Equilibrium of liquid-liquid extraction of 2-phenylbutyric acid enantiomers:Experiment and model☆
Weifeng Xu,Guilin Dai,Kewen Tang*,Panliang Zhang*,Biquan Xiong,Yu Liu
Department of Chemistry and Chemical Engineering,Hunan Institute of Science and Technology,Yueyang 414006,China
1.Introduction
Chirality,as an intrinsic property of the “building blocks of life”,exists extensively within nature[1,2].On an account of their sensitivity to stereochemistry,the enantiomers usually show large differences in bioactivity.Therefore,chirality is continuously an attractive issue for its essential role in chemical industry and pharmaceutical industry.
On account of the dramatic raise in applications of singleenantiomer of drugs in the past ten years,the development of new chiralseparation technologies keeps an active role in the areas offragrance,pharmaceutical,and field of special chemicals.Traditional methods of chiral separation include crystallization[3],chromatography[4],liquid membrane[5],and so forth.Given that there are certain advantages in some aspects such as efficiency,practicality,economy,etc.,these methods are increasingly more attractive;however,there still exists some shortcomings.When compared with aforementioned methods,liquid–liquid reactive extraction has been regarded as a promising method for enantiomers separation on account of higher feasibility economically and being easier to scale up with a wide range of applications.By the method of chiral solvent extraction,numerous investigations for the separation of optically active compound have been reported recently[6–27].There are abundant literatures available for enantioselective extraction;however,to our knowledge,only a few researches involve exploration both fundamentally and thoroughly about the reaction process and mechanism through a hybrid process of experimental research and mathematical modeling,which can be helpful for realizing the prediction and optimization of the extraction performance[10,11,13].
In chiral extraction process,a selective extraction agent(selector)in the extract phase is needed,which reacts with the solutes of enantiomers.Enantioselectivity(α)is the key argument for chiral extraction.For example[19],foran enantioselectivity of1.05,190 theoreticalstages are required to obtain a 99%pure product(R/S=100),whileαincreases to a value of 1.20,the number of stages will be reduced to 30.Chiral selectors may be obtained from natural sources or from the synthetic building blocks.The common chiralselectors include tartaric acid derivatives[6,7,27],chiral crown ethers[8,9],cinchona alkaloids[11,14,15],copper–amino acid complexes[20,21],chiral micelles[22,23],and cyclodextrins(CDs)or their derivatives[7,24–26].Among these chiral selectors,β-CD has the advantage that its cavity will be suitable to include a wide range of chemical compounds especially for that of pharmaceutical interest.In the process of inclusion–complexation,the analytes should fit either completely or with their hydrophobic part into the β-CD cavity[28],entering through one of the two openings,primary and secondary with 6-hydroxyl and 2-,3-hydroxyl groups,respectively[29].
The hydroxyl groups exist in the rim of the CDs can be easily modi fied by chemical reactions in order to obtain CD derivatives which are obtained by varying degree of substitution.The modi fied CDs can exhibit different properties than the native ones,which have a very wide range of applications for following advantages.On the one hand,the CDs can be applied to separate varieties of racemic enantiomers,and they can show a high selectivity of the enantiomers separation[26,30].On the other hand,the modi fied CDs can dissolve easily in water,which can increase the solubility of enantiomers in water.The increased solubility of enantiomers can improve the output of enantiomer production in industrial production.Additionally,the CDs are obtained from glucose,which are low-cost and environmental protective.
2-Phenylbutyric acid(2-PBA)is a hydroxy acid,which is important for pharmaceutical process.To our best knowledge,studying for enantiomer separation of2-PBAis little and only capillary electrophoresis has been employed to the resolution ofit;while capillary electrophoresis method is mainly used for analysis.In this paper,enantioselective liquid–liquid extraction is introduced to separate 2-PBA enantiomers,due to its easy scaling up.Additionally,a mathematical model for the reactive extraction of racemic 2-PBA is established to realize the prediction and optimization of the extraction process.Hydrophilic β-cyclodextrin(β-CD)derivatives were used as the chiral selector.Important extraction factors are investigated by experiment and modeling,such as types of organic solvents,β-CD derivatives,pH,concentration of cyclodextrin and temperature.
2.Experimental
2.1.Materials
The grades and suppliers of reagents are listed in Table 1.
