Novel kinetic model for the simulation analysis of the butanol productivity of Clostridium acetobutylicum ATCC 824 under different reactor configurations
Hugo I.Velázquez-Sánchez,Ricardo Aguilar-López*
Center of Research and Advances Studies of the National Polytechnic Institute(CINVESTAV),Biotechnology and Bioengineering Department,Av.National Polytechnic Institute 2508 Col.San Pedro Zacatenco,07360 Mexico City,Mexico
1.Introduction
Biofuels,which can be de fined as all compounds of organic nature derived from living beings and their metabolism that can potentially be utilized as fuels,lead a group of alternative energy sources aimed to provide solution to the environmental issues caused by the overexploitation of fossil ones[1,2].One of the most studied biofuels in the last decade is butanol,considering that it can offer better properties regarding fuel mileage yield,lower gaseous emissions,higher energy content and lower hygroscopicity versus the currently developed processes for the production of ethanol and biodiesel[3].
Traditionally,the methodology to obtaining butanolby fermentation is based on the degradation of various sugars(particularly glucose or sucrose)carried out by Gram-positive bacteria of the genus Clostridium,via a metabolic pathway called ABE(acetone–butanol–ethanol)[4].ABE fermentation presents inherent restrictions that have prevented its consolidation as a mature technology such as low production yields,inhibitory effect over the culture's growth due to both solvents and glucose accumulation and mechanisms of metabolic regulation own of Gram-positive bacilli such as the sporulation process[5].However,ABE fermentation by Clostridium bacteria is still the most employed biological process for the solvent and biofuel production due to the poor performance obtained by heterologous recombinant strains such as Escherichia coli,which don't achieve butanol titers over 7 g·L-1or volumetric productivities over 0.2 g·L-1·h-1[2].Furthermore,the petrochemical production of n-butanol is based on the hydrogenation of aldehydes obtained from the oxidation of propylene,making its production cost fluctuate alongside the crude oil market price[6].
One proposal that arises to overcome such challenges is the design and implementation of novel production strategies in order to find appropriate procedures to efficiently exploit the bacterial metabolism.From the above,traditional batch fermentations have been considered due their simple operating conditions and less prone to contamination,butthe maximumhistoricalbutanoltitre achieved in such configuration doesn't exceed 15 g·L-1due inhibition of the bacterial growth.Continuous regime has the advantage to alleviate the negative side effects of both substrate and product accumulation,but the slow growth rate of Clostridium cultures does impede to operate under high dilution rates that can help to improve productivity of the system,while also causing that the final butanol titre at the outlet of the reactor dilute,which has a negative impact over downstream operation cost and energy consumption of the fermentation system[7].Finally semi-continuous regimes applied to the ABE fermentation system had been successful only when they are coupled with in situ product recovery techniques,such as gas stripping or pervaporation.Nonetheless even if the experimental results show promising results towards product selectivity and culture growth maintenance there can be an opportunity to optimize the operational conditions in such complex systems to achieve lower costs and higher yields[8,9].
In this regard,mathematical modelling and simulation techniques can be used as the first step in novel process and bioreactor design,through the analysis of the behaviour of the culture under different operating conditions and regimes,where current literature has a wide variety of proposed structures for the description of the ABE fermentation process[10,11].
However,even considering that the mathematical modelling of the ABE fermentation system has at least 40 years of development,there is not a consensus about the most adequate structure to use in the scope of process design,control and optimization purposes,as the vast majority ofreported modelstructures,they are only valid under specific process conditions and regimes.Recently,the so-called unstructured phenomenological kinetic modelling approach,sought to find the middle point between the complexity of metabolomic or genomic level models and easiness ofuse and interpretation ofthe traditionalunstructured kinetic models,this approach has been successfully applied to the ABE system and is a solid prospect to evaluate the performance of this system under more complex regimes such as the ones involving medium optimization,multistage reactor clusters or integrative systems involving cell immobilization and in situ recovery strategies[20].
On other side,few information has been reported in the open literature regarding the effect of the operating regime in establishing ABE fermentation using Clostridium strains and the possible causes and effects associated to butanol production due to the use of one or another production strategy,as even experimental studies are generally limited to evaluate the performance of a particular operational regime under restrained conditions against classic batch methods,so many of the novel proposed processes are still open for analysis.
Therefore,in this work,a novel phenomenological unstructured kinetic model for ABE fermentation validated experimentally under batch and continuous operation is used to evaluate the theoreticalbutanoltitre and productivity ofa culture of Clostridium acetobutylicum ATCC 824 under the following four different production strategies:1)adding butyric acid at the beginning of a batch fermentation,2)single stage CSTR,3)Three stage CSTRs with and without biomass recirculation and 4)fed-batch culture.
2.Methodology
2.1.Model development
A phenomenological non-structured kinetic model was developed to describe the kinetic behaviour of ABE production in batch reactor.The mathematical model is based on classical mass balance approach and it was considered as a benchmark production plant by extending it to simulate continuous operation,as follows:
Biomass reaction rate:

Butanol reaction rate:

Butyric acid reaction rate:

Acetone reaction rate:
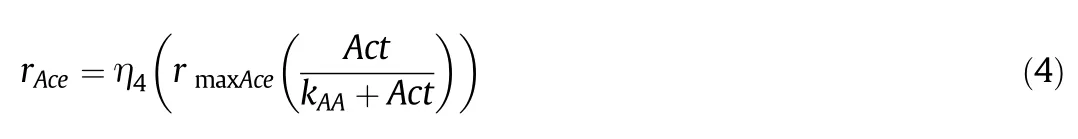
Acetic acid reaction rate:
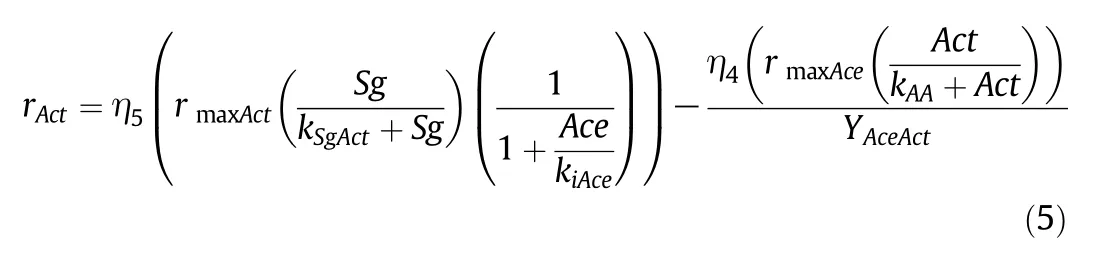
Ethanol reaction rate:

Glucose mass balance:

Biomass mass balance:

Butanol mass balance:

Butyric acid mass balance:

Acetic acid mass balance:

Acetone mass balance:

Ethanol mass balance:

