Atomic layer deposition of Al2O3 on porous polypropylene hollow fibers for enhanced membrane performances☆
Xiaojuan Jia ,Zexian Low ,He Chen ,Sen Xiong ,Yong Wang ,*
1 State Key Laboratory of Materials-Oriented Chemical Engineering,Jiangsu National Synergetic Innovation Center for Advanced Materials,and College of Chemical Engineering,Nanjing Tech University,Nanjing 210009,China
2 Department of Chemical Engineering,Monash University,Clayton,Australia
1.Introduction
Polypropylene hollow fiber(PPHF)membranes have been used in ultra filtration(UF)and microfiltration(MF)for water treatment in a number of fields,due to their brilliant mechanical properties,low cost,good chemical resistance and thermal stability[1–4].Compared to flat sheet membranes,hollow fiber configuration are preferred due to its highly effective filtration area per unit volume,simple module fabrication,no requirement of feed and permeance and less prone to fouling or concentration polarization[5].Recent studies have concentrated on the hollow fiber form of PP membranes due to its aforementioned advantages[6,7].For example,PPHF membranes were applied in the pervaporation separation of water/ethanol mixtures and the separation of anionic dye aqueous solution,both exhibiting remarkable long-term performance stability and anti-fouling performance[8,9].
However,PPHF membranes surface fouling remains an ongoing challenge due to the hydrophobicity and leads to decline of water permeance[10].A variety of strategies have been applied to modify surface properties of PPHF membranes to improve their anti-fouling properties[11–13].For instance,surface graft polymerization is applied to improve the anti-fouling and separation performances of the membranes without undermining their bulk properties[14–16].Surface graft polymerization requires the existence of reactive groups on the membrane surface,which is mainly generated from chemical treatments,such as plasma deposition,UV irradiation,and γ-ray irradiation[17–21].Unfortunately,these treatments require harsh pretreatment or complex synthesis procedure.In addition,the grafting reaction in liquid solution is difficult to control,which may lead to blockage of the membrane pores.More importantly,large volume oforganic wastewater was produced during these processes[22,23].Therefore,it is necessary to develop a green and simple processing alternative to enhance the permeability and anti-fouling property ofPPHF membrane.
Atomic layer deposition(ALD)is usually applied to deposit conformal and ultrathin films by the sequential surface reactions with atomic level control on membranes to modify membrane surface property and separation performance[24–26].ALD can be operated at low temperature and is particularly appropriate for the porous polymer membranes modi fication without degrading the polymer[27–29].Li etal.employed ALD on track-etched polycarbonate(PCTE)membranes to tailor the membrane pore size by altering the ALD cycles[30].Jung et al.[31]applied to deposit Al2O3on polypropylene separators to significantly improve their wettability towards electrolytes and thermal stability of the safety batteries.Previous work reported that ALD was certified to be significantly effective method to modify the polymeric membranes.The membrane hydrophilicity of the most common membranes including polytetra fluoroethylene[32],polyvinylidene fluoride[33],and PP[34,35]were enhanced with subsequent ALD of metal oxide,with simultaneous improvement to the water permeance and solute retention.However,ALD technique has only been used to modify flat sheet polymeric membranes so far[32–35].The feasibility of the use of ALD technique to improve the performance of hollow fiber membrane configuration is highly interested.
In this work,we applied ALD technique to deposit a thin layer of Al2O3on commercial porous PPHF membranes to improve their anti-fouling property,stability and separation performance.This is a proof-of-concept work to demonstrate the possibility of upgrading the performances of HF membranes by ALD.The ALD of Al2O3is the most extensively studied system in the ALD of different metal oxides,and it has been shown that ALD of Al2O3can be easily performed on different substrates,enabling us to have more references and comparisons in our efforts to upgrade PPHF membranes by ALD.Therefore,Al2O3is chosen as a model oxide to deposit on PPHF membranes,and other metal oxides are expected to be also applicable.To the best of our knowledge,this work denotes the first successful application of ALD technique.By controlling the ALD deposition parameters,we could retain the asymmetrical structure of the Al2O3-modi fied PPHF membranes.In addition,it is possible to prepare an appropriate Al2O3deposition layer on porous PPHF membranes with turning the thickness and morphology by controlling the deposition cycles.
2.Experimental
2.1.Materials
PPHF membranes(outer diameter:0.38 mm,inner diameter:0.33 mm,wall thickness:25 μm,mean pore size:200 nm,Nanjing O'Happure Membranes Co.)were applied as substrates for Al2O3ALD in present work.Trimethylaluminum(TMA,Metalorganic Center,99.99%,Nanjing University)and Deionized water(H2O)were used as oxygen supply for the Al2O3ALD process,respectively.Ultrahigh purity N2(Tianhong Gas Company,99.99%)was applied as the precursors carrier and purging gas.Ethanol(99.7%,Yasheng Chemical Co.LTD)was used as received.Silicon wafers(SY100W-01,Seyang Electronics)were cleaned with ethanol and used to monitor the growth rate of Al2O3thickness during ALD process.Bovine serum albumin(BSA),(Mw=67 kDa,97%,GM Corporation)solution was employed to assess the discrimination and fouling resistance of both the pristine and the modi fied PPHF membranes.