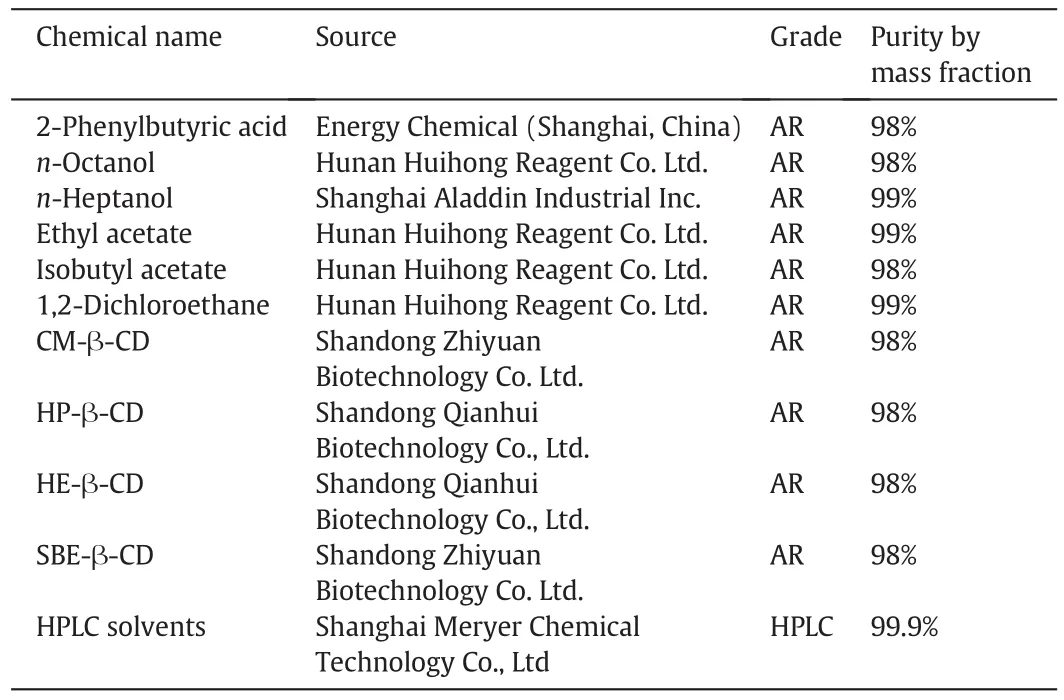
Table 1 Specifications for chemicals used
2.2.Analytical method
The determination of 2-PBA enantiomers concentrations in extraction phase was analyzed by HPLC(Agilent Technologies Corporation,Series 1260,USA).The quantitative analysis was performed by UV–Vis adsorption detector at the wavelength of 225 nm.An Inertsil ODS-3 C18column(250 mm formed by UV–Vis μm)was employed and the column temperature was maintained at 30.0°C.The mobile phase consisted of methanol and 0.025 mol·L-1HP-β-CD aqueous solution(pH=4.00,adjusted with triethylamine and glacial acetic acid),in which the volume ratio was 36:64.Other condition was flow rate of 1.0 ml·min-1.The retention time of(+)-2-PBA was less than that of(-)-2-PBA.This analytical method was adjusted according to the method of 2-phenylpropionic acid[31].
2.3.Extraction experiments
The aqueous phase containing 0.1 mol·L-1(HP-,HE-,CM-,or SBE-)β-CD was dissolved in 0.1 mol·L-1NaH2PO4/H3PO4buffer solution and the organic phase was obtained by dissolving 0.01 mol·L-1racemic 2-PBAin organic solvent.Each 3 mlofthe two phases were puttogether into a 10-mlplastic centrifuge tube and shaken suf ficiently(12 h)being kept in a water bath at a fixed temperature to reach equilibrium.Deposited after 2 h,the two phases would be separated.The HPLC was used to analyze the concentration of 2-PBA in the aqueous phase.Based on a mass balance,the concentration of(+)-and(-)-2-PBA in organic phase was determined.
2.4.Mechanism and model
2.4.1.Mechanism of reaction extraction
The extraction mechanism is the basis of understanding the separation process and building the mathematical model.As we all know,reactions may take place either in the aqueous phase,the organic phase,or at the interface for the reactive extraction system[30].In this extraction system,β-CDs are insoluble in organic liquids due to their super hydrophilia;thus,the reactions may only occur either in the aqueous phase or at the interface.Additionally,the solutes of 2-PBA enantiomers can partly be dissolved in both the two phases.Therefore,a homogeneous reaction mechanism is assumed to research the present extraction system.
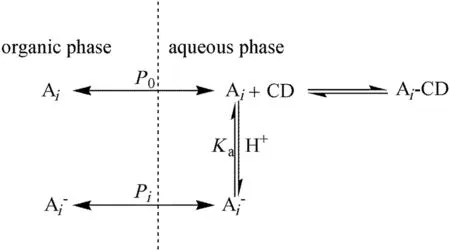
Fig.1.Diagram ofthe mechanism ofreactive extractive of2-PBAenantiomers by HE-β-CD.(A i=(+)-2-PBA or(-)-2-PBA,CD=HE-β-CD.)
Fig.1 shows the equilibrium of the reactive extraction system.Equilibrium of the system includes the physical partition of molecular and ionic 2-PBA between the two phases,the inclusion–complexation equilibrium between HE-β-CD and 2-PBA enantiomers and the acid–base dissociation equilibrium of 2-PBA.
The extraction efficiency can be evaluated by distribution ratio(k)and enantioselectivity(α),which are de fined by the following Eqs.(1)to(3):

where(CA+)o/(CA-)oand(CA+)w/(CA-)wrepresent the analytical concentrations of(+)-2-PBA/(-)-2-PBA in organic phase and aqueous phase,respectively.
2.4.2.Basic equations
The thermodynamic equilibrium of this extraction system can be modeled on the basis of the following equations.Eqs.(4)and(5)are the physical partition coefficients of molecular and ionic 2-PBA respectively,where,[]represents the equilibrium concentration of the corresponding species;the subscript of w and o represent the concentrations in aqueous and organic phases,respectively[30].

The dissociation constant of 2-PBA is described as the following Eq.(6)and the value of Kais 4.34 which is obtained by Science if nder.
