrophic bacteria was also decreased by 5.37%.In other studies,the fA,Ain suspended sludge was measured about 8 to 9%[20].Accordingly,the results presented that the autotrophic bacteria ratio in biofilm is higher than that in the suspended biomass.

3.3.3.Autotrophic maximum specific growth rate(μA,max)and the halfsaturation constant(KNH)
The autotrophic maximum specific growth rate(μA,max)and the half-saturation constant(KNH),have been calculated from the respirometric experiment using Eq.(6)[29].
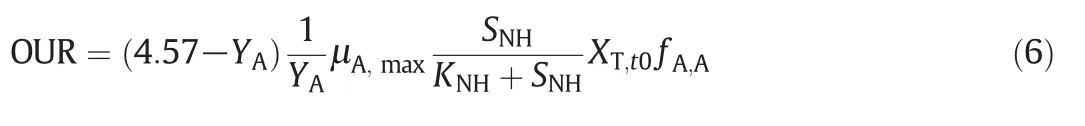
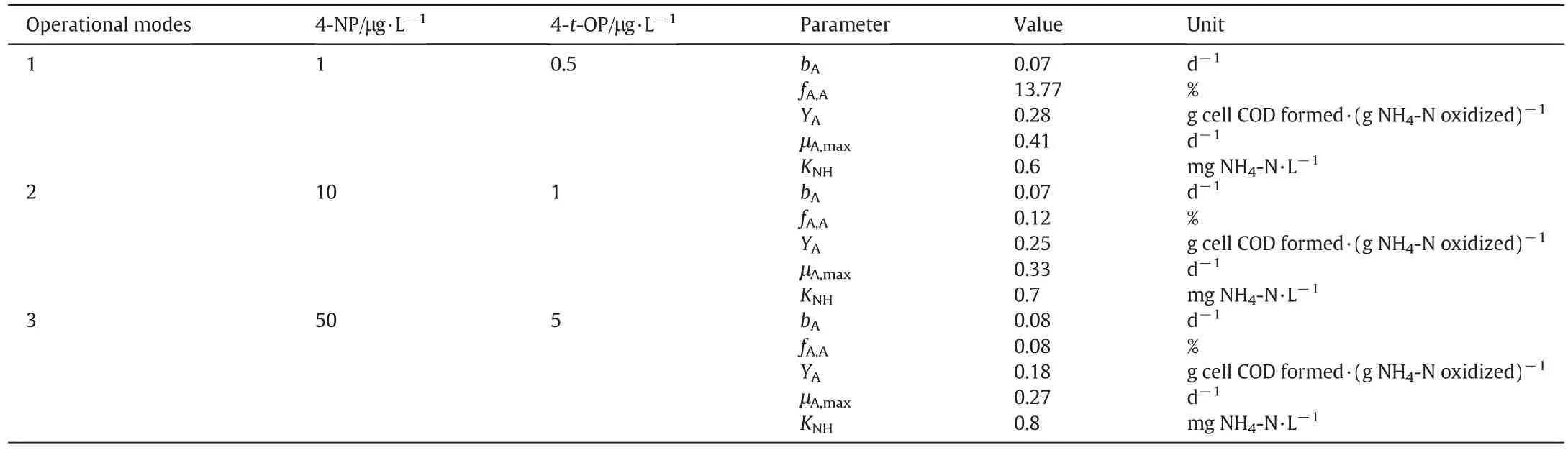
Table 5 Autotrophic kinetic coefficients calculated in this study
The ranges of μA,maxand KNHin ASM1 model have been proposed respectively as 0.2–1 d-1and 0.1–1 mg NH4-N·L-1.These results agree very well with the values proposed in the literature(Table 5)[14,24].The values of μA,maxand KNHcoefficients depend on the type of substrate.Slowly biodegradable and xenobiotic materials have inhibitory effects on autotrophic bacteria[31].According to Table 5,the μA,maxwas decreased and the KNHwas increased by increasing AP concentrations,obviously.Thus,it could be understood that 4-NP and 4-t-OP have strongly affected the growth of autotrophic bacteria and the biodegradability of the substrate.
3.4.Comparing the effect of APs on heterotrophic and autotrophic bacteria
Fig.7 was drawn to compare the effects of APs on growth rate,biomass production and decay coefficient in heterotrophic and autotrophic bacteria.Accordingly,by increasing the AP concentrations,the growth rates of heterotrophic and autotrophic bacteria were decreased respectively by 29.95%and 32.4%.The decay rates of heterotrophic and autotrophic bacteria were also increased by 16.6%and 17.7%,respectively.In addition,the amount of produced biomass per substrate utilization has decreased in heterotrophic and autotrophic bacteria by 23.3%and 32.4%,respectively.
Thus,it was concluded that heterotrophic bacteria have been less affected by the presence of APs,and these compounds had greater inhibitory effects on autotrophic bacteria.According to other conducted studies,autotrophic bacteria have shown more sensitivity to the presence of surfactants such as 4-n-NP than heterotrophic bacteria[32,33].