2.2.ALD of Al2O3 on PPHF membranes
Both the pristine membranes and silicon wafers were fixed in the ALD reactor(Savannah S100,Cambridge Nano-Tech)under vacuum(~26.66 Pa).The metal precursor(TMA)and oxidant precursor(H2O)vapors were delivered into the reactor sequentially by the nitrogen gas.Exposure mode was introduced in this work.In a typical ALD cycle,pulse times of both precursors were 0.015 s.After each pulse of precursors,to guarantee abundant precursor adsorption and diffusion,5-s exposure time was used,and then the system was purged under N2for 30 s to wipe out the excess precursor as well as the byproducts in the system.The reaction was repeated for different ALD cycles to produce Al2O3-PPHF membrane of different skin thickness as indicated.
2.3.Characterizations
The thicknesses and refractive indices of Al2O3film on the Si wafer were used a variable-angle spectroscopic ellipsometer(VASE,J.A.Wollam Co.).To investigate the changes in chemical structure and to confirm investigate the successful deposition of Al2O3between the pristine and the Al2O3-deposited PPHF membranes,Fourier Transform Infrared(FTIR)spectra of the PPHF membranes were measured using FTIR spectrometer in attenuated total re flection(ATR)mode(Nicolet 8700).The spectra were calculated within the wavenumber range of 500–4000 cm-1.The Energy Dispersive X-ray Spectrometer(EDS)was used to detect the distribution of the cross-sectional and surface elemental quantification of the membranes(EMAX X-act).The thermal analyzer was implemented to analyze thermalgravimetric(TG)of the PPHF membrane(NETZSCH TG209F1).A heating rate was set at 10 °C·min-1from 20 to 600 °C in O2atmosphere.The surface morphology of the PPHF membranes was inspected under a field emission scanning microscope(FESEM,Hitachi S4800)operated at 5 kV.To avoid electron charging,Au/Pd thin layer was sputtering-coated on the sample surface prior to SEM observation.The electronic tensile tester was applied to measure the mechanical properties of the PPHF membranes(CMT-6203,Shenzhen Sans Test Machine Co.).The specimens were used in the length of 50 mm.A tensile rate of the machine was 20 mm·min-1.Static protein adsorption was performed to estimate the anti-fouling properties of the Al2O3-deposited membranes and the pristine membrane was used as reference.The protein adsorption quantity on the membrane surface was measured by the decreased the BSA concentration.The concentrations of the BSA solutions were calculated using an ultraviolet(UV)spectrometer on the absorbance at 280 nm(Thermo Fisher NanoDROP 2000C).The pristine and the modi fied PPHF membranes were separately put into a test tube that was filled with 7 ml of 0.5 g·L-1BSA phosphate buffer.Both ends of the PPHF membranes were not submerged into the solution.Then,the glass tube was incubated in aqueous bath at 25°C for 12 h.The adsorption quantity of BSA on the membrane surface was measured from the declined BSA concentration.The following equation was used to obtain the amount of the membranes surface absorption M(μg·cm-2)

where V is the volume of BSA phosphate buffer(ml);Cpis the feed concentration of BSA solution,and Cfis the permeate concentration of BSA solution;A is the external surface area of membranes(cm2).
2.4.Membrane performances
The pure waterpermeance ofPPHF membranesand retention ofBSA were measured in a homemade apparatus at room temperature.The PPHF membranes with certain length were folded and their both ends were fixed in a transparent hose by epoxy resin,and the hose housing the PPHF membranes was connected to the homemade apparatus as testing module.The PPHF membranes were prewetted in ethanol for 1 min before the permeation test,then the membranes were compacted at 1 0.1MPa for 10 min to acquire a stable flux,before measuring the pure water permeance(PWP)at the same pressure.The following equation was used to obtain the 10PWP(L·m-2·MPa-1·h-1)of the membranes:

where Q is the net water permeability(L);A is the external surface area(m2);ΔP is the transmembrane pressure(MPa);t is the operation time(h).
The BSA solution was applied to measure the BSA retention of pristine and the modi fied PPHF membranes;0.5 g BSA was dissolved in 1 L phosphate buffer solution.The concentrations of BSA solution in both the feed and permeate were calculated by UV spectrometer.The rejection R(%)of BSA was de fined by following this equation:

where Cp(g·L-1)is the feed concentration ofBSAsolution;Cf(g·L-1)is the permeate concentration of BSA solution.
3.Results and Discussion
3.1.Deposition of Al2O3 on the PPHF membranes
The formation ofAl2O3layer on the PPHF membranes was measured by FTIR spectroscopy.In Fig.1,the peaks of PP(2800—2960 cm-1,and 1500—1402 cm-1)were observed in all samples before and after the Al2O3deposition.No detectable new peaks could be observed for the 50-cycle deposition on the membrane.However,a new absorption peak appeared around 650—700 cm-1after 100 or more deposition cycles,which can be attributed to the Al--O bond[36].It was viewed that the peak intensity increased with the numberofdeposition cycles,since the membrane with 400 deposition cycles demonstrated a considerably stronger peak compared to the samples with 100 and 200 deposition cycles,indicating that the quantity of Al2O3increased with the increment of ALD deposition cycles.In addition,the peak intensity of PP situated at 2800—2960 cm-1reduced after the Al2O3deposition,ascertaining the deposition of Al2O3on the surface of the membranes which diminished PP absorption peaks.