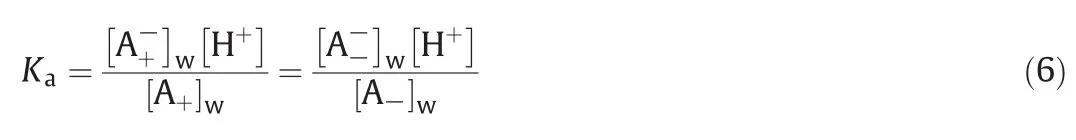
The inclusion–complexation equilibrium constants of HE-β-CD with 2-PBA enantiomers in aqueous phase can be written as follows:

The reactive extraction system is optimized aiming at enhancing the enantiomeric excess(ee),the fractions of solutes(φ)and performance factor(pf).The de finition of ee,φ,and pf are shown in Eqs.(9)–(11)[30].

where[Ai]wrepresents the total concentration of the solute i in aqueous phase at equilibrium,and[Ai]represents the initial total concentrations of the solute i.
3.Results and Discussion
According to the results of experiment and the method of acquiring the physical partition coefficients and chemical equilibrium constant[30],the values of P0and Pi,K+and K-are obtained experimentally and displayed in Table 2.Employing these parameters,the model could be employed to simulate the separation process.

Table 2 The values of physical partition coefficients(P0,P i)and chemical equilibrium constant(K+,K-)
3.1.Organic solvents effect
Investigation of the partition behavior of(+)-and(-)-2-PBA is performed in various organic/aqueous-phase systems(Table 3).As Table 3 shows,distribution ratio and enantioselectivity are affected obviously by the type of the organic solvent.Comparingwith other organic solvent,when 1,2-dichloroethane is used,the distribution ratios are suitable,and more importantly,the highest enantioselectivity of 2.096 is achieved.Therefore,1,2-dichloroethane is the optimal choice.
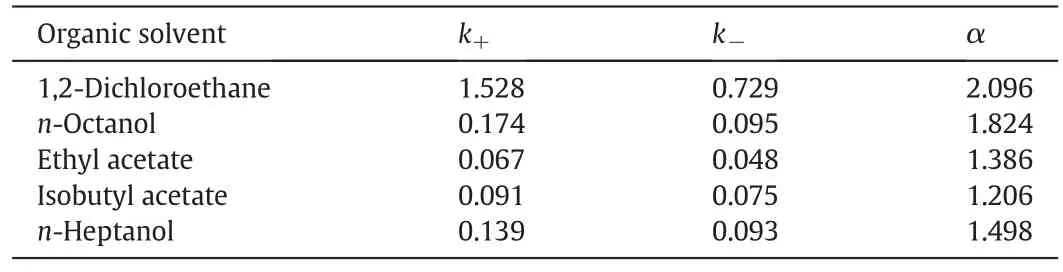
Table 3 In flence of organic solvent type(Aqueous phase:[HE-β-CD]=0.1 mol·L-1,pH=3.00.Organic phase:[2-PBA]=0.01 mol·L-1;T=5.0 °C.)
3.2.In fluence of β-CD derivatives
Investigation of the in fluence of β-CD derivatives on the distribution ratio and enantioselectivity for2-PBAenantiomers is carried outand the corresponding results are depicted in Table 4.

Table 4 In fluence of hydrophilic extractant type(Aqueous phase:pH=3.00.Organic phase:1,2-dichloroethane[2-PBA]=0.01 mol·L-1;T=5.0°C.)
As Table 4 shows,the in fluence of β-CD derivatives on the distribution ratios and enantioselectivity is apparent.Comparing with other β-CD derivative,when HE-β-CD is used as the selector,the distribution ratios are suitable,and more importantly,the highest enantioselectivity of 2.096 is achieved.Therefore,HE-β-CD is selected as the best extractant.
3.3.In fluence of pH value
The state of 2-PBA in aqueous phase varies with the pH value of aqueous phase.According to the mechanism hypothesis,HE-β-CD can form a complex with molecular 2-PBA enantiomers but not with ionic 2-PBA enantiomers.Therefore,the partition behavior of 2-PBA enantiomers may be affected by the pH value of aqueous phase in a liquid–liquid reactive extraction system.Fig.2 shows the results of in fluence of pH values on the separation efficiency.The dots represent the experimental data and the lines represent the model simulation.As shown in Fig.2,the simulated results are consistent with the experimental results.The mean relative errors are 2.27%for k+,2.13%for k-,and 1.86%for α.
As shown in Fig.2(A),k+and k-values are nearly not changed at pH≤4.00 and then increase rapidly with the further increase of pH value.The enantioselectivity shows a converse tendency(Fig.2(B)).This may be because of the fact that at low pH value(pH≤4.00),most of the 2-PBA are in their molecular form and HE-β-CD can selectively form inclusion complex with molecular 2-PBA.With the pH rising up,the molecular 2-PBA enantiomers start to be ionized and the nonselective partition behavior of these anions makes distribution ratios increased considerably and enantioselectivity decreased rapidly.Therefore,a low pH value(≤3.00)is needed for the extraction process.