The kinetic modelis composed of6 kinetic rates(Eqs.(1–6))and a set of seven differential equations(Eqs.(7–13))describing the corresponding mass balances for Glucose(Sg),Biomass(X),Butanol(But),Butyric acid(Sb),Acetic acid(Act),Acetone(Ace)and Ethanol(Et)respectively.F stands for the volumetric flow of the feeding solution,V stands for the volume of culture medium within the reactor and Sgaand Sbaare the glucose and butyric acid concentrations within the feeding solution.
The analysis of the simpli fied metabolic pathway of C.acetobutylicum reported by Haus et al.[6],which is shown in Fig.1,was used to propose the mathematical structures representing the reaction rates within the system.It describes that the metabolism of glucose oxidation is carried out in two sequential phases,one phase called acidogenesis,carried out by vegetative cells,which includes from the glycolysis pathway up to the formation of Acetyl CoA and its subsequent oxidation to organic acids such as butyric,lactic and acetic;and another metabolic pathway called solventogenesis,performed in conjunction with the metabolism thattriggers the process ofsporulation,which starts from the reincorporation ofthe organic acids mentioned above into the celland culminates with its transformation into acetone and butanol[21].In the case of ethanol,it is reported in the literature that its production is constitutive,regardless of the metabolic state of the crop.It is also important to note that the strain C.acetobutylicum ATCC 824 has not been genetically modi fied to inhibitits sporulation process,so this effectwas also considered within the equation construction.
In the case of the characterization of the system operating with immobilized cells,a pseudo-homogeneous approach was considered to characterize the effect that diffusion limitations have on the system based on the application of the so-called efficiency factor(ηi).This parameter is de fined as the quotient of the apparent reaction rate considering diffusion restrictions between the reaction rate observed in a system without limitation by mass transfer,so it can take values between 0 and 1[22].
Finally,to consider the fact that the biomass inside the reactor in the immobilized cellsystemis attached to the supportand this is suspended inside the reactor,it was considered to include an additional parameter in the mass balance for thatvariable,calledα.This parameter represents the massfraction ofbiomassleaving the reactordue to the input–output mass flow under continuous regime.Alfa value can range from 0 to 1,where α =0 represents packed-bed flow dynamics and α =1 represents the ideal case of the “perfect mixing”continuous reactor[23].
The parametric identification of the proposed model was made via the Marquardt algorithm into the software ModelMaker®3.0.3.Experimental data was obtained from a fermentation system reported by Yen and Li[24],which consisted of a stirred tank reactor of 1 L nominal volume working with 600 mL of P2 medium with glucose as the main carbon source and inoculated with cells of C.acetobutylicum ATCC 824,operated under four different operating regimes:batch and continuous with free cells and batch and continuous with immobilized cells respectively.The immobilization medium was a powdered brick with a particle diameter between 0.15 and 2.4 mm,which occupied 65 ml of reactor volume.
2.2.Numerical simulations
Allthe numericalsimulations were made into a PC equipped with an Intel® Core© i5 560 M processor and 4 GB of RAM into the MATLAB®2016a software,using the ODE Solver library's command ode15s to solve the system's set of differential equations by considering the following operational regimes:batch with organic acid feeding,single stage CSTR,three stage CSTR with and without biomass recirculation and fed-batch with constant feeding rate.The theoretical performance ofthe systemwas evaluated by considering butanoltitre and productivity at equilibrium state in batch culture and stationary state in all the CSTR ones.
In addition,to characterize the steady state behaviour of the systems operating under continuous regime,bifurcation diagrams were elaborated considering the dilution rate(D=F/V),glucose concentration in the feed stream(Sga)and concentration of butyric acid in the feed stream(Sba)as bifurcation parameters using the MATCONT version 5p0 program,which runs within the framework of MATLAB®2016a.
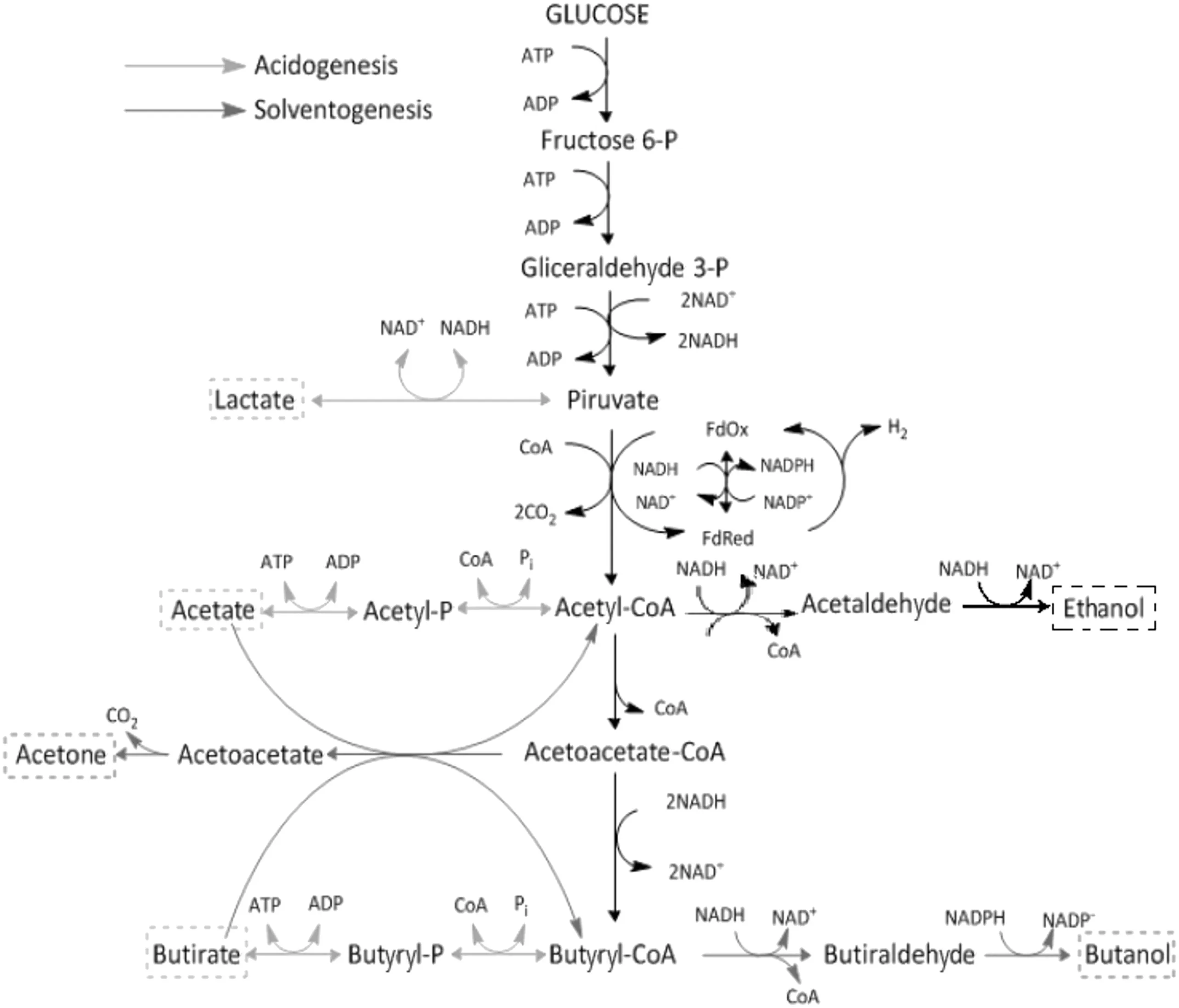
Fig.1.Diagram of the simpli fied ABE fermentation pathway of Clostridium acetobutylicum reported by Haus et al.[6].
3.Results
3.1.Model validation
Table 1 summarizes the values obtained by the results of the parametric identification.The validation of the model was made by simulation considering the following initial conditions:X0=0.2 g·L-1,Sg0=60 g·L-1,But0=0.01 g·L-1,Sb0=0.01 g·L-1,Ace0=0.01 g·L-1,Act0=0.01 g·L-1and Et0=0.01 g·L-1(Fig.2),where the mathematical model represents the dynamic behaviour of the analysed variables with a linear correlation index r2=0.9952 and r2=0.9710 over experimental data for free and immobilized cells respectively,and a p-value<0.001,which ensures that there is not a significant difference between the predicted behaviour described by the proposed model and the experimental data[25].It should be noted that the parameters ηiand α only take the value reported when using the structure to model the system with immobilized cells,both in batch and continuous regime,otherwise they are assigned the value of one(Table 2).
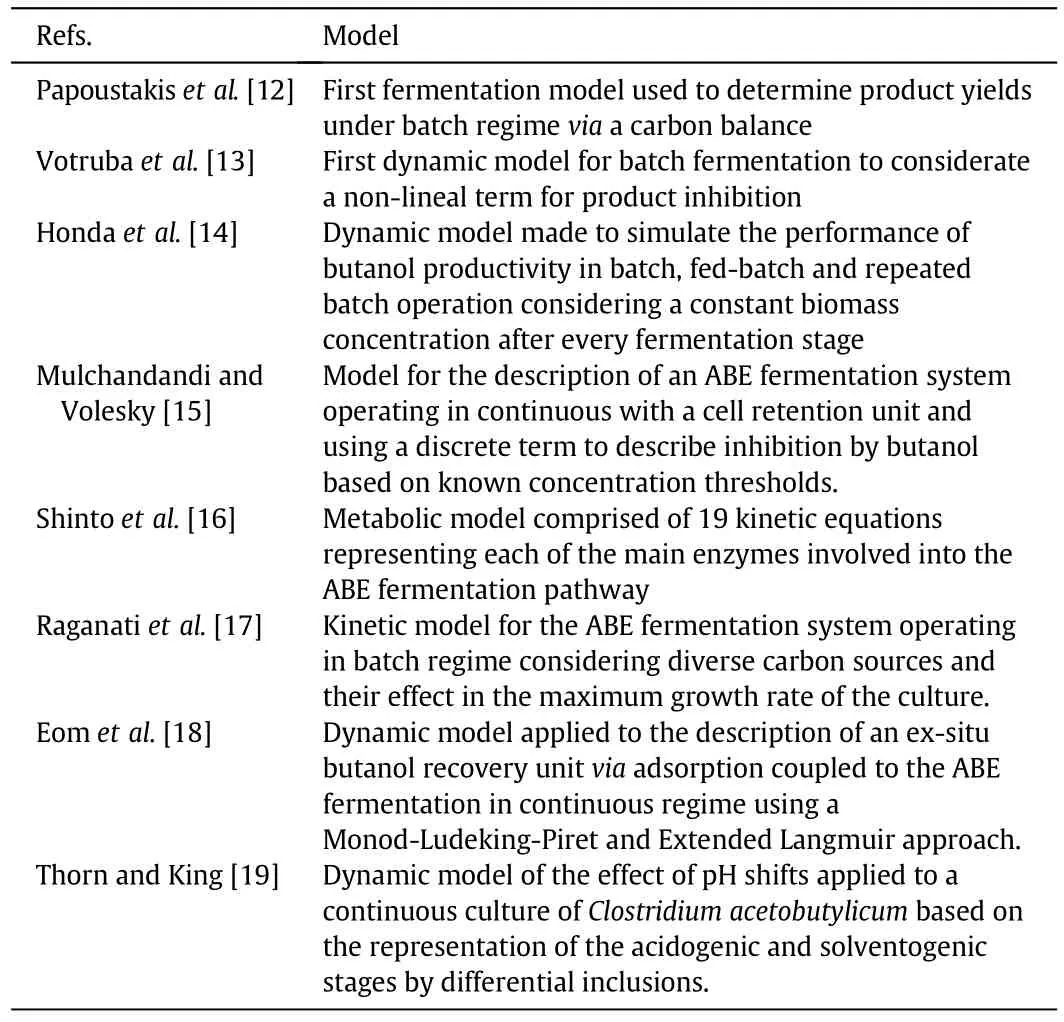
Table 1 Previous reported mathematical models for the description of the ABE fermentation system