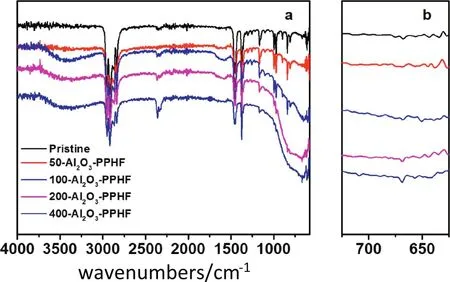
Fig.1.(a)FTIR spectra of the pristine and Al2O3-PPHF membranes with varying ALD deposition cycles.(b)shows the enlarged wavenumbers located around the 600–700 cm-1.
The EDS result shows the distribution of Al element on both sides of the membrane surfaces and indicates the selective coverage of Al2O3on the membrane surfaces.In Fig.2b and c,EDS mapping of the crosssectionalsamples veri fied thatalumina was also deposited in the interior of the membranes.Signals of Al element dated from the Al2O3-PPHF membrane were presented throughout the entire cross section with 200 deposition cycles,with the Alelementdensity decreasing gradually from the outersurface to the inner surface(Fig.2b).The result was confirmed from the EDS mapping of the outer and inner surface of PPHF(Fig.S1).The gradient distribution of Al2O3was due to the decreasing surface pores with increasing ALD cycles,resulting in the decreased diffusion of precursors through the membrane and into the inner regions of the membrane.
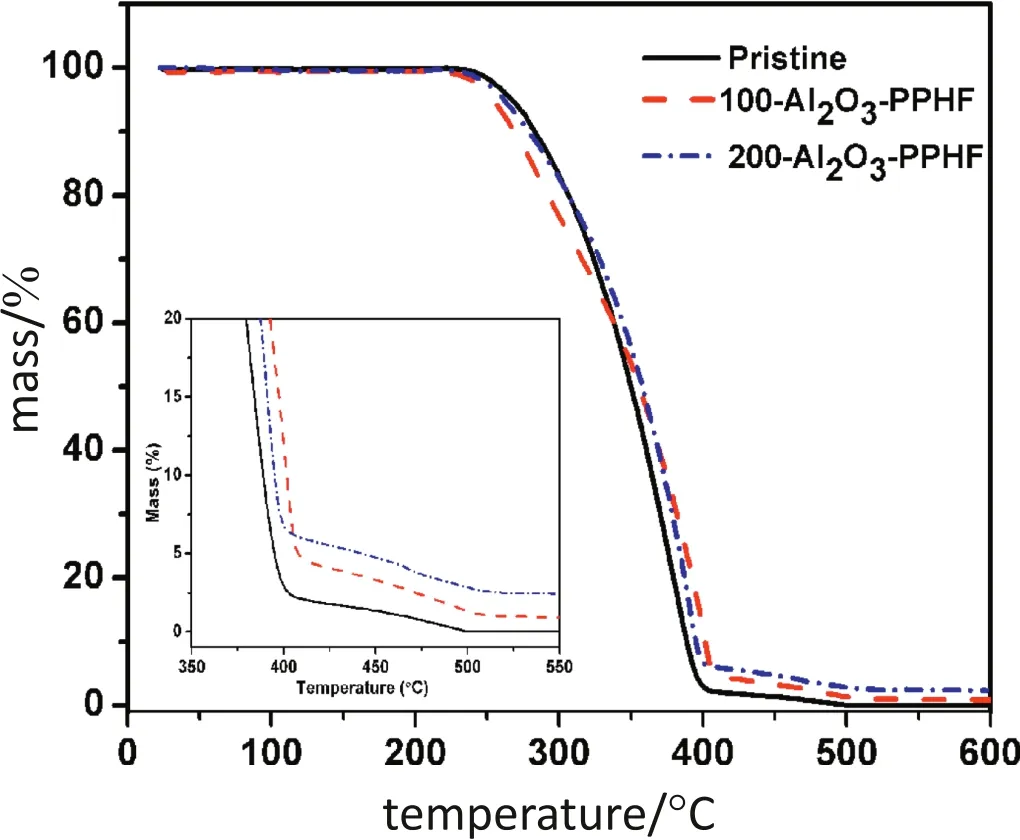
Fig.3.TGA curves of the pristine and the Al2O3-PPHF membranes.Inset shows the mass change around 350—550 °C.
TG analysis was applied to verify the Al2O3layer successful deposition on the PPHF membranes.The residual mass of the pristine membranes and the Al2O3-PPHF membranes were measured.As shown in Fig.3,the degradation of Al2O3-PPHF membranes occurred in three steps.In the first step,Al2O3-PPHF membranes barely displayed any mass change from 20 °C to 230 °C.In the second step,the membranes started to degrade at the temperature higher than 230°C until completion at420°C.In the third step,the Al2O3-PPHF exhibited minorchanges to residual mass,suggesting that the PP components were completely degraded before 600°C.The residual mass of the pristine PPHF membranes after pyrolysis at 600°C was nearly negligible while the membranes with 100 and 200 deposition cycles possessed a residual mass rate of 0.87%and 2.35%,respectively.The increased residual mass of the membranes subjected to ALD deposition cycles once again confirmed that Al2O3was successfully deposited onto the membranes.The per cycle of mean mass in the first 100 cycles,and 100—200 cycles were determined to be 0.0087%and 0.0148%,respectively.The lower mean mass uptake per cycle was due to the deposition of Al2O3in the inner region of the polymer matrix,which restricted the growth of the deposited Al2O3.Continuous deposition of Al2O3leads to surface deposition of Al2O3on the surface which can grow more rapidly than the Al2O3precursors in the polymer matrix[27,32].Furthermore,the TGA data revealed that both the pristine and Al2O3-PPHF membranes exhibited similar mass loss at 230°C.So the Al2O3layer retained the thermal stability of the membranes.
