Porous g-C3N4 with enhanced adsorption and visible-light photocatalytic performance for removing aqueous dyes and tetracycline hydrochloride☆
Junlei Zhang ,Zhen Ma ,2,*
1 Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention(LAP3),Department of Environmental Science and Engineering,Fudan University,Shanghai 200433,China
2 Shanghai Institute of Pollution Control and Ecological Security,Shanghai 200092,PR China
1.Introduction
New photocatalysts have been actively sought in the field of photocatalysis.The development of such types of photocatalysts can provide new opportunities for us to remove aqueous and gaseous pollutants and to elucidate the fundamental aspects of photocatalysts and photocatalytic mechanisms.In particular,visible-light-driven photocatalysts are very interesting because they can use solar energy more efficiently.
In 2009,Wang et al.reported that graphite-like carbon nitride(g-C3N4)can be used as a visible-light-driven photocatalyst for hydrogen production[1].Since then,g-C3N4has attracted much attention for its application in photocatalytic water splitting[2–5],contaminant degradation[6–10],CO2reduction[11],and NOxremoval[12–14].However,g-C3N4was often prepared by thermal polymerization of melamine powders without the supply of fresh air/O2during calcination(to avoid the total oxidation of melamine),thus leading to the formation of bulk(non-porous)g-C3N4with low surface areas.The low specific surface area,insuf ficient solar-light absorption,and fast recombination of photogenerated electrons and holes caused by the π–π conjugated electronic system may severely limit the photocatalytic performance of bulk g-C3N4.
To solve these problems,many strategies have been adopted to modify g-C3N4.These strategies include ion/element(e.g.Fe3+[15],S2-[16,17],O[18–20],B[21],C[22],P[23,24],Br[25])doping,metal(e.g.Pt[26],Au[27],Ag[28],Bi[29])deposition,and constructing semiconductor heterojunctions[30,31]such as Ag2MoO4/g-C3N4[32]and Ag6Mo10O33/g-C3N4[33].Alternatively,the textures of g-C3N4can be tuned to form g-C3N4nanoparticles[34,35],nanosheets[36,37],nanorods[38],nanoribbons[39],hollow spheres[40,41],and “seaweed”[4].
Porous g-C3N4can exhibitimproved photocatalytic activity forhydrogen production due to the large specific surface area as well as more active sites and adsorption sites[42–44].However,the preparation of porous g-C3N4is usually assisted by additional hazardous/toxic reagents and/or gas atmosphere,thus entailing high costs.Yang et al.prepared porous g-C3N4by heating bulk g-C3N4in O2at a designated temperature for 30 min,and porous g-C3N4showed enhanced photocatalytic performance for hydrogen production[45].Nevertheless,the addition of pure O2still limits the large-scale synthesis and application of g-C3N4.A more convenient method for the synthesis of porous g-C3N4is needed.
Motivated by this,we prepared porous g-C3N4by simply heating bulk g-C3N4in a muf fle oven(in static air)without involving additional conditions(e.g.,tubular oven and pure O2).These porous g-C3N4samples exhibited significantly upgraded visible-light photocatalytic abilities for removing organic dyes and tetracycline hydrochloride compared with bulk(non-porous)g-C3N4.