Table 2 Parametric identification of the proposed model considering experimental data reported by Yen and Li[24]
The high parametric uncertainty observed in some estimated values can be attributed to the fact that experimental data is measured with finite accuracy and only a subset of the state variables is accessible experimentally in an “in-line”manner,as samples must be obtained from the culture and processed of fline,this coupled with the inherent non-linearity nature of biological systems can be the main causes for such results[26];however key parameters like the maximum specific growth rate(μmax)are indeed within values reported into literature for Clostridium bacteria[27,17].
3.2.Batch culture with butyric acid supplementation
In a previouswork,Chang[28]evaluated both theoretically and experimentally the effect of the addition of butyric acid on batch fermentation systems of Clostridium beijerinckii,concluding that supplementation of the culture medium using initial concentrations of butyric acid in a range between 2 and 5 g·L-1could offer an increase of up to 20%in the butanol titre at the end of the fermentation;Chang also showed that values higher than the aforementioned range negatively impacted butanol generation since the crop could suffer a shortage of ATP,which reduced biomass growth.Fig.3 and Fig.4 show the behaviour ofthe fermentation system with both free and immobilized cells respectively,operating under batch regime with and without butyric acid supplementation.The simulation analysis done to evaluate the system response under such conditions was performed considering F=0 L·h-1and Sb0=4 g·L-1.The results show that the effect of butyric acid addition in fact increases the final butanol concentration in both the free and immobilized cell systems by about 20%,however there is a sensitive delay ofabout10 h to reach the kinetic equilibriumstate in the system;This may be due to the excess production of butanol during the start of the fermentation that causes the phenomenon of inhibition by productto manifestitselfin a shortertime than ifthere were no acid addition,so this has a negative impact on the productivity of the system.It should be mentioned that in spite of the above information,it can be corroborated that C.acetobutylicum ATCC 824 is also able to tolerate initial concentrations of butyric acid between 0 and 4 g·L-1without exhibiting the phenomenon known as“acid-crash”,which is the inhibition of the activation of the sporulation phase ofthe microorganism due to a very pronounced drop of pH below 4 units.
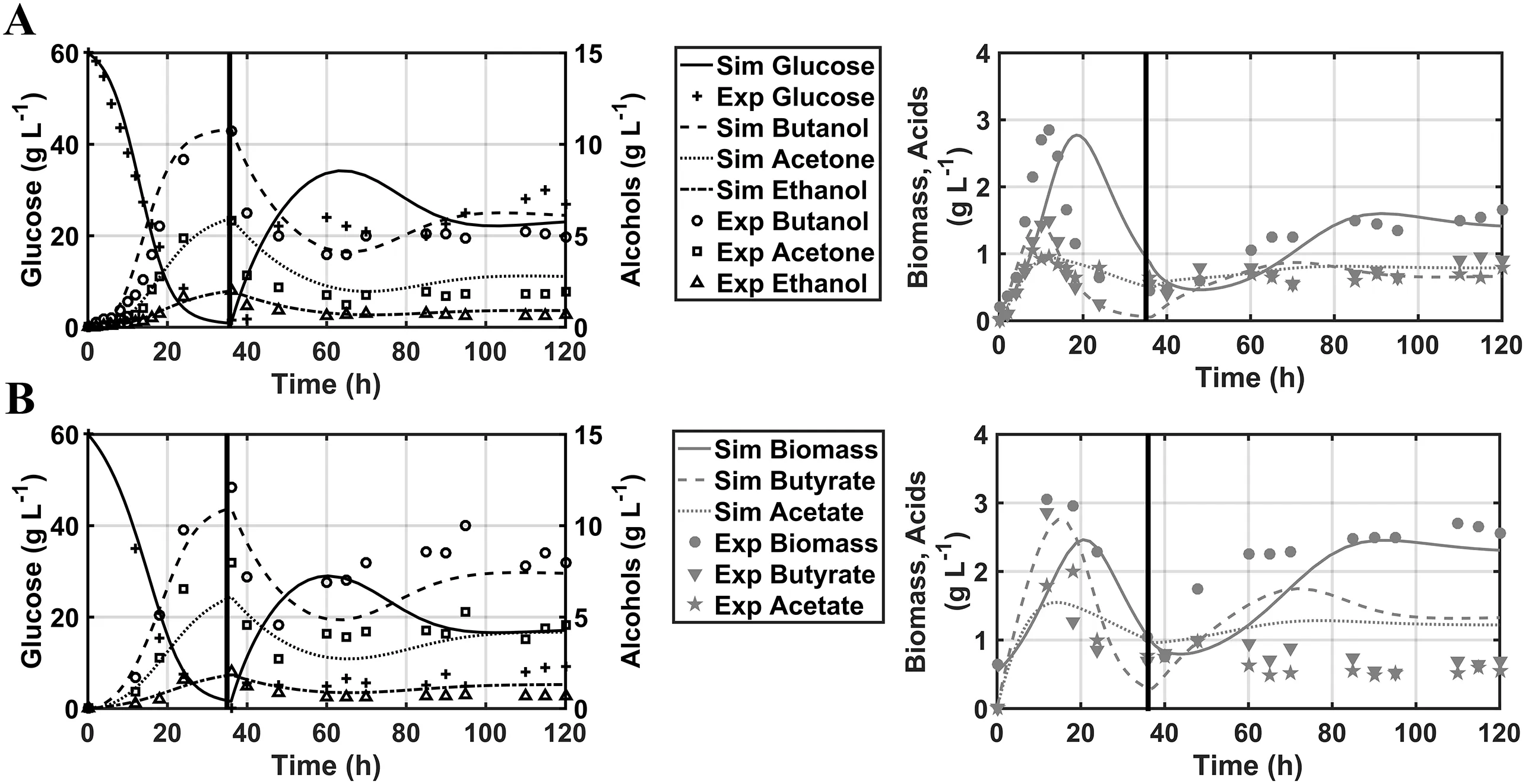
Fig.2.Validation of the proposed kinetic model versus Clostridium acetobutylicum ATCC 824 experimental data reported by Yen and Li[24]:A)Free cells fermentation,r2=0.9995 and B)Immobilized cells fermentation,r2=0.9710.Horizontal line indicates the switch between batch and continuous regime(36 h).
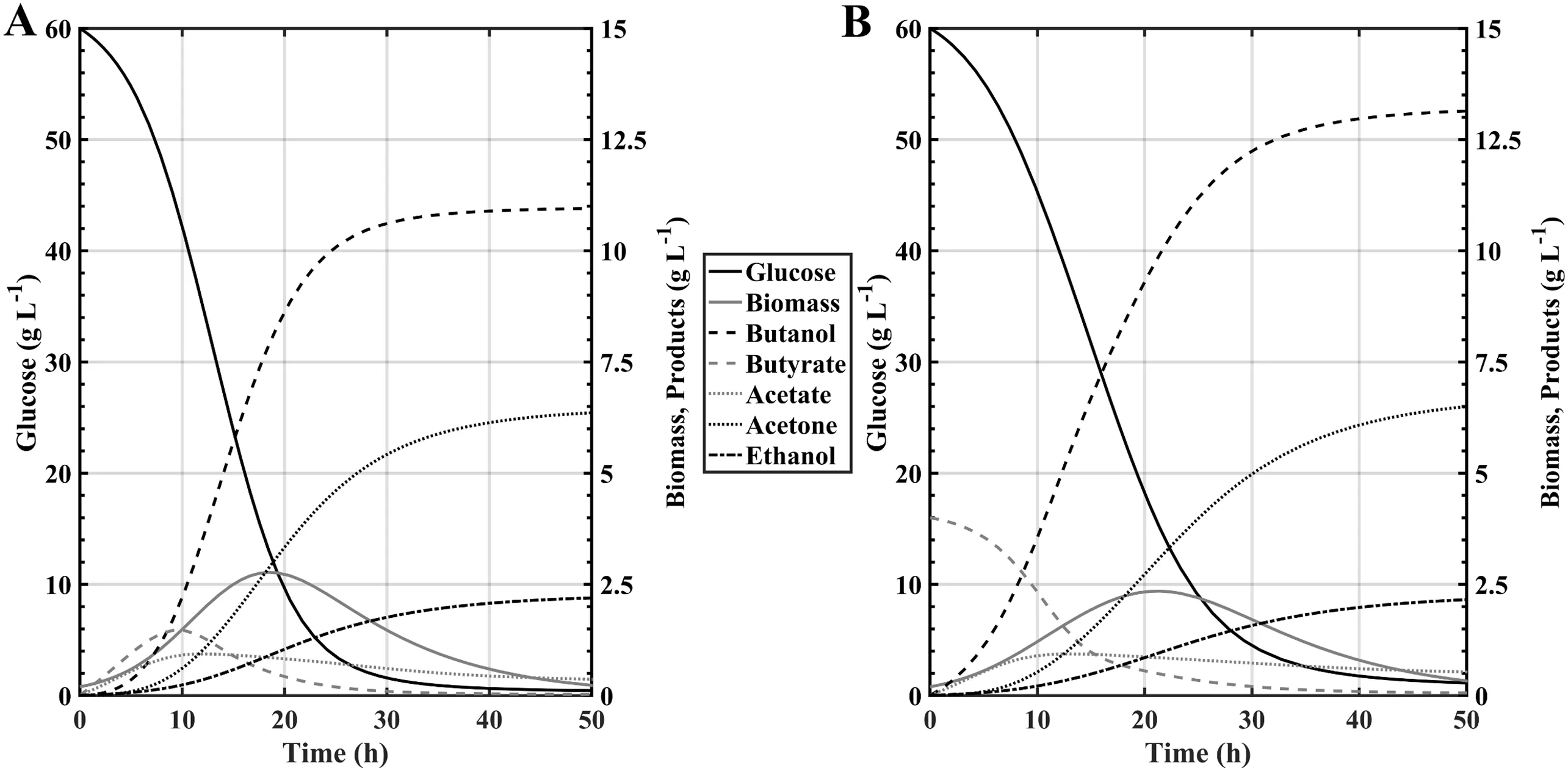
Fig.3.Kinetic comparison of Clostridium acetobutylicum ATCC 824 under batch regime between:A)Free cells without butyric acid supplementation and B)Free cells with Sb0=4 g·L-1.