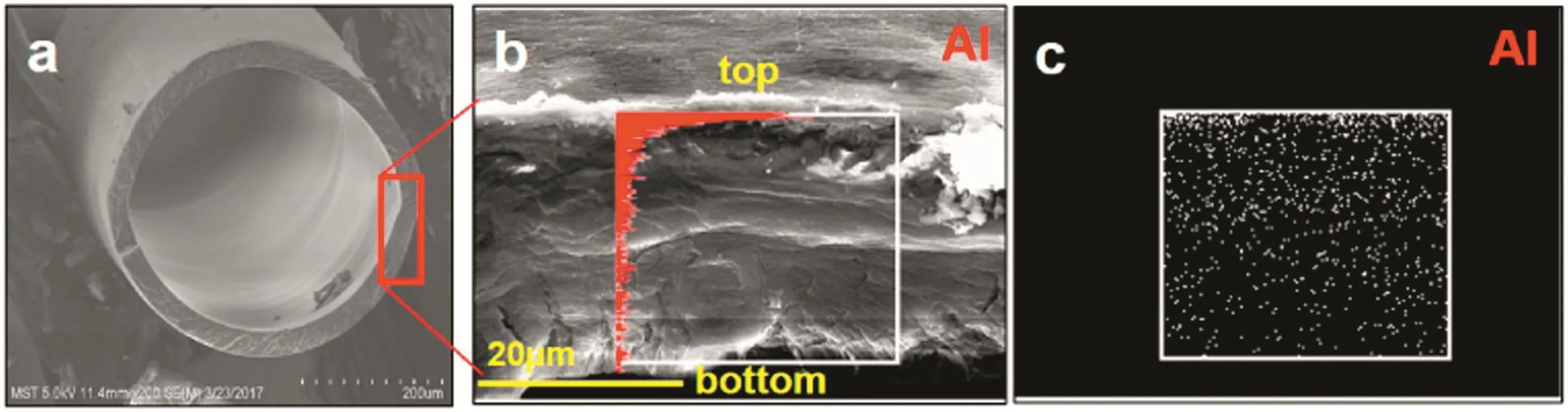
Fig.2.Cross section of PPHF with 200 ALD cycles(a,b).Inset shows the distribution of Al element across the membrane.(c)EDS mapping of the region highlighted in(b).
3.2.Tailorability of PPHF membranes morphology
SEM was performed to inspect the outer surface morphology and pore structure of the pristine and the Al2O3-PPHF membranes.The outer surface of the pristine membranes exhibited a smooth net-like structure without any noticeable particulates(Fig.4a).Compared to the pristine membrane,after 50 deposition cycles of Al2O3(Fig.4b),there was no obvious change in the outer surface morphology.Nevertheless,as the number ofALDcycles was increased,some smallgranules appeared on the outer surface of the membranes(Fig.4c).When ALD of Al2O3increased to 100 cycles,Al2O3particulates with larger diameter were observed.The outer surface of the membrane was gradually covered by Al2O3nanoparticles and became coarser after 200 and 300 deposition cycles of Al2O3(Fig.4d and e).The pores of the membranes were almost completely blocked after 400 deposition cycles of Al2O3since no pores can be observed at the same magnification(Fig.4f),indicating the mean pore size of PPHF membranes can be controlled by changing the ALD cycles.In Fig.4,there were additional particulates on the outer surface of the Al2O3-PPHF membranes,implying that the growth of surface reaction mechanism followed the self-limiting of ALD technique[29].In addition to the pore sizes,the outer surface morphologies and the shape of pores were retained.It was previously observed that the Al2O3grew conformably along the pore wall during ALD[37,38].As shown in Fig.S2,the growth of Al2O3on silicon wafer was linearly and the calculated growth rate was~0.13 nm per cycle.Therefore,the outer surface pore size ofthe membranes can be precisely tailored by controlling the ALD cycles.
3.3.Significantly upgrading of mechanical property of PPHF membranes
The mechanical properties of membranes are one of the significant factors in membrane application processes.The membranes needed suf ficient mechanical strength to withstand membrane operating and cleaning conditions.Fig.5 shows the strain curves of pristine and the Al2O3-PPHF membranes.The ultimate stress was determined to be~2.0 MPa and ~1.9 MPa for PPHF membranes that underwent 100 and 200 ALD cycles,respectively,as compared to~1.7 MPa of the pristine membrane.Furthermore,the membranes exhibited significant increase in elongation at break(Table S1),the maximum elongation of 100 and 200 Al2O3-PPHF membranes were increased to~234.8%and~233.4%,respectively,which was superior to the pristine membrane(~37%).After Al2O3deposition,as we discussed before,there was a subsurface nucleation stage during the ALD process,the high reactivity TMA precursors can in filtrate into PPHF,locating in the defect sites and chain-end parts with--CH3[39].Subsequently,the absorbed TMA will react with water vapor and produce Al2O3nucleation clusters or react with polymer chains to form the C--Al--C bond[40].Thus,the mechanicalpropertiesofthe Al2O3-PPHFwere enhanced,and the ductility ofthe Al2O3-deposited membranes was highly increased[41].This result could potentially play a part in improving the strength of Al2O3-PPHF membranes.