4.Conclusions
In this study,the effects ofAlkylPhenolcompounds on heterotrophic and autotrophic bacterial activity and kinetic coefficients were evaluated in the MBBR system by using the respirometric technique.The yield coefficients(YH,YA),maximum specific growth rate(μH,max,μA,max),the half-saturation constant(KS,KNH),decay coefficient(bH,bA)and active fraction in total suspended solids(fA,H,fA,A)were calculated for autotrophic and heterotrophic bacteria at different concentrations of 4-NP and 4-t-OP compounds.With increasing AP concentration,the maximum specific growth rate,yield coefficient and the active fraction in the total suspended solids were decreased for heterotrophic and autotrophic bacteria,while their decay coefficient and half saturation constant were increased.The inhibitory effects of APs on bacterial activity in the MBBR process which consists of attached and suspended growth is quite evident.The AP effect on autotrophic biological activities has been more than their impact on heterotrophic bacteria.
Acknowledgments
This article is the resultofPhDthesis approved in the Isfahan University of Medical Sciences(IUMS).The authors wish to acknowledge to Vice Chancellery of Research of IUMS for the financialsupport,Research Project,#394773.

Fig.7.Comparison of AP effects;a)heterotrophic bacteria;b)autotrophic bacteria.
References
[1]S.Deshayes,V.Eudes,M.Bigourie,C.Droguet,R.Moilleron,Alkylphenol and phthalate contamination of all sources of greywater from French households,Sci.Total Environ.599(2017)883–890.
[2]D.Liu,J.Liu,M.Guo,H.Xu,S.Zhang,L.Shi,C.Yao,Occurrence,distribution,and risk assessment of alkylphenols,bisphenol A,and tetrabromobisphenol A in surface water,suspended particulate matter,and sediment in Taihu Lake and its tributaries,Mar.Pollut.Bull.112(2016)142–150.
[3]S.Massart,B.Redivo,E.Flamion,S.N.Mandiki,E.Falisse,S.Milla,P.Kestemont,The trenbolone acetate affects the immune system in rainbow trout,Oncorhynchus mykiss,Aquat.Toxicol.163(2015)109–120.
[4]W.Zheng,S.R.Yates,S.A.Bradford,Analysis of steroid hormones in a typical dairy waste disposal system,Environ.Sci.Technol.42(2008)530–535.
[5]M.V.F.Andrade,I.K.Sakamoto,J.J.Corbi,E.L.Silva,M.B.A.Varesche,Effects of hydraulic retention time,co-substrate and nitrogen source on laundry wastewater anionic surfactant degradation in fluidized bed reactors,Bioresour.Technol.224(2017)246–254.
[6]G.-G.Ying,B.Williams,R.Kookana,Environmental fate of alkylphenols and alkylphenol ethoxylates—a review,Environ.Int.28(2002)215–226.
[7]A.Bergé,M.Cladière,J.Gasperi,A.Coursimault,B.Tassin,R.Moilleron,Meta-analysis of environmental contamination by alkylphenols,Environ.Sci.Pollut.Res.19(2012)3798–3819.
[8]H.-B.Lee,T.E.Peart,Determination of 4-nonylphenol in effluent and sludge from sewage treatment plants,Anal.Chem.67(1995)1976–1980.
[9]C.Li,Z.Zhang,Y.Li,J.Cao,Study on dyeing wastewater treatment at high temperature by MBBR and the thermotolerant mechanism based on its microbial analysis,Process Biochem.50(2015)1934–1941.
[10]E.Ahmadi,M.Gholami,M.Farzadkia,R.Nabizadeh,A.Azari,Study of moving bed biofilm reactor in diethyl phthalate and diallyl phthalate removal from synthetic wastewater,Bioresour.Technol.183(2015)129–135.
[11]Y.Luo,Q.Jiang,H.H.Ngo,L.D.Nghiem,F.I.Hai,W.E.Price,J.Wang,W.Guo,Evaluation of micropollutant removal and fouling reduction in a hybrid moving bed biofilm reactor–membrane bioreactor system,Bioresour.Technol.191(2015)355–359.
[12]L.Gunnarsson,M.Adolfsson-Erici,B.Björlenius,C.Rutgersson,L.Förlin,D.G.J.Larsson,Comparison of six different sewage treatment processes—reduction of estrogenic substances and effects on gene expression in exposed male fish,Sci.Total Environ.407(2009)5235–5242.
[13]V.Hoang,R.Delatolla,E.La flamme,A.Gadbois,An investigation of moving bed biofilm reactor nitrification during long-term exposure to cold temperatures,Water Environ.Res.86(2014)36–42.
[14]D.Di Trapani,M.Capodici,A.Cosenza,G.Di Bella,G.Mannina,M.Torregrossa,G.Viviani,Evaluation of biomass activity and wastewater characterization in a UCTMBR pilot plant by means of respirometric techniques,Desalination 269(2011)190–197.