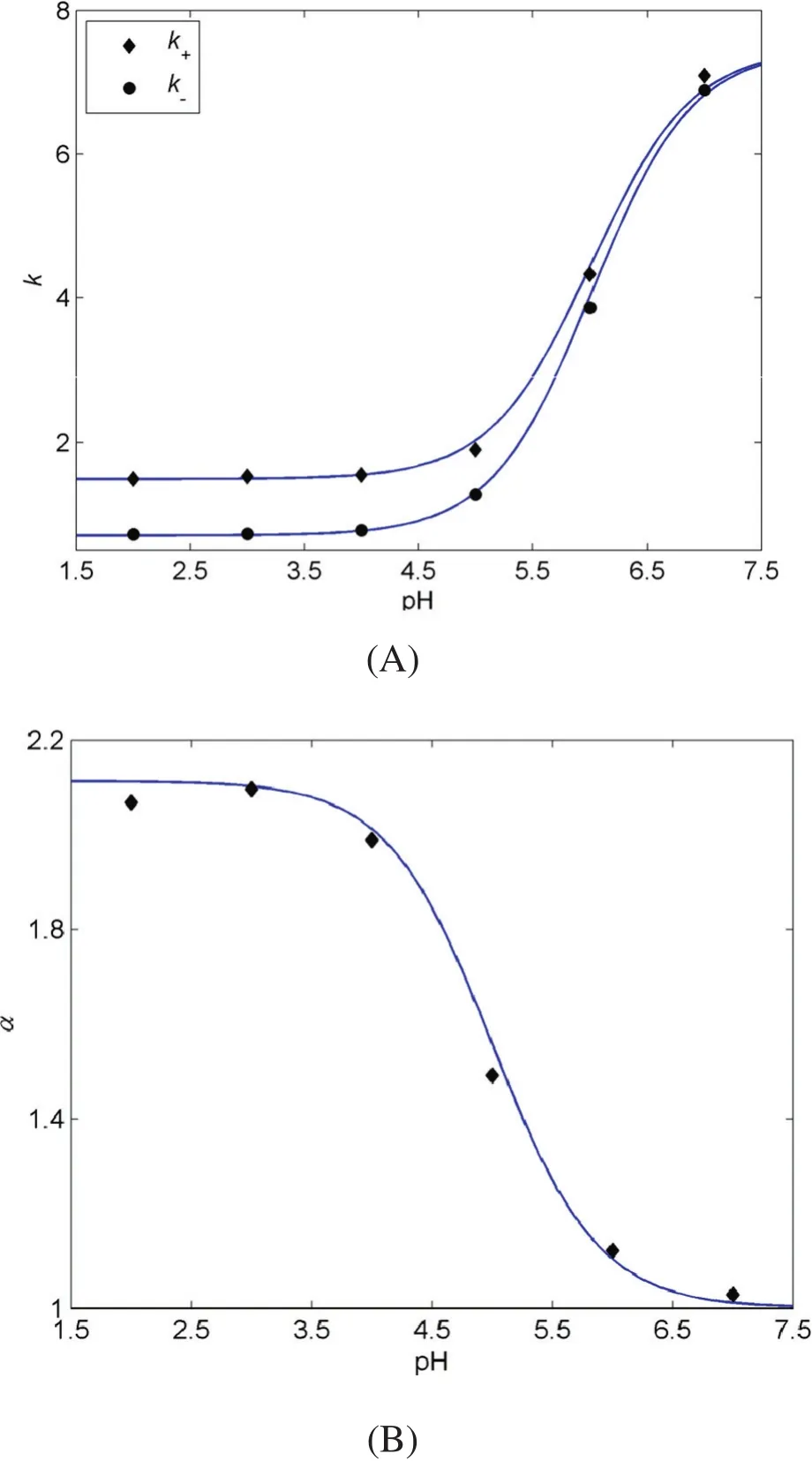
Fig.2.In fluence of pH on k(A)and α (B).([2-PBA]=0.01 mol·L-1,[HE-β-CD]=0.1 mol·L-1,T=5.0 °C.)
3.4.HE-β-CD concentration in fluence
The effect of the concentration of HE-β-CD on separation of 2-PBA enantiomers is researched with the concentration of HE-β-CD varying from 0 to 0.2 mol·L-1.A comparison between the experimental values and the model predictions on distribution ratio and enantioselectivity is also studied.Results show that the model predictions are consistent well with the experimental values as shown in Fig.3,and as shown by mean relative errors of 11.66%for k+,14.02%for k-,and 2.66%for α.
Italso can be seen from Fig.3(A)that k+and k-are oflinear proportion to the concentration of HE-β-CD.Fig.3(B)reveals that the operational enantioselectivity increases rapidly when the concentration of HE-β-CD is under 0.1 mol·L-1and then keeps nearly unchanged.Since HE-β-CD can include 2-PBA enantiomers to form complexes,the increase of the quantity of HE-β-CD promotes the generation of the inclusion complexes,which results in the increase of distribution ratio.Meanwhile the recognition ability of HE-β-CD toward 2-PBA enantiomers could be enhanced with a relatively higher concentration.Therefore,the increase of HE-β-CD concentration can make the enantioselectivity for 2-PBA enantiomers increase.

Fig.3.In fluence of HE-β-CD concentration on k(A)and α (B).([2-PBA]=0.01 mol·L-1,pH=3.00,T=5.0°C.)