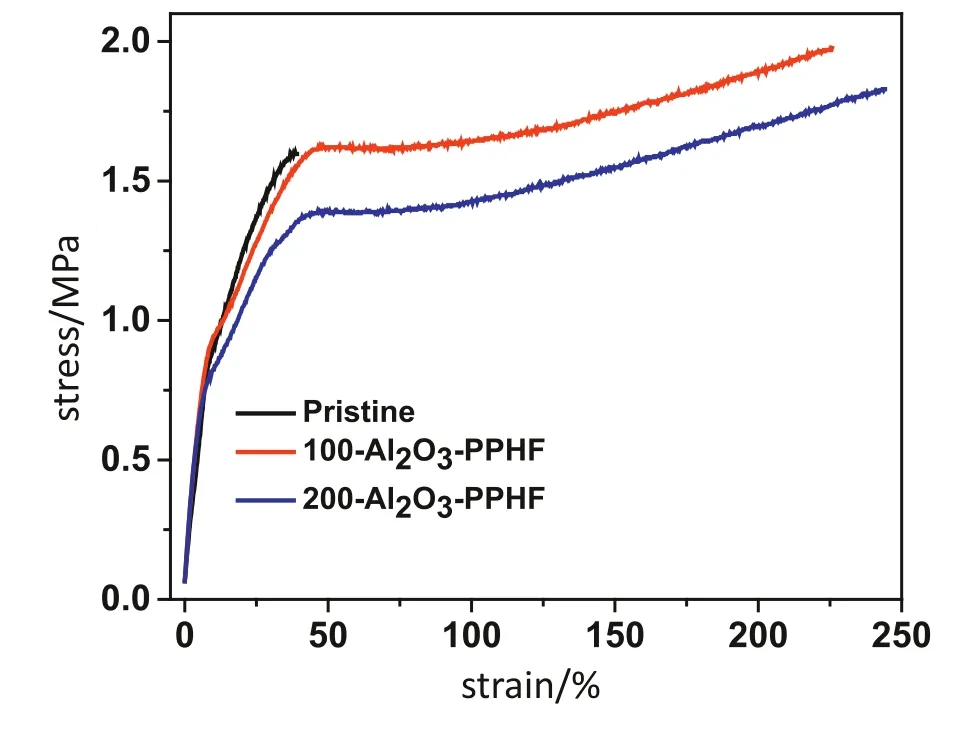
Fig.5.Mechanical properties of the pristine and the Al2O3-PPHF membranes subjected to 100 and 200 ALD.
3.4.Enhanced separation performances of Al2O3-PPHF membranes
Fig.6 presents the permeance and the BSA retention of the PPHF membranes with different ALD cycles.The permeance of the pristine membrane was about 2438 L·m-2·h-1·MPa-1,and the BSA retention was ~15.6%.Maximum permeance of 2849 L·m-2·h-1·MPa-1(increment of 17%)and one-fold increase in BSA rejection to 28%were attained after 50 ALD cycles.Interestingly,both the permeability and BSA retention were enhanced simultaneously with the increasing ALD cycles.However,higher deposition cycles(>150 cycles)resulted in a significant permeability loss.The permeance of Al2O3-PPHF membranes were governed by the pore size and the surface hydrophilicity[32].Our previous work indicated that the permeability of the porous membrane can be improved by increasing ALD cycles[33].However,further increasing the ALD cycles led to lower mean pore size of the Al2O3-PPHF membranes.Beyond 150 cycles,the reduction of the pore size offset the increase in membrane surface hydrophilicity,resulting in a lower permeance.On the other hand,the BSA retention increased with the ALD deposition cycles due to the smaller pore sizes.The BSA retention was increased to~86.1%after the 300 cycles of ALD.The results were consistent with that of the other works[30,33,35].