[15]M.Amin,H.Farokhzaddeh,A.Fatehizadeh,M.Ghasemian,H.Moradpour,M.Nikaeen,A.Sha fiea,R.Molayi,A.Sabouri,Biodegradation performance of anaerobic sequencing batch biofilm reactor for oil with polychlorinated biphenyls,Int.J.Environ.Health Eng.2(2013)19.
[16]A.M.Mansouri,A.A.L.Zinatizadeh,A.Akhbari,Kinetic evaluation of simultaneous CNP removal in an up- flow aerobic/anoxic sludge fixed film(UAASFF)bioreactor,Iran.J.Energy Environ.5(3)(2014)323–336.
[17]K.Trojanowicz,W.Styka,T.Baczynski,Experimental determination of kinetic parameters for heterotrophic microorganisms in biofilm under petrochemical wastewater conditions,Pol.J.Environ.Stud.18(2009)913–921.
[18]APHA/AWWA/WEF,Standard Methods for the Examination of Water and Wastewater,American Water Works Assn,USA,2012.
[19]M.Shakerkhatibi,H.Ganjidoust,B.Ayati,E.Fatehifar,Performance of aerated submerged fixed- film bioreactor for treatment of acrylonitrile-containing wastewater,J.Environ.Health Sci.Eng.7(2010)327–336.
[20]V.Torretta,M.Ragazzi,E.Trulli,G.De Feo,G.Urbini,M.Raboni,E.C.Rada,Assessment of biological kinetics in a conventional municipal WWTP by means of the oxygen uptake rate method,Sustainability 6(2014)1833–1847.
[21]C.P.L.Grady,G.T.Daigger,H.C.Lim,Biological Wastewater Treatment,Second Marcel Dekker,Inc.,New York,1999.
[22]K.Chandran,B.F.Smets,Estimating biomass yield coefficients for autotrophic ammonia and nitrite oxidation from batch respirograms,Water Res.35(2001)3153–3156.
[23]M.Henze,M.C.M.van Loosdrecht,G.A.Ekama,D.Brdjanovic,Biological Wastewater Treatment,IWA Publishing,London,2008.
[24]U.Jeppsson,A General Description of the IAWQ Activated Sludge Model No.1,1987 1–14.
[25]P.Taylor,R.T.Alqahtani,M.I.Nelson,A.L.Worthy,Analysis of a Chemostat Model With Variable Yield Coefficient and Substrate Inhibition:Contois Growth Kinetics,202,2015 37–41.
[26]E.Metcalf,H.Eddy,Wastewater Engineering:Treatment and Reuse,4th ed.Tata McGraw-Hill Publ.Co.Limited,New Delhi,India,2003 1819.
[27]H.Gullicks,H.Hasan,D.Das,C.Moretti,Y.T.Hung,Bio film fixed film systems,Water(Switzerland)3(2011)843–868.
[28]S.Milia,M.Perra,G.Cappai,A.Carucci,SHARON process as preliminary treatment of re finery wastewater with high organic carbon-to-nitrogen ratio,Desalin.Water Treat.57(2016)17935–17943.
[29]E.W.A.Liwarska-Bizukojc,Determination of kinetic and stoichiometric parameter of activated sludge models,Environ.Prot.Eng.37(2011)73.
[30]M.Capodici,S.Fabio Corsino,F.Di Pippo,D.Di Trapani,M.Torregrossa,An innovative respirometric method to assess the autotrophic active fraction:application to an alternate oxic–anoxic MBR pilot plant,Chem.Eng.J.300(2016)367–375.
[31]S.N.Dokianakis,M.E.Kornaros,G.Lyberatos,On the effect of pharmaceuticals on bacterial nitrite oxidation,Water Sci.Technol.50(2004)341–346.
[32]A.S.Stasinakis,D.Mamais,N.S.Thomaidis,E.Danika,G.Gatidou,T.D.Lekkas,Inhibitory effect of triclosan and nonylphenol on respiration rates and ammonia removal in activated sludge systems,Ecotoxicol.Environ.Saf.70(2008)199–206.
[33]K.K.Brandt,M.Hesselsoe,P.Roslev,K.Henriksen,J.Sorensen,Toxic effects of linear alkylbenzene sulfonate on metabolic activity,growth rate,and microcolony formation of Nitrosomonas and Nitrosospira strains,Appl.Environ.Microbiol.67(2001)2489–2498.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- CFD modeling of turbulent reacting flow in a semi-batch stirred-tank reactor☆
- Synergistic and interference effects in coaxial mixers:Numerical analysis of the power consumption☆
- Atomic layer deposition of Al2O3 on porous polypropylene hollow fibers for enhanced membrane performances☆
- Experimental investigation and cost assessment of the salt production by solar assisted evaporation of saturated brine☆
- Equilibrium of liquid-liquid extraction of 2-phenylbutyric acid enantiomers:Experiment and model☆