3.5.In fluence of temperature
The effect of temperature on separation of 2-PBA enantiomers is investigated with a range of temperature from 5.0 °C to 35.0 °C.Fig.4 shows that the partition behavior is affected strongly by temperature.Both distribution ratios and enantioselectivity decrease with the temperature.The possible reason for this phenomenon is that the increase of temperature makes the host-guest interaction between HE-β-CD and 2-PBA enantiomers gradually weakened and the recognition ability of HE-β-CD is consequently reduced.In addition,the results are consistent with the van't Hoff model,indicating that the conformation and enantioselective interactions keep unchanged in the studied temperature range[32].
3.6.Model predictions
According to the above results,the modelestablished can predictthe separation efficiency of 2-PBA accurately.Therefore,the model can be employed to study how multifactor technical conditions affect the extraction efficiency in a single stage.
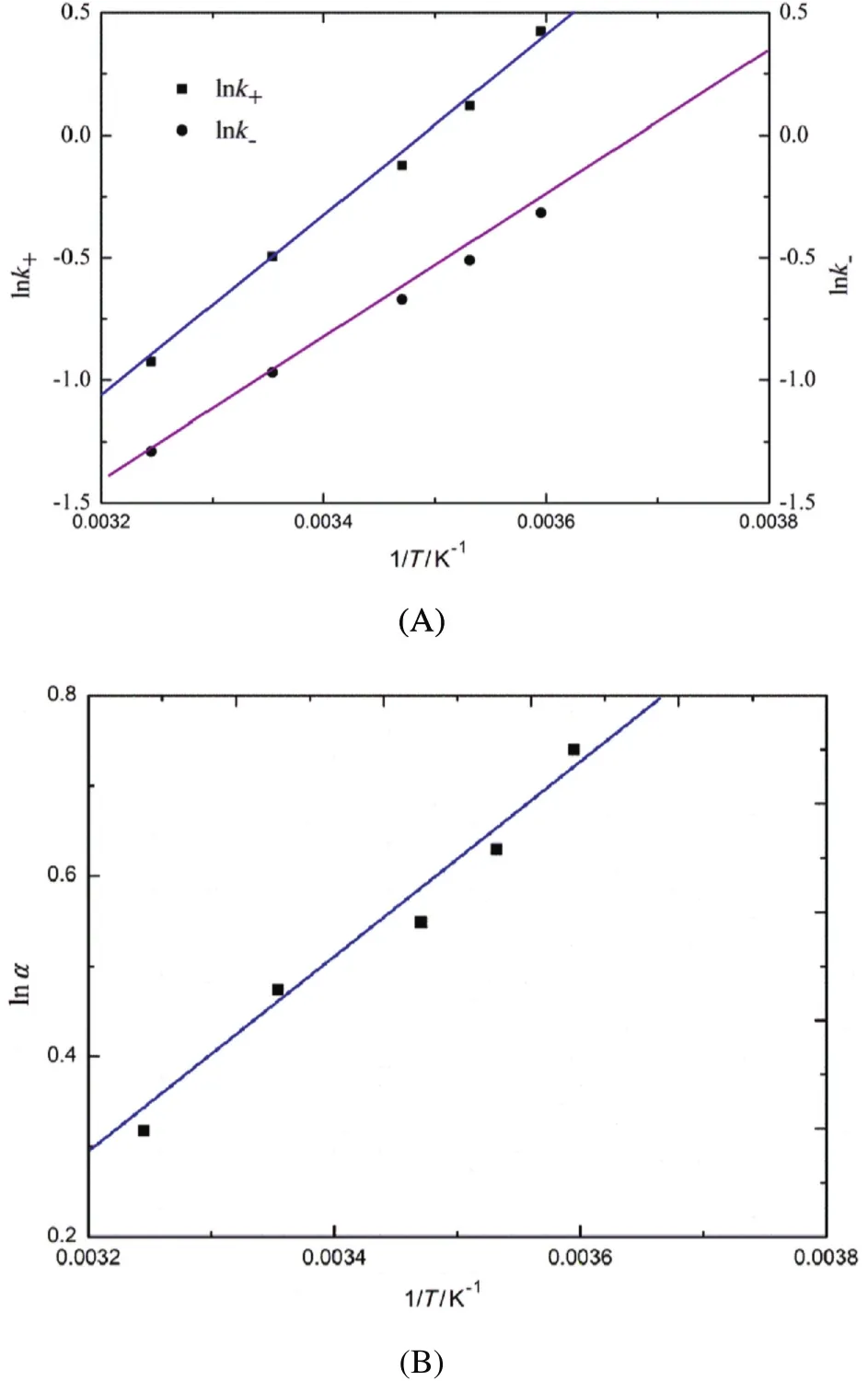
Fig.4.Plots ofln k+,ln k-(A)and lnα (B)versus 1/T.([2-PBA]=0.01 mol·L-1,[HE-β-CD]=0.1 mol·L-1,pH=3.00.)
Fig.5 depicts the tendency of the distribution ratios and enantioselctivity for 2-PBA enantiomers where they act as a function of pH and HE-β-CD concentration.As shown in Fig.5(A)and(B),a similar tendency could be observed in k+and k-when the pH value and HE-β-CD concentration are changed.The distribution ratios increase with the increase of pH and HE-β-CD concentration.What's more,a region with a high enantioselctivity will be obtained in which pH value is low and HE-β-CD concentration is high.