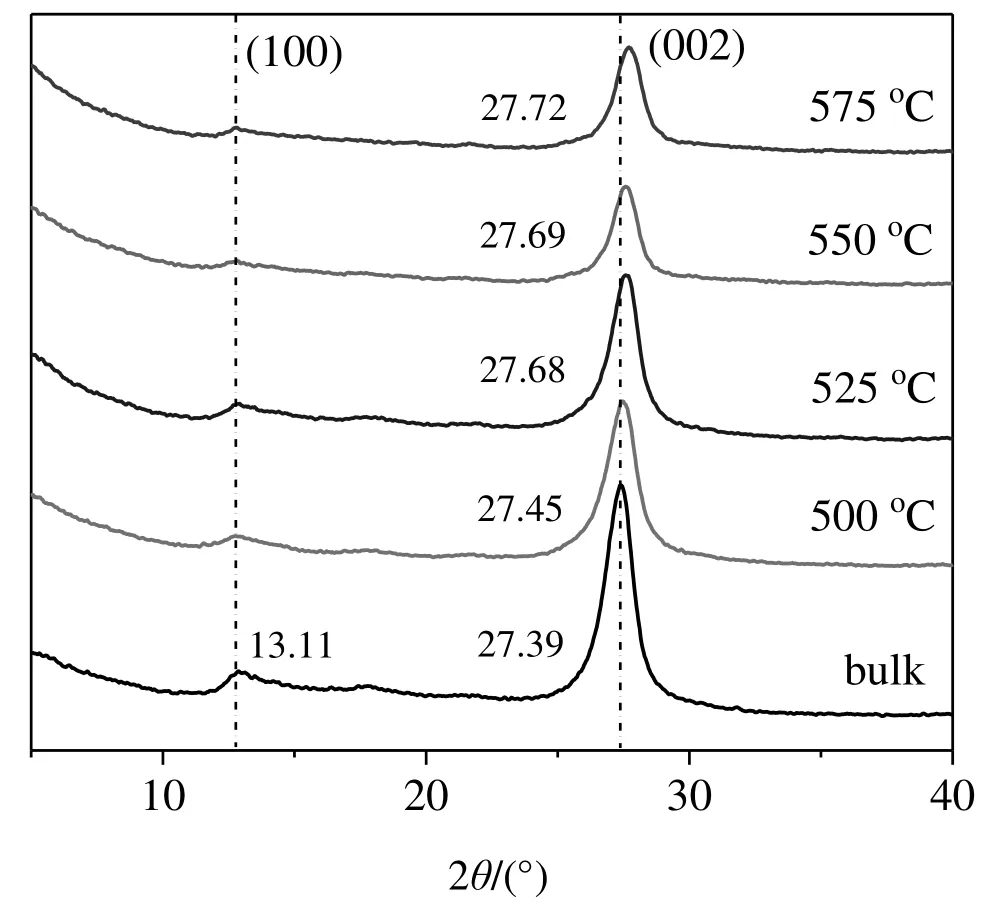
Fig.1.XRD patterns of g-C3N4 samples.
2.Experimental
2.1.Synthesis of g-C3N4 samples
Bulk g-C3N4was prepared by thermal polymerization[46].Typically,10.0 g melamine powders were put into an Al2O3crucible,and the crucible was then covered with an Al2O3cover(otherwise,melamine would be combusted in air upon heating).The crucible was heated to 550 °C in a muf fle oven at a rate of 3 °C·min-1,kept at 550°C for 4 h,and then cooled down to room temperature in the muf fle oven.Then,0.5 g bulk g-C3N4was grinded into powders,placed in an Al2O3crucible without a cover,and re-calcined in the muf fle oven at a designated temperature(500,525,550,or 575°C)for 2 h to obtain porous g-C3N4.
2.2.Characterization
X-ray diffraction(XRD)patterns of the samples were obtained on MSAL XD2 X-ray diffractometer with CuKαradiation at 40 kV and 30 mA.The scanning speed was 8(°)·min-1.X-ray photoelectron spectroscopic(XPS)data were acquired using a multifunctional photoelectron spectroscopy instrument(Axis Ultra Dld,Kratos).Scanning electron microscopy(SEM)data images were recorded on a Shimadzu SUPERSCAN SSX-550 field emission scanning electron microscope.Optical diffuse re flectance spectra were recorded on a UV–VIS–NIR scanning spectrophotometer (Lambda 35, Perkin-Elmer).Photoluminescence(PL)spectra were collected using a LabRAM HR Evolution(HORIBA JY company,France)instrument with an excitation wavelength at 355 nm.Electrochemical impedance spectroscopy(EIS)and Mott-Schottky measurements were conducted using an electrochemical analyzer(CHI 660B Chenhua Instrument Company).
2.3.Photocatalytic activity evaluation
The photocatalytic degradation of methylene blue(MB),methyl orange(MO),or tetracycline hydrochloride(TC)was conducted under visible lightat room temperature.A Xe lamp(300 W,HSX-F300,Beijing NBeT Technology Co.,Ltd.)with a UV-cutoff filter(420 nm)was used as the light source(Fig.S1 in the Supporting Material)[32,33,47–50].

Fig.2.C 1s(A)and N 1s(B)XPS spectra of C3N4 samples.