Fig.4.Kinetic comparison of Clostridium acetobutylicum ATCC 824 under batch regime between:A)Immobilized cells withoutbutyric acid supplementation and B)Immobilized cells with Sb0=4 g·L-1.
3.3.Fed-batch fermentation
Semi-continuous operation regime is generally used to reduce the impact of the substrate inhibition phenomenon on a culture,generally carried out by starting with a batch fermentation until either a desired substrate concentration or an equilibrium condition is reached,then a concentrated substrate solution is fed at a constant,linear,exponential or intermittent rate[29].In ABE fermentation,fed batch culture is recommended only when there is the possibility of coupling a product recovery process in line with the fermentation due to the strong effects of butanol inhibition[30,31].
In order to study the behaviour of fed-batch culture an additional differential equation must be added to the system,as in this regime the reaction volume is not constant:

Then,a theoretical scenario was set-up considering a theoretical bioreactor of 1000 L of capacity,initiating batch operation with half of its operation volume(V0=500 L).As a first simulation essay,there was proposed to evaluate the behaviour of the fermentation system using an arbitrary initial F=5 L·h-1and beginning the pumping of the inlet solution,containing a Sgaof 60 g·L-1,after 36 h of batch operation.Fig.5 indicates that constant feeding rate of the reactor,at least with the proposed flow rate and glucose concentration,is not able to fully mitigate the effectof bacterialgrowth inhibition due butanol accumulation,even if its concentration in the reactor lowers from 10.95 to 7.83 g·L-1;however when the reactor volume reaches its final value of 1000 L after 136 h there is a slight recovery of bacterial growth that was able to consume the remaining glucose within the medium to produce enough butanol to reach 10.02 g·L-1.
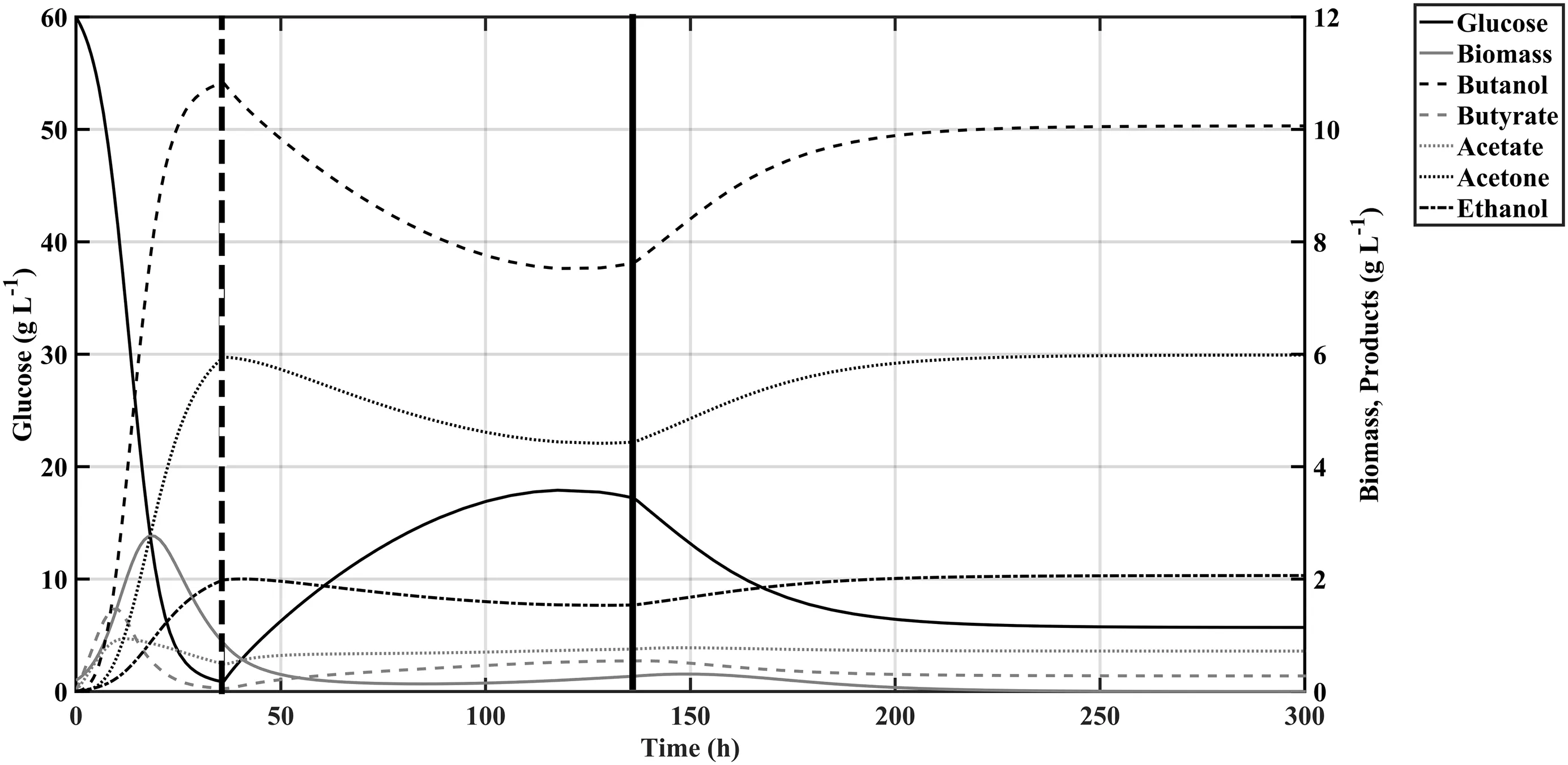
Fig.5.Simulation results obtained with the modelproposed in this work considering fed-batch operation regime with a constant feeding rate F=5 L·h-1 containing glucose at60 g·L-1;the dotted vertical line indicates the beginning of the inlet flow and the solid line indicates where the flow stops due to maximum volume reached.
The results ofsimulationscondensed in Fig.6 representthe dynamic of the fed-batch system manipulating the inlet flow F between 2 and 10 L·h-1with a constant Sgaof 60 g·L-1,these conditions were chosen to avoid a drop in reaction rate by possible substrate inhibition and to ensure that the process had a minimum fermentation time of 50 h.It can be observed that for substrate feed rates less than 4 L·h-1the amount of glucose entering the reactor is not suf ficient for the crop to maintain a steady growth rate to counteract the dilution effect caused by the increase in reaction volume.However,at feed rates above 6 L·h-1there is an increase in the production of butanol which allows to continue increasing its concentration in spite of the increase of volume,which together with the reduction in the total process time due to limitation by volume allows to increase the productivity of the system by 10%.
If the performance of the fed-batch system is measured in raw mass production then the reactor operating with immobilized cells and butyric acid supplementation at a F=8 g·L-1produced 5.105 kg of butanol within the initial 36 h and after 120 h it produced 11.33 kg.Nonetheless,as butanol is traditionally recovered via distillation,the dilution effect of the product compared with the batch-only titre(13.60 vs.11.33 g·L-1)and also the increase in process time should be evaluated to determine the viability of this production strategy.
3.4.Single stage CSTR
For the analysis ofthe systemoperating in single-stage CSTR,the use of bifurcation analysis was used as a tool to describe the fermentation dynamics considering the dilution rate(D=F/V),the concentration of glucose in the stream(Sga)and the concentration of butyric acid in the feed stream(Sba)as bifurcation parameters.Within the main objective ofdeveloping bifurcation diagrams considering the aforementioned process parameters was to identify as a first step the reactor washing rate(Dw),which allowed to identify the limits of the operating region of the reactor under analysis;as well as to find out the range of values in the substrate feed concentration that can be used without promoting the inhibition of bacterialgrowth,this is because as reported by Qureshi et al.[31]Clostridium cells exhibitgrowth inhibition by glucose when its concentration in the medium reaches values higher than 175 g·L-1.The initialconditions chosen to carry outthese simulations,both forfree and immobilized cells systems were those reported by Yen and Li[24]:X0=0.2 g·L-1,Sg0=60 g·L-1,But0=0.01 g·L-1,Sb0=0 g·L-1,Et0=0.01 g·L-1,D=0.054 h-1and Sga=60 g·L-1.Additionally,the supplementation of the feeding solution with a Sba=4 g·L-1was brie fly evaluated.