Fig.6 describes the variation of the enantiomeric excess(ee)in aqueous phase for 2-PBA enantiomers where it acts as a function of pH and HE-β-CD concentration.The decrease of the pH value always makes the ee value increased.However,the variation of ee with HE-β-CD concentration was not a simplex.With the augment of the HE-β-CD concentration,the ee value has a general uptrend firstly,and then begins to decrease after reaching the maximum.This phenomenon indicates that increasing the HE-β-CD concentration could bring out a surge in enantioselectivity but not always in enantiomeric excess.Thus,the pH value should be kept low and the HE-β-CD concentration should remain a suitable level to obtain relatively high ee.
It is difficult to apply Figs.5(C)and 6 to determine the optimized technical conditions for the resolution of 2-PBA enantiomers on account of the opposite tendency of the enantiomeric excess and enantioselectivity.Therefore,the performance factor(pfi)is recommended in this paper to further optimize this reactive extraction system,which is de fined as the Eq.(11).A high performance factor presents a higher purity and higher yield of the given enantiomer.

Fig.5.Calculated k+(A),k-(B),and α(C)for 2-PBA enantiomers as a function of pH and HE-β-CD concentration.
The effects of pH and HE-β-CD concentration on the performance factor are simulated and the results are revealed in Fig.7.The simulated results show thatpHand HE-β-CDconcentration affectthe performance factor obviously.As shown in Fig.7(A),pfichanges slowly when pH value is under 4.00,and then decreases rapidly with the pH value increased.In Fig.7(B),the rising of HE-β-CD concentration makes the pfiincreased firstly,and then decreased gradually.Thus,the lower pH and suitable HE-β-CD concentration should be kept aiming at large pfi.According to the simulation and optimization,the ideal experimental conditions are as follows:pH of 3.00,HE-β-CD concentration of 0.1 mol·L-1and temperature of 5.0 °C.

Fig.6.Calculated ee w as a function of pH and HE-β-CD concentration.
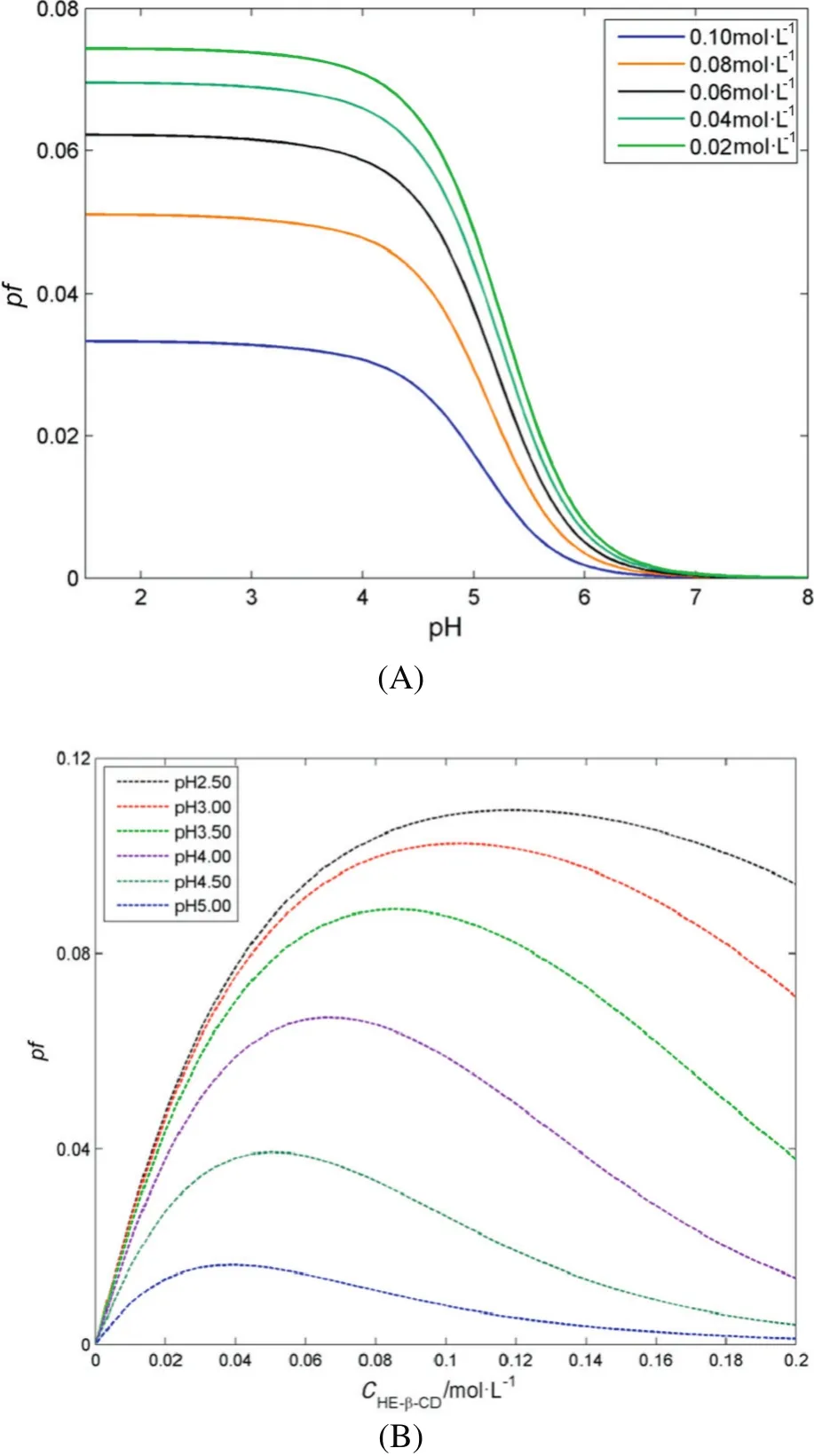
Fig.7.Calculated pf as a function of pH(A)and HE-β-CD concentration(B).([2-PBA]=0.01 mol·L-1,T=5.0 °C.)