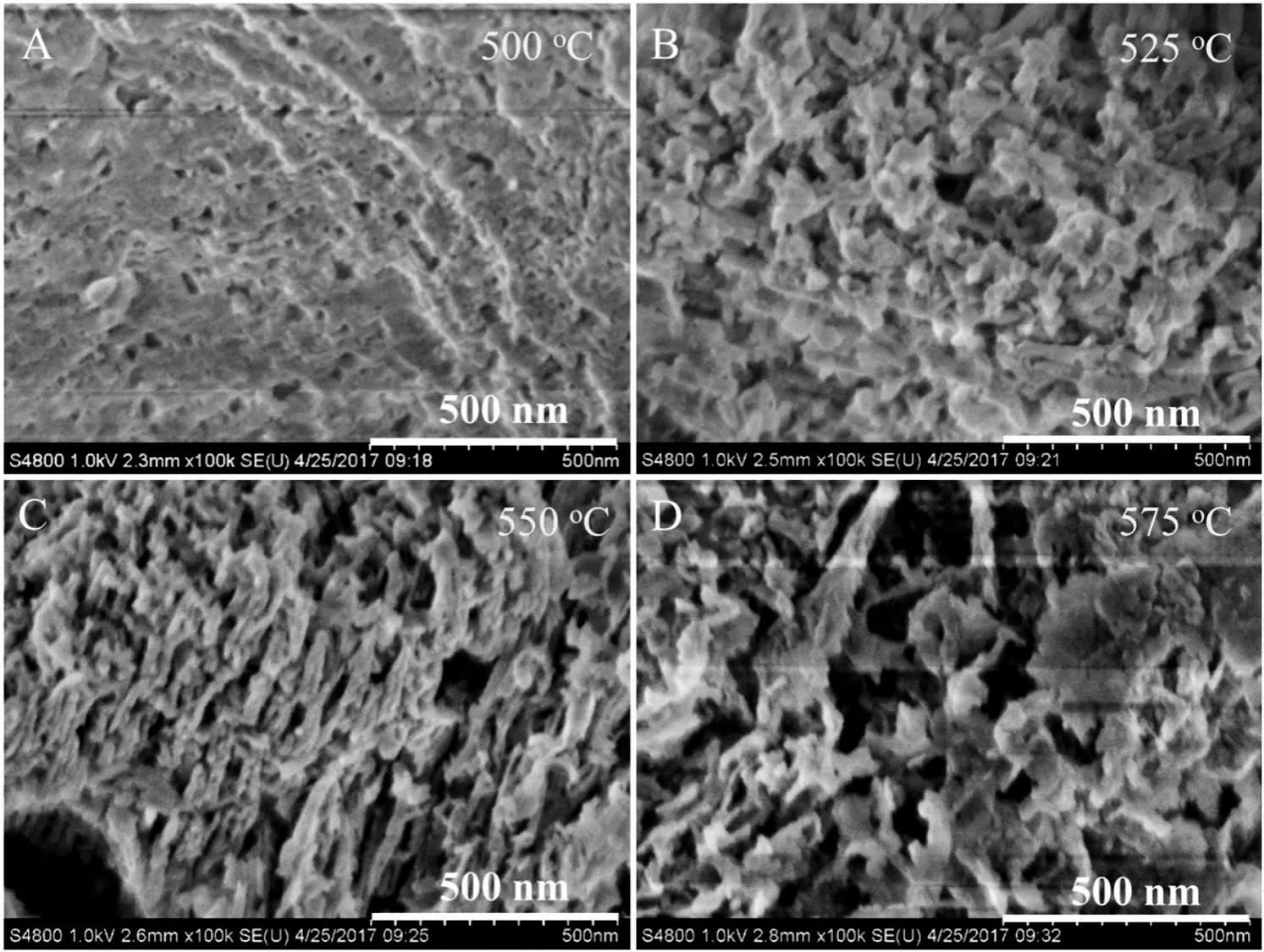
Fig.3.SEM images of the re-calcined g-C3N4 samples.
Typically,30 mg photocatalyst,in the form of fine powders,was suspended in 50 ml MB(20 mg·L-1),MO(10 mg·L-1),or TC(20 mg·L-1)solution in a 100 ml beaker by a brief exposure to ultrasound.The solution was stirred at a rate of 800 r·min-1in the dark for 30 min to allow for the adsorption/desorption equilibrium,and the Xe light was turned on.During the photocatalytic reaction,a small portion of the solution was sampled at certain intervals and centrifuged,and the supernatant was analyzed by a UV-5200PC spectrometer(Shanghai Metash Instruments Co.,Ltd.).