Fig.6.Comparison between butanol production(markers)and reactor volume dynamics(lines)in cultures of Clostridium acetobutylicum ATCC 824 under fed-batch regime:A)free cells without butyric acid supplementation,B)free cells with Sb a=4 g·L-1;C)immobilized cells without butyric acid supplementation and D)immobilized cells with Sb a=4 g·L-1.
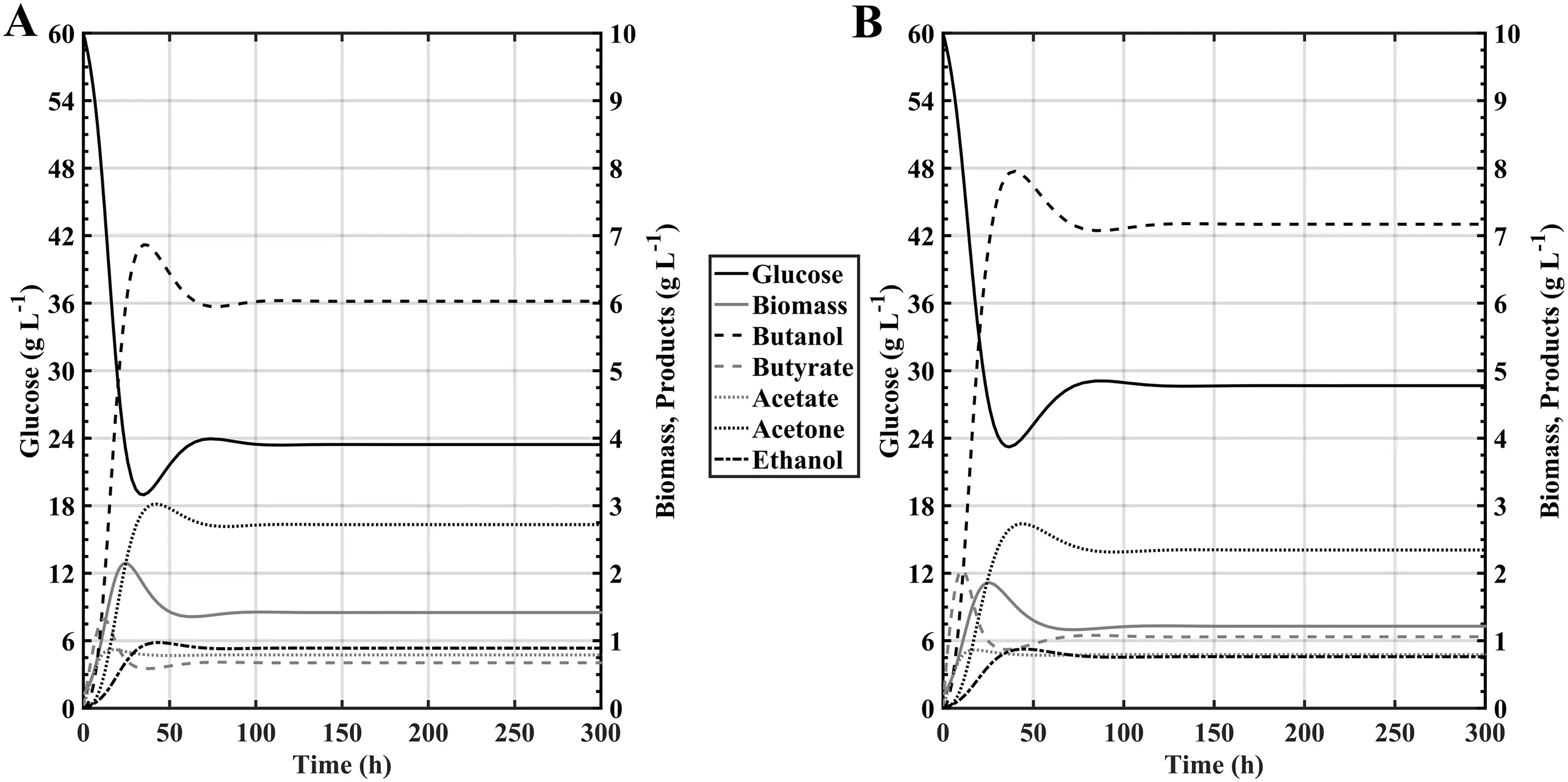
Fig.7.Kinetic comparison of Clostridium acetobutylicum ATCC 824 under continuous regime between:A)Free cells without butyric acid supplementation and B)free cells with Sb a=4 g·L-1.
Simulation results made for that purpose considering the free cells reactor are shown in Fig.7,where the reactor reached steady-state after 150 h,and it was observed that the effect of addition of butyric acid within the feeding solution improved butanol titre from 6.01 to 7.18 g·L-1,even if the biomass concentration in the reactor decreased 15%.This phenomenon is congruent with the observations made by Chang[28]and Sandoval-Espinola et al.[5],where it was demonstrated that a higher butyric acid pool within the medium re-routed the metabolic carbon flow towards solvent production instead of cell formation to avoid a shortage of ATP and accumulation of NADH within the cells.
The response for the systems considering the use of immobilized biomass show similar results compared to the free cells,as the butyric acid supplementation induces an increase into butanol titre from 7.30 to 8.91 g·L-1.It should be noted that as the cells are immobilized the biomass is able to remain within the reactor up to a certain extend due to the consideration ofthe non-idealsolubility constantαdiscussed into the ModelDevelopmentsection,therefore the increase in both productivity and titre are higher than in the free cells scenery(19.47 vs.22.05%respectively).