4.Conclusions
Hydrophobic 2-PBA enantiomers can be enantioselectively extracted by hydrophilic selector β-CD derivatives.Through the physical partition equilibrium,chemical reaction equilibrium and mass balance,a mathematical model is built and this model can accurately predict the in fluence of experimental conditions on separation effects.The effect of single impact factor(e.g.,types of organic solvents and β-CD derivatives,concentration of the selector,pH,and temperature)on the separation efficiency was investigated experimentally and thatofmultifactor parameter on the separation efficiency was studied by model.Combining the experimental results and the simulated results,the optimized operation condition is obtained.Under the optimum conditions,the best enantioselectivity is obtained at 2.096.In order to make the 2-PBA enantiomers separated completely,the multistage extraction research will be performed in the future work.
Nomenclature
A+,-(±)-2-PBA
A+-CD complex of(+)-2PBA with HE-β-CD
A—CD complex of(+)-2PBA with HE-β-CD
C analytical concentration
CD cyclodextrin
CM-β-CD carboxymethyl-β-cyclodextrin
ELLE enantioselective liquid–liquid extraction
ee enantiomeric excess
HE-β-CD hydroxyethyl-β-cyclodextrin
HP-β-CD hydroxypropyl-β-cyclodextrin
K complexation equilibrium constants
Kadissociation constant
k distribution ratio
Piphysical partition coefficient of ionic(+)-and(-)-2-PBA
P0physical distribution coefficient of molecular(+)-and(-)-2-PBA
2-PBA 2-phenylbutyric acid
pf performance factor
SBE-β-CD sulfobutylether-β-cyclodextrin
T temperature
α enantioselectivity
[] equilibrium concentration
Subscripts
i index for+,-
w aqueous phase
o organic phase
0 initial value
+ (+)-2-PBA
- (-)-2-PBA
References
[1]I.W.Wainer,Drue stereochemistry,2nd ed.,Anal.Methods Pharmacol.,Marcel Dekker,New York,1993.
[2]H.Y.Aboul-Enein,I.W.Wainer,The ImpactofStereochemistry on Drug Development and Use,Wiley,New York,1997.
[3]G.T.Liu,K.Nagahama,Application of rapid expansion of supercritical solutions in the crystallization separation,Ind.Eng.Chem.Res.35(1996)4626–4634.
[4]T.J.Ward,B.A.Baker,Chiral separations,Anal.Chem.80(2008)4363–4372.
[5]C.A.M.Afonso,J.G.Crespo,Recent advances in chiral resolution through membranebased approaches,Angew.Chem.Int.Ed.43(2004)5293–5295.
[6]V.Prelog,M.Kovakevic,M.Egli,Lipophilic tartaric acid esters as enantioselective ionophores,Angew.Chem.Int.Ed.Engl.28(1989)1147–1152.
[7]K.W.Tang,J.M.Yi,Y.B.Liu,X.Y.Jiang,Y.Pan,Enantioselective separation of R,S-phenylsuccinic acid by biphasic recognition chiral extraction,Chem.Eng.Sci.64(2009)4081–4088.
[8]M.Steensma,N.J.Kuipers,A.B.de Haan,G.Kwant,In fluence of process parameters on extraction equilibria for the chiral separation of amines and amino-alcohols with a chiral crown ether,J.Chem.Technol.Biotechnol.81(2006)588–597.
[9]M.Pietraszkiewicz,M.Kozbia,O.Pietraszkiewicz,Chiral discrimination of amino acids and their potassium or sodium salts by optically active crown ether derived from D-mannose,J.Membr.Sci.138(1998)109–113.
[10]J.Koska,C.A.Haynes,Modelling multiple chemical equilbria in chiral partition systems,Chem.Eng.Sci.56(2001)5853–5864.
[11]B.Schuur,J.G.M.Winkelmam,H.J.Heeres,Equilibrium studies on enantioselective liquid-liquid amino acid extraction using a cinchona alkaloid extractant,Ind.Eng.Chem.Res.47(2008)10027–10033.
[12]H.I.Kim,K.W.Lee,D.Mishra,K.M.Yi,J.H.Hong,M.K.Jun,H.K.Park,Separation of molybdenum and vanadium from oxalate leached solution of spent residue hydrodesulfurization(RHDS)catalyst by liquid–liquid extraction using amine extractant,J.Ind.Eng.Chem.21(2015)1265–1269.