3.Results and Discussion
3.1.Characterization of g-C3N4 samples
Figs.1 and S2 show the XRD patterns of g-C3N4samples.They all show(100)and(002)peaks at around 13.1°and 27.5°,respectively,attributed to the in-plane structural packing motif and interlayer stacking re flection of conjugated aromatic segments,respectively[4,20,43,51].The data confirm that g-C3N4is stable after being re-calcined in air at 500–575 °C in a muf fle oven.Compared to bulk g-C3N4,the(002)peak of the re-calcined samples slightly shifts toward larger 2θ values,indicating a slightly decreased interlayer spacing[45].We also attempted to calcine bulk g-C3N4at600°C,only to find thatthe material was totally gone after calcination.
Fig.S3 shows the XPS survey spectra.Allg-C3N4samples contain two sharp peaks ataround 288 and 398 eV,assigned to C 1s and N 1s signals,respectively.The data indicate that the composition and chemical state of g-C3N4are largely retained after re-calcination.
High-resolution C 1s spectra(Fig.2A)show the presence of three kinds of carbon species in bulk g-C3N4.The peak at 284.6 eV is from C(sp2)-C(sp2)in graphitic carbon species[52],the peak around 287.8 eV is assigned to C atoms attached to N inside the aromatic ring(N=C—(N)2)[53],and the peak at 290.2 eV is for C—O[20].The peak at around 290.2 eV disappears after re-calcination at 525°C,indicating the oxidation ofsurface C—Ospecies.Asimilarphenomenon is observed in other re-calcined g-C3N4samples(Fig.S4A).

Fig.4.(A)Nitrogen adsorption–desorption isotherms and(B)the corresponding pore size distribution curves of g-C3N4 samples.
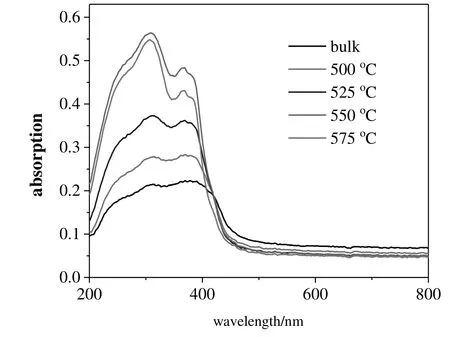
Fig.5.UV–Vis diffuse-re flectance spectra of g-C3N4 samples.
N 1s high-resolution spectra(Fig.2B)ofbulk g-C3N4show three core levels ataround 398.3,400.0,and 402.5 eV,attributed to sp2-hybridized aromatic nitrogen atoms bonded to carbon atoms(C=N—C))[53],tertiary nitrogen(N—(C)3)groups linking structuralmotif(C6N7)[54],and amino functional groups(C—N—H)from the defective condensation of heptazine substructures[43,51],respectively.After re-calcination of bulk g-C3N4,the peak at around 402.5 eV disappears,indicating that C—N—H is oxidized in air(Figs.2B and S4B).
Figs.3 and S5 show the SEM images of g-C3N4samples.Bulk g-C3N4has a typical stacked lamellar structure(Fig.S5A).For comparison,the surfaces of re-calcined samples appear as irregular porous structures(Figs.3 and S5B-E),and the diameters of these pores range from several nanometers to several hundred nanometers.The layered structures become looser and looser as the re-calcination temperature increases(Fig.3).
Fig.4 shows the N2adsorption–desorption isotherms and pore size distribution curves of g-C3N4samples.The BET specific surface areas of g-C3N4re-calcined at 500,525,550,and 575°C are 71.2,194.2,213.9,and 225.5 m2·g-1,respectively,much higher than that of bulk g-C3N4(8 m2·g-1)(Fig.4A).Correspondingly,the re-calcined g-C3N4samples possess much larger pore volumes (0.29,1.02,1.50,and 0.92 cm3·g-1)compared to bulk g-C3N4(0.066 cm3·g-1).The much higher BET surface area and pore volumes ofre-calcined g-C3N4samples may result from the creation of more pores.The pore size distribution curves(Fig.4B)indicate that the g-C3N4samples are rich in pores of sizes between 1 and 40 nm.These facts are consistent with the SEM observations.