Fig.8.Dynamics of the ABE fermentation system of Clostridium acetobutylicum ATCC 824 operating as CSTR with respect to changes in dilution rate(D)and glucose feed concentration(Sg a):A)Butanol concentration in free cell system,B)Butanol productivity in the system with free cells,C)Butanol concentration in the system with immobilized cells and D)Butanol productivity in system with free cells.
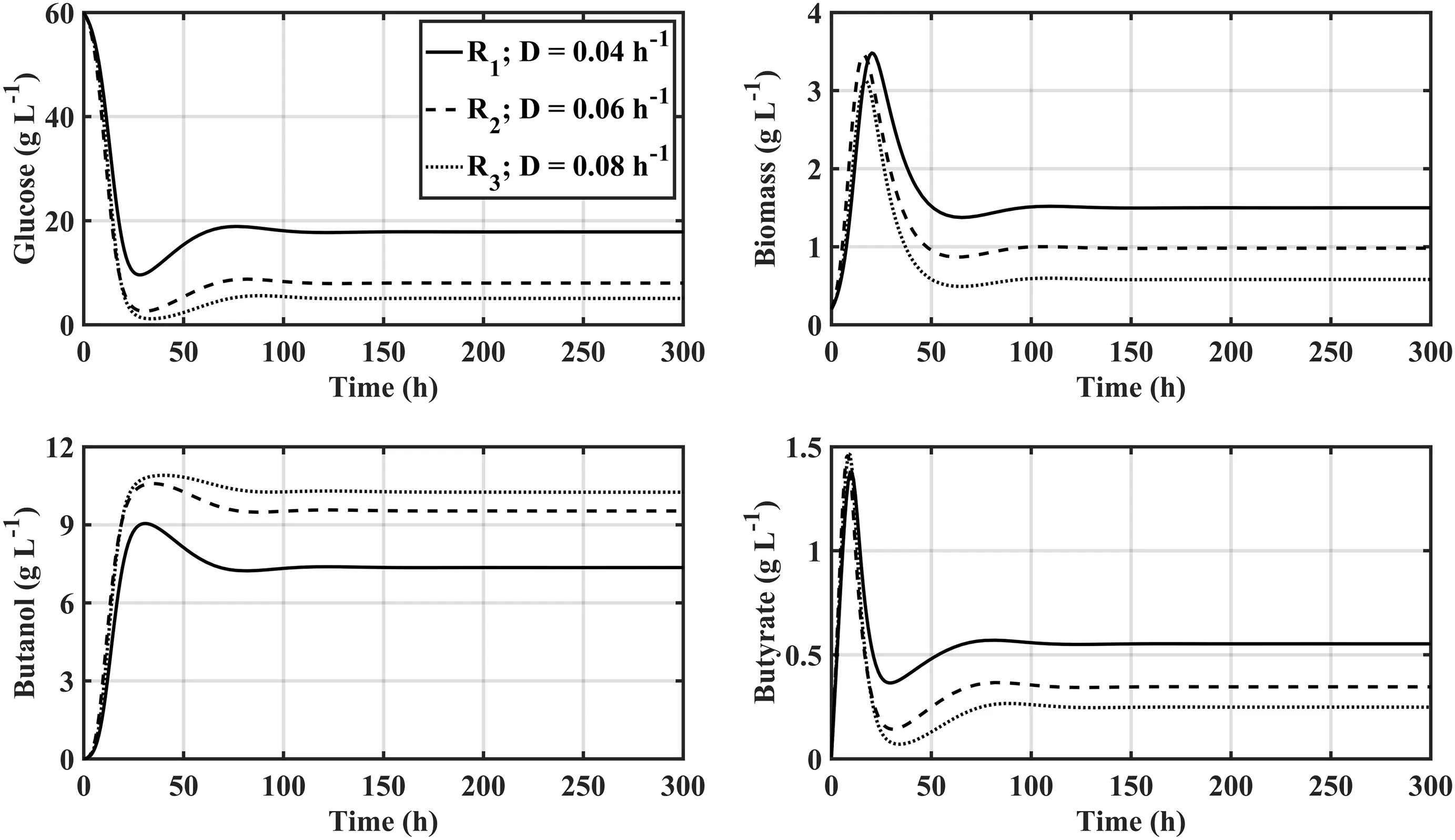
Fig.9.Dynamic of a 3-staged CSTR system of free cells of Clostridium acetobutylicum ATCC 824 with biomass recirculation from the 3rd to the 1st reactor considering ascending dilution rates after every vessel.
As a first step in the construction of the aforementioned bifurcation diagrams,simulations were performed to find steady state points on which the continuation algorithms of the MATCONT software could perform the calculation of the equilibrium points reached by the fermentation system under different operational conditions.Fig.8 shows the information regarding the bifurcation diagrams obtained for both evaluated systems(free and immobilized cells),considering as performance metrics both the steady-state butanol concentration and the butanol volumetric productivity.The large difference in concentration and productivity obtained between the reactorwith free and immobilized cells is appreciated in a tangible way,this is natural considering that the proposed kinetic modelincludes a non-ideal flow term(α)in the biomass balance(Eq.(9))derived from the hypothesis that the immobilization support,despite being suspended in the culture broth,does not leave the tank with the same speed as the free cells do,so that the washing effect induced by the input and output mass flow is attenuated.This same phenomenon in turn allows the reactor with immobilized cells to operate at dilution rates of up to an order of magnitude above those that can support the system with free cells,so that its performance in both metrics evaluated is considerably higher.
Another point to note is that the proposed model has the ability to predict what glucose concentration can be exploited in the system without the occurrence of the phenomenon of inhibition by substrate(190 g·L-1for free cells and 160 g·L-1for immobilized cells),while also serves to refute the theory that indicates that a higher concentration of substrate in any fermentation will always lead to a higher concentration of product.The obtained results also defy the hypothesis that a system operating with immobilized cells must be more resistant to inhibitory phenomena due to diffusional difficulties that are imposed under such conditions,nevertheless for this particular case the explanation derives from the fact that the system with immobilized cells,by producing a greateramountofbutanol,isinhibited by the accumulation ofthis before the inhibition by glucose can be presented as such,considering that the immobilization method used in the Yen and Li[24]is based on adsorption to a porous matrix,so that as such only a portion of the immobilized cell population is subject to diffusion limitations that protect them from the action of the solvent.
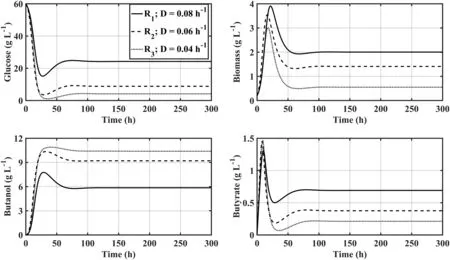
Fig.10.Dynamic of a 3-staged CSTR system of free cells Clostridium acetobutylicum ATCC 824 with biomass recirculation from the 3rd to the 1st reactor considering descending dilution rates after every vessel.
In addition,in this particular operating regime it is evidentthat both metrics evaluated present con flicting behaviours:the conditions that allow to increase the productivity of the system force the concentration of butanol to be reduced,which is counterproductive from the point of viewofthe costofdownstreamoperations in this process,so finding operating regions that allow a balance between the two metrics could require the use of multi-objective optimization tools.
3.5.Multiple stage CSTR
According to the proposals made by de Gooijer et al.[32]a stepwise fermentation becomes favourable when the inhibition by product is strong,which in fact is ideal for the production of butanol;on the other hand a three-stage process is the closest to the ideal CSTR followed by a piston flow reactor.Some researchers have tried to take advantage of Clostridium's two-phase metabolism(acidogenesis and solventogenesis)to propose continuous reactor operation in serial policies[33,34],considering the hypothesis thathigh dilution rates established in the firstreactor to maximize acid production are favourable for a second stage of butanol production ata lower dilution rate,however there is strong experimental evidence which suggests that the dominant metabolism during the first culture stage becomes the control metabolism of the whole process,regardless of the dilution rate and pH set in the remaining reactors[35,36].
In this study,the simulations were carried out considering the free-cellsystem in a three-stage process under two differentconditions,one without biomass recirculation and one with biomass recirculation,and the immobilized one just considering the scenario without biomass recirculation,mirroring a previous experimental essay by Setlhaku et al.[37].The selected operational regime was the one in which the dilution rate increases after each stage:D1=0.04 h-1,D2=0.06 h-1and D3=0.08 h-1by considering a fixed F=10 L·h-1but descending reactor volumes equal to V1=250 L,V2=167 L and V3=125 L,with 60 g·L-1glucose in the feed in the first stage.For this purpose an additional equation was constructed which indicates that the output biomass of the third stage is recirculated to the first reactor with the restriction that the amount of recycled biomass can only be as much as the biomass present at the outlet(DiXi):
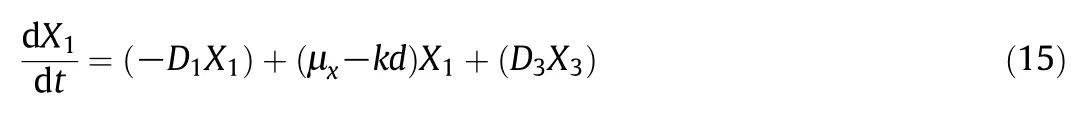