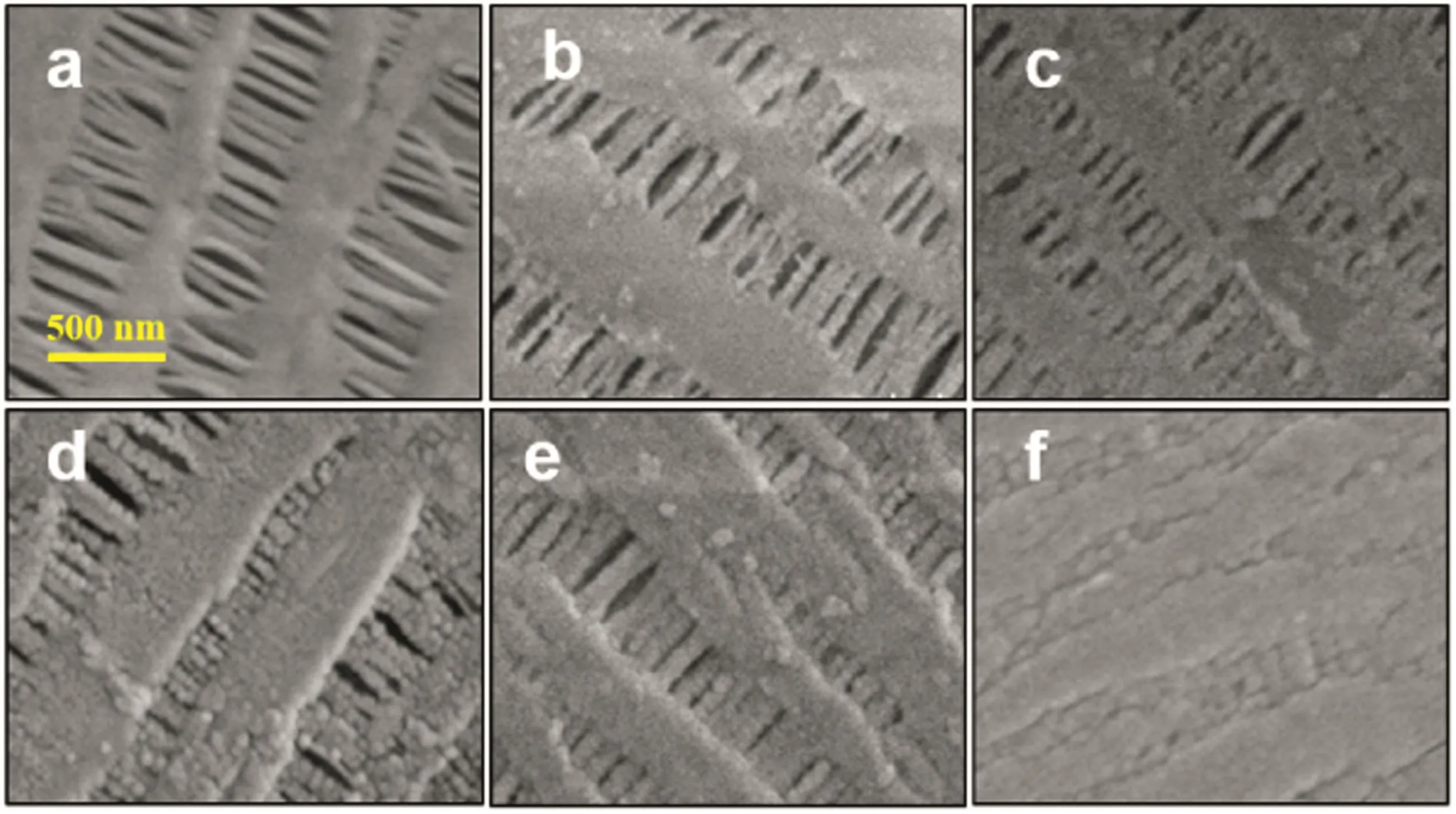
Fig.4.SEM pictures of the pristine(a)and the Al2O3-deposited PPHF membranes with a deposition cycle number of(b–f)50,100,200,300,400.All pictures have the same scale bar as shown in(a).
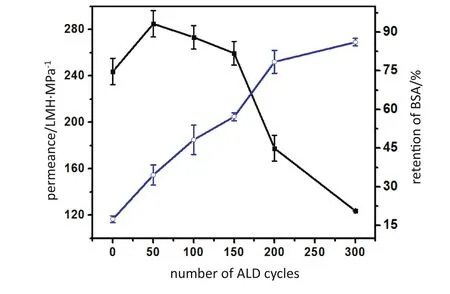
Fig.6.Permeance and BSA retention of the pristine membrane and Al2O3-PPHF membranes with varying ALD cycles.
3.5.Improvement of fouling-resistance of the Al2O3-PPHF membranes
The static protein adsorption was performed using BSA solution to estimate the anti-fouling property of Al2O3-PPHF membranes.Fig.7 shows the BSA adsorption of the Al2O3-PPHF membranes under increasing ALD cycles.The BSA adsorption of Al2O3-PPHF membranes decreased under increasing number of ALD cycle.Our previous work reported that the contact angle of Al2O3-PP flat membranes dropped to 70°after 400 cycles[35].BSA adsorption of 77 μg·cm-2was observed for the pristine membranes which can be contributed to the strong hydrophobic surface interaction of proteins.After ALD deposition of Al2O3,there was a progressive reduction in BSA adsorption with rising cycle numbers.A minimal BSA adsorption of 32 μg·cm-2was obtained at ALD cycle of 400,indicating the anti-fouling property of membranes was improved.Therefore,the anti-fouling property of PPHF membranes can be improved by increasing the deposition of Al2O3.
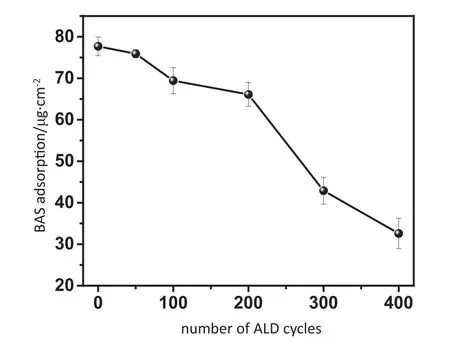
Fig.7.Adsorption of BSA onto Al2O3-PPHF membranes with increasing ALD cycles.