Fig.5 shows the UV–Vis–NIR DRS data of g-C3N4samples.Compared to that of bulk g-C3N4,the absorption edges of the re-calcined g-C3N4samples display a slightly blue shift,but they still have absorption in the visible-light region.The optical absorption near the band edge can be described by the Tauc equation:

In this equation,A is constant,α is the absorption coefficient,h is the Planck's constant,ν is the light frequency,and Egis the band gap.The power index n depends on the type of electronic transition,i.e.,n equals to 1 for a directband-gap material and 4 for an indirectband-gap material.The square of absorption coefficient of a semiconductor is usually linearly proportional to the energy(hν)in the absorption edge region during the direct optical transition process.Fig.S6 shows the plots of(αhν)1/2versus energy(hν).The Egvalue of bulk g-C3N4is calculated to be 2.70 eV,whereas the Egvalues of g-C3N4samples re-calcined at 500,525,550,or 575°C are 2.77,2.78,2.78,and 2.80 eV,respectively.

Fig.6.(A)Absorption spectra of MB with reaction time in the presence of 30.0 mg g-C3N4(525°C);The adsorption and degradation curves of(B)MB or(D)MO in aqueous solution with time in the absence of photocatalyst or in the presence of g-C3N4(30 mg);(C)The constants of MB photodegradation rates using g-C3N4.
3.2.Photocatalytic activity evaluation
The photocatalytic degradation of cationic MB and anionic MO dyes were studied.Prior to the visible-light irradiation,the mixture containing a dye solution and a catalyst was kept in the dark for 30 min to achieve the adsorption–desorption equilibrium.
Fig.6Ashows the absorption spectra ofMB with time in the presence of 30.0 mg g-C3N4(525°C).The porous sample exhibits much higher adsorption capacity(11.4%)in the dark than bulk g-C3N4.Subsequently,the absorption peak at 664 nm quickly decreases as the time of visiblelight irradiation increases,suggesting the destruction of the conjugated structure.After 60 min of irradiation,MB photodegradation efficiency reaches 73.2%for g-C3N4(525°C),much higher than that(16.9%)using bulk g-C3N4(Figs.6B and S7A).
The adsorption capacity gradually increases with the increase of recalcination temperature(Figs.6B and S7A),due to the enlarged surface area(Fig.4A).MB photodegradation efficiencies(50.4%,59.2%,and 55.8%)for g-C3N4re-calcined at 500,550,and 575°C,respectively,are also much higher than that(16.9%)using bulk g-C3N4,but lower than that(73.2%)using g-C3N4(525°C),confirming that525 °C is the optimal re-calcination temperature.
The reaction testing data were fitusing the pseudo- first-ordermodel(Fig.S7B and Table S1)[55,56]:-ln(C/C0)=Kt,where C is the concentration of MB at time t,C0is the original concentration of MB,and K is the reaction rate constant.The photodegradation rate(0.0289 min-1)using g-C3N4(525°C)is higher than those obtained by using bulk g-C3N4(0.00304 min-1)or other re-calcined g-C3N4samples(0.0126,0.0201,and 0.0201 min-1for g-C3N4re-calcined at 500,550,and 575°C,respectively,Fig.6C).
A similar phenomenon is observed when using anionic dye MO(Figs.6D,S8,and Table S2)as a modelpollutant.g-C3N4(525°C)stillexhibits the best visible-light photocatalytic performance among all g-C3N4samples.
Tetracycline hydrochloride(TC),as a broad-spectrum antibacterial agent,has been widely applied in human medicine,livestock,and in the treatmentofbacterialinfection,posing serious threats to aquatic environments[57].TC was thus used as a model pollutant to evaluate the visible-light photocatalytic performance of g-C3N4samples(Figs.7 and S9).
Fig.7A displays the evolution of the UV–vis spectra during TC degradation over g-C3N4(525°C)under visible irradiation.The main absorption peak of TC is located at 357 nm.It decreases rapidly with the extension ofthe irradiation time.After 60 min ofirradiation,TC removal efficiency reaches 91.8%,higher than those obtained by using other g-C3N4samples(34.9%for bulk g-C3N4;79.5%,91.1%,and 85.5%for g-C3N4re-calcined at 500,550,and 575°C,respectively,Fig.7B and S9A).