Simulation results in all the tested conditions show a characteristic behaviour of increase of butanol concentration after each stage,which is consistentwith the behaviourofCSTR reactors in series[37],nonetheless the best performance was obtained by the configuration operating with free cells and biomass recirculation,which achieved a steadystate butanol titre of 10.25 g·L-1(Fig.9)versus the obtained by the immobilized cells reactor of 9.45 g·L-1.This dynamic is expected due to the fact that the system with free cells carry over more biomass at the outlet of every stage to the next one,which allows the system to maintain a higher and more uniform conversion rate through the fermentation.
The opposing dilution rate regime(descending values)was also evaluated,and as although the increase in butanol concentration between the successive reactors does indeed rise,the performance gains between stages is more pronounced.This can be explained considering that starting the fermentation with a high dilution rate cells are less prone to suffer inhibition by butanol accumulation as this componentis having a relative low retention time to trigger sporulation,which can mean the successive stages get more viable cells than the ones present within the ascending dilution rate regime(Fig.10).The best performance obtained under such regime was achieved when there was biomass recirculation,where the finalbutanoltitre and productivity were 10.39 g·L-1and 103.9 g·h-1.
To complete the analysis,a comparative table of the performance of the operating regimes evaluated against the metrics obtained by the commercial fermentation system reported by Jiang et al.[7],where it can be seen that the use of reactors with immobilized cells can be an alternative of application to improve said industrial process(Table 3).
4.Conclusions
In this work,the performance of the ABE fermentation under differentreactors configurations is analysed.For this,studio is considered as a class of unstructured phenomenological kinetic model which is able to describe the system behaviour under a greater array of operational conditions and regimes,considering only the nominal parametric identification,which sets it as an important advance towards the use of numerical simulation for the analysis and synthesis of novel butanolproducing bioprocesses,the simulation results obtained in this work are congruent with experimental reports.
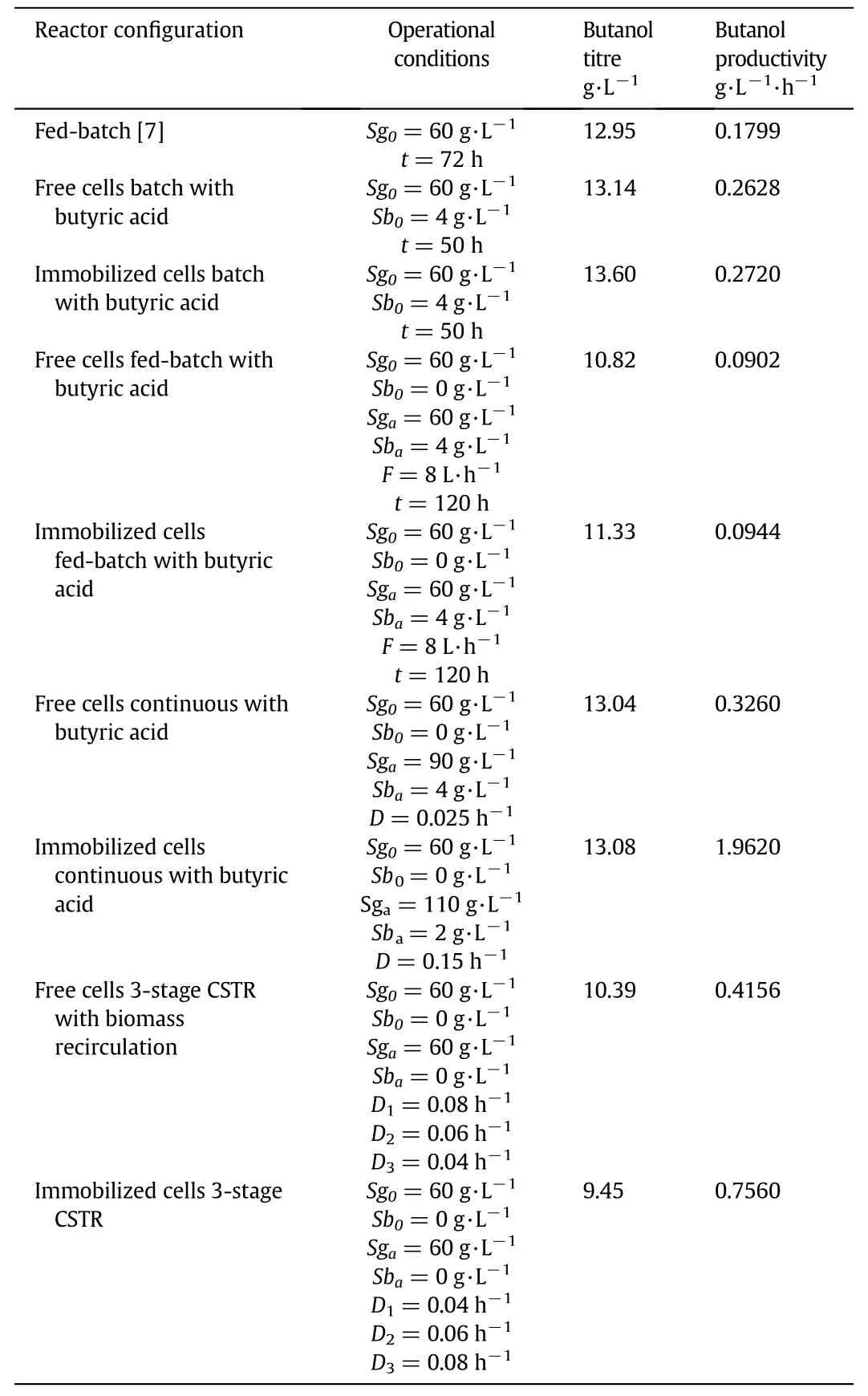
Table 3 Performance comparison between an industrial ABE fermentation systems versus simulation results obtained in this work via numerical simulation under different operational regimes
As a notorious result itwas interesting to find thatthe supplementation of the culture medium for ABE fermentation with butyric acid does indeed provide a slight increase in butanol concentration in both batch and fed-batch regimes,however further economic studies should be made to determine if the addition of such organic acid is viable regarding feedstock costs,as the obtained butanolconcentration enhancement lies between 16%and 20%.
Additionally,it was shown that ABE fermentations operating in continuous regime and using immobilized cells have a very positive impacton the butanoltitre and the productivity ofthe system,obtaining enhancement of both metrics accounting for an increase in 1.01%and 909%over the most recent industrial process reported to date respectively.This result is also consistent with previous experimental work made under such process conditions and can help to establish new design and process synthesis criteria to improve the viability of biological butanol production as fuel extender.
Nomenclature
DwReactor's washout dilution rate,h-1
kAAAcetic acid-acetone affinity constant,g·L-1
kButButanol growth inhibition constant,g·L-1
kdSpecific cell death rate,h-1
kSbButanol-butyric acid affinity constant,g·L-1
kSbSgButyric acid-glucose affinity constant,g·L-1
kSgGlucose affinity constant,g·L-1
kSgActGlucose-acetic acid affinity constant,g·L-1
kSgbGlucose-butanol affinity constant,g·L-1
kSgEtGlucose-ethanol affinity constant,g·L-1
kSiGlucose growth inhibition constant,g·L-1
YAceSgAcetone per glucose mass yield,g·g-1
YButSbButanol per butyric acid mass yield,g·g-1
YButSgButanol per glucose mass yield,g·g-1
YEtSgEthanol per glucose mass yield,g·g-1
YXSgBiomass per glucose mass yield,g·g-1
μmaxMaximum specific cell growth rate,h-1
νmaxAceMaximum specific acetone production rate,h-1
νmaxActMaximum specific acetic acid production rate,h-1
νmaxButMaximum specific butanol production rate,h-1
νmaxEtMaximum specific ethanol production rate,h-1
νmaxSbMaximum specific butyric acid production rate,h-1
Acknowledgements
H.I.Velázquez-Sánchez is very grateful with Consejo Nacional de Ciencia y Tecnologia for the financial support via the postgraduate scholarship No.277760,and with CINVESTAV-IPN for supplying the research facilities to develop this work.
References
[1]A.Ranjan,V.S.Moholkar,Biobutanol:Science,engineering,and economics,Int.J.Energy Res.36(3)(2011)277–323.
[2]K.A.K.Khöler,J.Rühl,L.M.Blank,A.Schmid,Integration of biocatalyst and process engineering for sustainable and efficient n-butanol production,Eng.Life Sci.15(2015)4–19.
[3]A.Ranjan,V.S.Moholkar,Biobutanol:A viable gasoline substitute through ABE fermentation,Proc.World Acad.Sci.Eng.Technol.51(2009)497–503.
[4]P.Maddipati,H.K.Atiyeh,D.D.Bellmer,R.L.Huhnke,Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract,Bioresour.Technol.102(11)(2011)6494–6501.
[5]W.J.Sandoval-Espinola,M.Chinn,J.M.Bruno-Barcena,Inoculum optimization of Clostridium beijerinckii for reproducible growth,FEMS Microbiol.Lett.362(2015)fnv164.
[6]S.Haus,S.Jabbari,T.Millat,H.Janssen,R.J.Fischer,H.Bahl,J.R.King,O.Wolkenhauer,A systems biology approach to investigate the effect of pH-induced gene regulation on solvent production by Clostridium acetobutylicum in continuous culture,BMC Syst.Biol.5(2011)5–10.
[7]Y.Jiang,J.Liu,W.Jiang,Y.Yang,S.Yang,Current status and prospects of industrial bio-production of n-butanol in China,Biotechnol.Adv.33(2015)1493–1501.