4.Conclusions
In the present study,we have provided a green and simple method to upgrade the performances of hydrophobic hollow fiber membranes based on atomic layer deposition of metal oxides.Asymmetric pore structure with tailorable pore size is successfully fabricated on the PPHF membranes.The PPHF membranes surface has been modi fied by producing a uniform coating of Al2O3layer on the outer surface of membrane.The mechanical properties(tensile strength and rate of elongation)are increased after the Al2O3-deposited PPHF membranes.Especially,the break of elongation of the PPHF membranes is signi ficantly improved from~37%for the pristine membrane to~235%after 100 cycles ALD deposition.The membrane permeance and rejection can be tailored by controlling the ALD cycle.The water permeance is increased and then decreased while the BSA retention keeps increasing with rising ALD cycle numbers.That is,water permeance and rejection are simultaneously enhanced at moderate ALD cycle numbers.Modi fied the membrane surface by Al2O3deposition is endowed less adsorption of BSA.Based on the striking effects of Al2O3deposition on PPHF membranes,the oxide-coated polymer hollow fiber membranes exhibit enhanced separation performance and membrane stability,we anticipate that the ALD could be used to deposit other metal oxides with superior chemical stability,such as TiO2or ZrO2,on the PPHF or other hollow- fiber membranes to further enhance their separation performances and also to broaden their applications in different fields.
Supplementary Material
Supplementary data to this article can be found online athttps://doi.org/10.1016/j.cjche.2017.10.008.
References
[1]T.Gullinkala,Isabel Escobar,Study of the hydrophilic enhanced ultra filtration membrane,Environ.Prog.27(2)(2008)210–217.
[2]H.Strathmann,Membrane separation processes:current relevance and future opportunities,AIChE J.47(5)(2001)1077–1087.
[3]K.G.S.T.A.Beltsios,K.L.Stefanopoulos,Membrane Science and Applications.Handbook of Porous Solids,2002 2281–2433.
[4]P.Vandezande,L.E.Gevers,I.F.Vankelecom,Solvent resistant nano filtration:separating on a molecular level,Chem.Soc.Rev.37(2)(2008)365–405.
[5]J.Mulder,Basic Principles of Membrane Technology,Springer Science&Business Media,2012.
[6]F.Yao,G.D.Fu,J.Zhao,E.T.Kang,K.G.Neoh,Antibacterial effect of surfacefunctionalized polypropylene hollow fiber membrane from surface-initiated atom transfer radical polymerization,J.Membr.Sci.319(1–2)(2008)149–157.
[7]Z.Xu,J.Wang,L.Shen,D.Men,Y.Xu,Microporous polypropylene hollow fiber membrane:part I.Surface modi fication by the graft polymerization of acrylic acid,J.Membr.Sci.196(2)(2002)221–229.
[8]S.Yu,Z.Chen,Q.Cheng,Z.Lü,M.Liu,C.Gao,Application of thin- film composite hollow fiber membrane to submerged nano filtration of anionic dye aqueous solutions,Sep.Purif.Technol.88(2012)121–129.
[9]Z.Xu,Microporous polypropylene hollow fiber membranes part II.Pervaporation separation of water/ethanol mixtures by the poly(acrylic acid)grafted membranes,J.Membr.Sci.214(1)(2003)71–81.
[10]I.C.Escobar,E.M.Hoek,C.J.Gabelich,F.A.DiGiano,Committee report:recent advances and research needs in membrane fouling,Am.Water Work.Assoc.J.97(8)(2005)79.
[11]F.Penacorada,A.Angelova,H.Kamusewitz,J.Reiche,L.Brehmer,Scanning force microscopy and wetting study of the surface modi fication of a polypropylene membrane by means of Langmuir-Blodgett film deposition,Langmuir 11(2)(1995)612–617.
[12]C.G.P.H.Schroën,M.C.Wijers,M.A.Cohen Stuart,A.van der Padt,K.van't Riet,Membrane modi fication to avoid wettability changes due to protein adsorption in an emulsion/membrane bioreactor,J.Membr.Sci.80(1)(1993)265–274.
[13]H.Yu,Y.Xie,M.Hu,J.Wang,S.Wang,Z.Xu,Surface modi fication of polypropylene microporous membrane to improve its antifouling property in MBR:CO2plasma treatment,J.Membr.Sci.254(1–2)(2005)219–227.
[14]K.Kato,E.Uchida,E.Kang,Y.Uyama,Y.Ikada,Polymer surface with graft chains,Prog.Polym.Sci.28(2)(2003)209–259.
[15]B.Boutevin,J.J.Robin,N.Torres,J.Casteil,Graft copolymerization of styrene onto ozonized polyethylene,Macromol.Chem.Phys.203(1)(2002)245–252.
[16]E.Ruckenstein,Z.Li,Surface modi fication and functionalization through the selfassembled monolayer and graft polymerization,Adv.Colloid Interf.Sci.113(1)(2005)43–63.
[17]X ZK.,Covalent attachment of phospholipid analogous polymers to modify a polymeric membrane surface:a novel approach,Langmuir 20(2004)1481–1488.
[18]Y.Yang,Y.Li,Q.Li,L.Wan,Z.Xu,Surface hydrophilization of microporous polypropylene membrane by grafting zwitterionic polymer for anti-biofouling,J.Membr.Sci.362(1–2)(2010)255–264.
[19]R.Kou,Z.Xu,H.Deng,Z.Liu,P.Seta,Y.Xu,Surface modi fication of microporous polypropylene membranes by plasma-induced graft polymerization of α-allyl glucoside,Langmuir 19(17)(2003)6869–6875.
[20]Y.Wang,J.Kim,K.Choo,Y.Lee,C.Lee,Hydrophilic modi fication of polypropylene microfiltration membranes by ozone-induced graft polymerization,J.Membr.Sci.169(2)(2000)269–276.