The rate constants(K)of TC photodegradation were obtained by applying the pseudo- first-order model(Fig.7C,S9B,and Table S3).The photodegradation rate(0.0409 min-1)using g-C3N4(525°C)is higher than those obtained by using bulk g-C3N4(0.00686 min-1)or other recalcined g-C3N4samples(0.0255,0.0387,and 0.0306 min-1for g-C3N4re-calcined at 500,550,and 575°C,respectively).
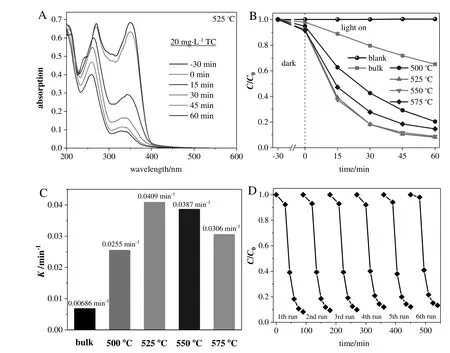
Fig.7.(A)Absorption spectra ofTC with reaction time in the presence of30.0 mg g-C3N4(525°C);(B)The adsorption and degradation curves ofTCin aqueous solution with time in the absence of photocatalyst or in the presence of g-C3N4 samples(30 mg);(C)The constants of TC photodegradation rates using g-C3N4 samples;(D)Recycling runs of TC removal using g-C3N4(525°C).

Fig.8.(A)XRD patterns and(B)survey XPS spectra of g-C3N4(525°C)before and after six runs.
The recyclability of g-C3N4(525°C)was studied through the degradation of TC.No obvious decline of the degradation efficiency is found after six recycling runs(Fig.7D).XRDpatterns of the used and fresh catalysts are identical(Fig.8A).The survey(Fig.8B)and high-resolution(Fig.S10)XPS data confirm the stable elementcomposition and the stability of C and N valence states.The morphology of catalyst is still maintained(Fig.S11).
The effectofinitialconcentration and pHvalue ofTC solutions on the photocatalytic activity ofg-C3N4was studied(Fig.S12).The degradation efficiency of TC decreases with the increase of TC concentration,and it goes up with the decrease of pH from 9 to 3.
The photocatalytic performance of g-C3N4(525°C)sample was further compared with commercial TiO2(P25).As shown in Fig.S13,g-C3N4(525°C)exhibits much higher removalcapability than P25 toward contaminants(MB,MO,and TC).
3.3.Photocatalytic reaction mechanism
The photodegradation of pollutants usually relies on active species(e.g.,h+,·OH,·O2-)in photocatalytic reaction systems[56,58].Thus,radical-capture experiments were carried out to identify the primary active species.The addition of isopropanol(IPA,1 mmol)has virtually no in fluence on TC photodegradation(Figs.9 and S14),demonstrating that·OH is not the active species.The TC removal efficiency is slightly inhibited in the presence of ammonium oxalate(AO,1 mmol),suggesting that h+is not the main active species.However,the photodegradation of TC is completely inhibited in the presence of benzoquinone(BQ,0.02 mmol).Therefore,·O2-is the primary active species.
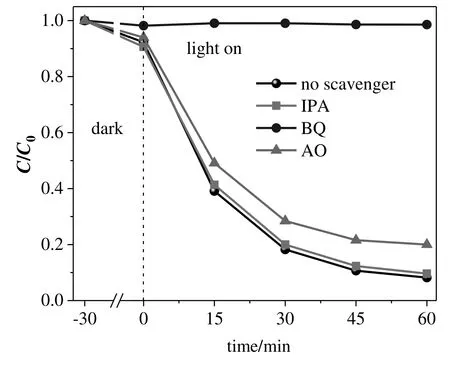
Fig.9.Effect of scavengers on photocatalytic performance of g-C3N4(525°C)in the photodegradation of TC under visible light.
The interfacial charge transfer and separation efficiency of photogenerated electrons and holes were studied by photoluminescence(PL)analysis.A higher recombination of photogenerated carriers is indicated by higher PL intensity[56].Fig.10A shows the PL spectra of bulk and re-calcined g-C3N4samples.The main emission peak is centered at around 460 nm for bulk g-C3N4,as attributed to the band-to-band PL phenomenon due to the down-transfer ofthe photo-induced charge carriers of g-C3N4[59].The re-calcined g-C3N4shows much lower PL intensity.Moreover,the emission peak experiences a slight blue-shift,consistentwith the extension ofthe absorption edge toward shortwavelength(Fig.5).