[13]N.Sunsandee,P.Ramakul,M.Hronec,U.Pancharoen,N.Leepipatpiboon,Mathematical model and experimental validation of the synergistic effect of selective enantioseparation of(S)-amlodipine from pharmaceutical wastewater using a HFSLM,J.Ind.Eng.Chem.20(2014)1612–1622.
[14]A.J.Hallett,G.J.Kwant,J.G.de Vries,Continuous separation of racemic 3,5-dinitrobenzoyl-amino acids in a centrifugal contact separator with the aid of cinchona-based chiral host compounds,Chem.A Eur.J.15(2009)2111–2120.
[15]B.Schuur,J.G.M.Winkelmam,H.J.Heeres,Equilibrium studies on enantioselective liquid-liquid amino acid extraction using a cinchona alkaloid extractant,Ind.Eng.Chem.Res.47(2008)10027–10033.
[16]X.Chen,J.Wang,F.Jiao,Ef ficient enantioseparation of phenylsuccinic acid enantiomers by aqueous two-phase system-based biphasic recognition chiral extraction:phase behaviors and distribution experiments,Process Biochem.9(2015)1468–1478.
[17]P.Zhang,J.Luo,K.Tang,J.Yi,C.Yang,Kinetics study on reactive extraction of d-p-hydroxyphenylglycine by BINAP–palladium complex in Lewis cell,Chem.Eng.Process:Process Intensif.93(2015)50–55.
[18]S.E.Evans,P.Davies,A.Lubben,B.Kasprzyk-Hordern,Determination of chiral pharmaceuticals and illicit drugs in wastewater and sludge using microwave assisted extraction,solid-phase extraction and chiral liquid chromatography coupled with tandem mass spectrometry,Anal.Chim.Acta 882(2015)112–126.
[19]A.Holbach,J.Godde,R.Mahendrarajah,N.Kockmann,Enantioseparation of chiral aromatic acids in process intensi fied liquid–liquid extraction columns,AIChE J.61(2015)266–276.
[20]B.J.Verkuijl,A.J.Minnaard,J.G.de Vries,B.L.Feringa,Chiral separation of underivatized amino acids by reactive extraction with palladium-BINAP complexes,J.Org.Chem.74(2009)6526–6533.
[21]K.Tang,G.Wu,P.Zhang,C.Zhou,J.Liu,Experimental and model study on enantioselective extraction of phenylglycine enantiomers with BINAP-metal complexes,Ind.Eng.Chem.Res.51(2012)15233–15241.
[22]M.Asztemborska,M.Miśkiewicz,D.Sybilska,Separation of some chiral flavanones by micellar electrokinetic chromatography,Electrophoresis 24(2003)2527–2531.
[23]C.Akbay,S.A.A.Rizvi,S.A.Shamsi,Simultaneous enantioseparation and tandem UV-MS detection of eight β-blockers in micellar electrokinetic chromatography using a chiral molecular micelle,Anal.Chem.77(2005)1672–1683.
[24]S.Fanali,Enantioselective determination by capillary electrophoresis with cyclodextrins as chiral selectors,J.Chromatogr.A 875(2000)89–122.
[25]K.Tang,H.Zhang,Y.Liu,Experimental and simulation on enantioselective extraction in centrifugal contactor separators,AIChE J.59(2013)2594–2602.
[26]G.Wang,L.Yang,F.Wu,N.Deng,Carboxymethyl-β-cyclodextrin enhanced TiO2removal of acid red R and lead ions in suspended solutions,J.Chem.Technol.Biotechnol.89(2014)297–304.
[27]Z.Ren,Y.Zeng,Y.Hua,Y.Cheng,Z.Guo,Enantioselective liquid-liquid extraction of racemic ibuprofen by L-tartatic acid derivatives,J.Chem.Eng.Data 59(2014)2517–2522.
[28]J.Szejtli,Cyclodextrins and their Inclusion Complexes,Akademiai Kiado,Budapest,1982.
[29]Y.Yamashoji,T.Ariga,S.Asano,M.Tanaka,Chiral recognition and enantiomeric separation of alanine β-naphthylamide by cyclodextrins,Anal.Chim.Acta 268(1992)39–47.
[30]K.Tang,P.Zhang,C.Pan,H.Li,Equilibrium studies on enantioselective extraction of oxybutynin enantiomers by hydrophilic-β-cyclodextrin derivatives,AIChE J.57(2011)3027–3036.
[31]Y.Zheng,J.Yan,S.Tong,X.Li,C.Chu,High performance liquid chromatographic enantioseparation of 2-phenylpropionic acid chiral mobile phase additive,Chin.J.Pharm.Anal.33(2013)827–830.
[32]T.O'Brien,L.Crocker,R.Thompson,K.Thompson,P.H.Toma,D.A.Conlon,B.Feibush,C.Moeder,G.Bicker,N.Grinberg,Mechanistic aspects of chiral discrimination on modi fied cellulose,Anal.Chem.69(1997)1999–2007.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- CFD modeling of turbulent reacting flow in a semi-batch stirred-tank reactor☆
- Synergistic and interference effects in coaxial mixers:Numerical analysis of the power consumption☆
- Atomic layer deposition of Al2O3 on porous polypropylene hollow fibers for enhanced membrane performances☆
- Experimental investigation and cost assessment of the salt production by solar assisted evaporation of saturated brine☆
- Preparation of PVC/PVP composite polymer membranes via phase inversion process for water treatment purposes