[21]Z.Liu,Z.Xu,J.Wang,J.Wu,J.Fu,Surface modi fication of polypropylene microfiltration membranes by graft polymerization of N-vinyl-2-pyrrolidone,Eur.Polym.J.40(9)(2004)2077–2087.
[22]D.Garg,W.Lenk,S.Berwald,K.Lunkwitz,F.Simon,K.Eichhorn,Hydrophilization of microporous polypropylene Celgard®membranes by the chemical modi fication technique,J.Appl.Polym.Sci.60(12)(1996)2087–2104.
[23]E.Gabriel,G.Gillberg,In situ modi fication ofmicroporous membranes,J.Appl.Polym.Sci.48(12)(1993)2081–2090.
[24]S.M.George,Atomic layer deposition:an overview,Chem.Rev.110(2010)111–131.
[25]B.Gong,G.N.Parsons,Quantitative in situ infrared analysis of reactions between trimethylaluminum and polymers during Al2O3atomic layer deposition,J.Mater.Chem.22(31)(2012)15672.
[26]A.Niskanen,K.Arstila,M.Leskelä,M.Ritala,Radical enhanced atomic layer deposition of titanium dioxide,Chem.Vap.Depos.13(4)(2007)152–157.
[27]C.A.Wilson RKG,S.M.George,Nucleation and growth during Al2O3atomic layer deposition on polymers,Chem.Mater.17(2005)5625–5634.
[28]M.Kemell,E.Färm,M.Ritala,M.Leskelä,Surface modi fication of thermoplastics by atomic layer deposition of Al2O3and TiO2thin films,Eur.Polym.J.44(11)(2008)3564–3570.
[29]G.N.Parsons,S.E.Atanasov,E.C.Dandley,C.K.Devine,B.Gong,J.S.Jur,et al.,Mechanisms and reactions during atomic layer deposition on polymers,Coord.Chem.Rev.257(23–24)(2013)3323–3331.
[30]F.Li,L.Li,X.Liao,Y.Wang,Precise pore size tuning and surface modi fications of polymeric membranes using the atomic layer deposition technique,J.Membr.Sci.385–386(2011)1–9.
[31]Y.S.Jung,A.S.Cavanagh,L.Gedvilas,N.E.Widjonarko,I.D.Scott,S.Lee,et al.,Improved functionality of lithium-ion batteries enabled by atomic layer deposition on the porous microstructure of polymer separators and coating electrodes,Adv.Energy Mater.2(8)(2012)1022–1027.
[32]Q.Xu,Y.Yang,X.Wang,Z.Wang,W.Jin,J.Huang,et al.,Atomic layer deposition of alumina on porous polytetra fluoroethylene membranes for enhanced hydrophilicity and separation performances,J.Membr.Sci.415–416(2012)435–443.
[33]Q.Wang,X.Wang,Z.Wang,J.Huang,Y.Wang,PVDF membranes with simultaneously enhanced permeability and selectivity by breaking the tradeoff effect via atomic layer deposition of TiO2,J.Membr.Sci.442(2013)57–64.
[34]Q.Xu,J.Yang,J.Dai,Y.Yang,X.Chen,Y.Wang,Hydrophilization of porous polypropylene membranes by atomic layer deposition of TiO2for simultaneously improved permeability and selectivity,J.Membr.Sci.448(2013)215–222.
[35]H.Chen,L.Kong,Y.Wang,Enhancing the hydrophilicity and water permeability of polypropylene membranes by nitric acid activation and metal oxide deposition,J.Membr.Sci.487(2015)109–116.
[36]A.Roy Chowdhuri,C.G.Takoudis,R.Klie,N.Browning,Metalorganic chemical vapor deposition ofaluminum oxide on Si:evidence of interface SiO2formation,Appl.Phys.Lett.80(22)(2002)4241–4243.
[37]M.Groner,F.Fabreguette,J.Elam,S.George,Low-temperature Al2O3atomic layer deposition,Chem.Mater.16(4)(2004)639–645.
[38]A.Ott,J.Klaus,J.Johnson,S.George,Al2O3thin film growth on Si(100)using binary reaction sequence chemistry,Thin Solid Films 292(1)(1997)135–144.
[39]D.Y.Li,J.Hu,Z.X.Low,Z.X.Zhong,Y.Wang,Hydrophilic ePTFE membranes with highly enhanced water permeability and improved efficiency for multipollutant control,Ind.Eng.Chem.Res.55(10)(2016)2806–2812.
[40]S.M.Lee,V.Ischenko,E.Pippel,A.Masic,O.Moutanabbir,P.Fratzl,et al.,An alternative route towards metal-polymer hybrid materials prepared by vapor-phase processing,Adv.Funct.Mater.21(16)(2011)3047–3055.
[41]S.M.Lee,E.Pippel,U.Gosele,C.Dresbach,Y.Qin,C.V.Chandran,et al.,Greatly increased toughness of in filtrated spider silk,Science 324(5926)(2009)488–492.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- CFD modeling of turbulent reacting flow in a semi-batch stirred-tank reactor☆
- Synergistic and interference effects in coaxial mixers:Numerical analysis of the power consumption☆
- Experimental investigation and cost assessment of the salt production by solar assisted evaporation of saturated brine☆
- Equilibrium of liquid-liquid extraction of 2-phenylbutyric acid enantiomers:Experiment and model☆
- Preparation of PVC/PVP composite polymer membranes via phase inversion process for water treatment purposes