[8]C.Xue,J.Zhao,C.Lu,S.T.Yang,F.Bai,I.C.Tang,High-titer n-butanol production by Clostridium acetobutylicum JB200 in fed-batch fermentation with intermittent gas stripping,Biotechnol.Bioeng.109(11)(2012)2746–2756.
[9]C.Xue,F.Liu,M.Xu,J.Zhao,L.Chen,J.Ren,F.Bai,S.T.Yang,A novel in situ gas stripping-pervaporation process integrated with acetone-butanol-ethanol fermentation for hyper n-butanol production,Biotechnol.Bioeng.113(1)(2016)120–129.
[10]R.Mayank,A.Ranjan,S.Moholkar,Mathematical models of ABE fermentation:Review and analysis,Crit.Rev.Biotechnol.33(4)(2013)419–447.
[11]T.Millat,K.Winzer,Mathematical modelling of clostridial acetone–butanol–ethanol fermentation,Appl.Microbiol.Biotechnol.101(2017)2251–2271.
[12]E.T.Papoutsakis,Equations and calculations for fermentations of butyric acid bacteria,Biotechnol.Bioeng.26(2)(1984)174–187.
[13]J.Votruba,B.Volesky,L.Yerushalmi,Mathematical model of a batch acetone–butanol fermentation,Biotechnol.Bioeng.28(2)(1986)247–255.
[14]H.Honda,T.Mano,M.Taya,K.Shimizu,M.Matsubara,T.Kobayashi,A general framework for the assessment of extractive fermentations,Chem.Eng.Sci.42(1987)493–498.
[15]A.Mulchandani,B.Volesky,Modeling of the acetone–butanol fermentation with cell retention,Can.J.Chem.Eng.64(1986)625–631.
[16]H.Shinto,Y.Tashiro,M.Yamashita,G.Kobayashi,T.Sekiguchi,T.Hanai,Y.Kuriya,M.Okamoto,K.Sonomoto,Kinetic modeling and sensitivity analysis ofacetone–butanol–ethanol production,J.Biotechnol.131(1)(2007)45–56.
[17]F.Raganati,A.Procentese,G.Olivieri,P.Götz,P.Salatino,A.Marzocchella,Kinetic study of butanol production from various sugars by Clostridium acetobutylicum using a dynamic model,Biochem.Eng.J.99(2015)156–166.
[18]M.H.Eom,B.Kim,H.Jang,S.H.Lee,W.Kim,Y.A.Shin,J.H.Lee,Dynamic modelling of a fermentation process with ex situ butanol recovery(ESBR)for continuous biobutanol production,Energy Fuel 29(2015)7254–7265.
[19]G.J.Thorn,J.R.King,Modelling the role of CtfA/B in reverse shift continuous culture experiments of Clostridium acetobutylicum,Math.Biosci.276(2016)101–113.
[20]H.I.Velázquez-Sánchez,M.C.Montes-Horcasitas,R.Aguilar-López,Development of a phenomenological kinetic model for butanol production using Clostridium beijerinckii,Rev.Mex.Ing.Quim.13(1)(2014)103–112.
[21]C.Xue,J.Zhao,L.Chen,S.T.Yang,F.Bai,Recent advances and state-of-the-art strategies in strain and process engineering for biobutanol production by Clostridium acetobutylicum,Biotechnol.Adv.35(2017)310–322.
[22]K.R.Rout,H.A.Jakobsen,Reactor performance optimization by the use of a novel combined pellet re flecting both catalyst and adsorbent properties,Fuel Process.Technol.99(2012)13–34.
[23]H.O.Méndez-Acosta,D.U.Campos-Delgado,R.Femat,V.González-Alvarez,A robust feedforward/feedback control for an anaerobic digester,Comput.Chem.Eng.29(2005)1613–1623.
[24]H.W.Yen,R.J.Li,The effects of dilution rate and glucose concentration on continuous acetone-butanol-ethanol fermentation by Clostridium acetobutylicum immobilized on bricks,J.Chem.Technol.Biotechnol.86(2011)1399–1404.
[25]T.Sellke,M.J.Bayarri,J.O.Berger,Calibration of p values for testing precise null hypotheses,Am.Stat.55(1)(2001)62–71.
[26]J.Vanlier,C.A.Tiemann,P.A.J.Hilbers,N.A.W.van Riel,Parameter uncertainty in biochemical models described by ordinary differential equations,Math.Biosci.246(2013)305–314.
[27]A.Procentese,F.Raganati,G.Olivieri,M.E.Russo,P.Salatino,A.Marzocchella,Continuous xylose fermentation by Clostridium acetobutylicum—Kinetics and energetics issues under acidogenesis conditions,Bioresour.Technol.164(2014)155–161.
[28]W.Chang,Acetone–Butanol–Ethanol Fermentation by Engineered Clostridium beijerinckii and Clostridium tyrobutyricum.PhD Thesis,Ohio State University,USA,2010.
[29]T.C.Ezeji,N.Qureshi,H.P.Blanschek,Acetone butanol ethanol(ABE)production from concentrated substrate:reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping,Appl.Microbiol.Biotechnol.63(6)(2004)653–658.
[30]S.Lee,M.O.Cho,C.H.Park,Y.Chung,J.H.Kim,Continuous butanol production using suspended and immobilized Clostridium beijerinckii NCIMB 8052 with supplementary butyrate,Energy Fuel 13(2008)3459–3464.
[31]N.Qureshi,B.C.Saha,M.A.Cotta,Butanol production from wheat straw hydrolysate using Clostridium beijerinckii,Bioprocess Biosyst.Eng.30(2007)419–427.
[32]C.D.De Gooijer,et al.,Bioreactors in series:An overview of design procedures and practical applications,Enzym.Microb.Technol.18(3)(1996)202–219.
[33]H.Bahl,W.Andersch,G.Gottschalk,Continuous production of acetone and butanol by Clostridium acetobutylicum in a two-stage phosphate limited Chemostat,Appl.Microbiol.Biotechnol.15(4)(1982)201–205.
[34]M.C.Lai,R.W.Traxler,A coupled two-stage continuous fermentation for solvent production by Clostridium acetobutylicum,Enzym.Microb.Technol.16(12)(1994)1021–1025.
[35]G.Godin,M.Engasser,Two-stage continuous fermentation of Clostridium acetobutylicum:Effects of pH and dilution rate,Appl.Microbiol.Biotechnol.33(3)(1990)269–273.
[36]O.Mutschlechner,H.Swoboda,J.R.Gapes,Continuous two-stage ABE-fermentation using Clostridium beijerinckii NRRL B592 operating with a growth rate in the first stage vessel close to its maximal value,J.Mol.Microbiol.Biotechnol.2(1)(2000)101–105.
[37]M.Setlhaku,et al.,Improvement in the bioreactor specific productivity by coupling continuous reactor with repeated fed-batch reactor for acetone–butanol–ethanol production,J.Biotechnol.161(2)(2012)147–152.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- CFD modeling of turbulent reacting flow in a semi-batch stirred-tank reactor☆
- Synergistic and interference effects in coaxial mixers:Numerical analysis of the power consumption☆
- Atomic layer deposition of Al2O3 on porous polypropylene hollow fibers for enhanced membrane performances☆
- Experimental investigation and cost assessment of the salt production by solar assisted evaporation of saturated brine☆
- Equilibrium of liquid-liquid extraction of 2-phenylbutyric acid enantiomers:Experiment and model☆