The interfacialcharge transferproperties and the separation efficiency of the photogenerated carriers can be investigated using electrochemical impedance spectroscopy(EIS)[60].Fig.10B shows the EIS Nyquist plots of typical samples.A smaller arc radius of the EIS Nyquist plot can re flect a higher efficient charge transfer at the interface[61].g-C3N4(525°C)exhibits more effective separation of photo-generated charge carriers due to its smaller arc radius.Thus,suitable re-calcination of bulk C3N4can dramatically enhance the separation and transfer ef ficiency of photo-generated electron–hole pairs.
The flat-band potentialsofg-C3N4samples were measured using the electrochemical method and the Mott-Schottky plots(Fig.11).The positive slopes of the linear plots apparently disclose the typical n-type inorganic semiconductors[45],and the conduction band(CB)potentialof the samples can be obtained from the intercept on the abscissa of MS plots.Fig.S15 shows the schematic energy level diagrams of the bulk and re-calcined g-C3N4.The CB potential of the re-calcined g-C3N4samples increases with the increase of calcination temperature and is more negative than thatofbulk g-C3N4.The higher position ofCB willresultin stronger reducibility bene ficial for photocatalytic degradation of pollutants.
A possible mechanism of pollutant degradation is proposed(Fig.S16).Under visible light irradiation,the electrons(e-)in g-C3N4will be photoexcited from the VB to CB due to the suitable band-gap(~2.70 eV).Because the CB potential of g-C3N4is higher than that of O2/O2-(-0.33 eV)[62],the electrons on the CB of g-C3N4can reduce O2to generate·O2-.The photogenerated holes(h+)left in the CB of g-C3N4could not oxidize H2O to generate·OH due to the more positive potential of·OH/H2O(2.68 eV)[62].Hence,·O2-and h+will react with the target pollutant molecules(MB,MO,and TC).

Fig.10.(A)Photoluminescence(PL)spectra and(B)electrochemical impedance spectroscopy(EIS)curves of g-C3N4 samples.

Fig.11.Mott–Schottky plots for g-C3N4 samples.
4.Conclusions
A series of porous g-C3N4samples were prepared by simply calcining bulk(non-porous)g-C3N4at different temperatures in a muffle oven(in the presence of static air).They all possess much larger BET specific surface areas than bulk g-C3N4.All the re-calcined g-C3N4samples exhibit much better adsorption and photocatalytic performance than bulk g-C3N4in the photocatalytic degradation of MB,MO,and TC under visible irradiation,and 525°C is the optimal re-calcination temperature.Recycling runs of TC degradation demonstrate the stability of g-C3N4.Such stable and upgraded photocatalytic activity can be attributed to the effective separation of photogenerated charge carriers.Moreover,g-C3N4(525°C)sample exhibits much higher removal capability than P25 toward contaminants(MB,MO,and TC).Superoxide radical anions(·O-2)are the primary active species for photocatalytic degradation.These porous g-C3N4materials not only show significant potential for the puri fication of wastewater but also may be useful for the construction of g-C3N4-based heterojunction catalysts in future studies.
Supplementary Material
Supplementary data to this article can be found online athttps://doi.org/10.1016/j.cjche.2017.10.010.
References
[1]X.C.Wang,K.Maeda,A.Thomas,K.Takanabe,G.Xin,J.M.Carlsson,K.Domen,M.Antonietti,A metal-free polymeric photocatalyst for hydrogen production from water under visible light,Nat.Mater.8(2009)76–80.
[2]Y.Wang,X.C.Wang,M.Antonietti,Polymeric graphitic carbon nitride as a heterogeneous organocatalyst:From photochemistry to multipurpose catalysis to sustainable chemistry,Angew.Chem.Int.Ed.51(2012)68–89.
[3]X.Q.Fan,L.X.Zhang,R.L.Cheng,M.Wang,M.L.Li,Y.J.Zhou,J.L.Shi,Construction of graphitic C3N4-based intramolecular donor-acceptor conjugated copolymers for photocatalytic hydrogen evolution,ACS Catal.5(2015)5008–5015.
[4]Q.Han,B.Wang,Y.Zhao,C.G.Hu,L.T.Qu,A graphitic-C3N4“seaweed”architecture for enhanced hydrogen evolution,Angew.Chem.Int.Ed.54(2015)11433–11437.
[5]F.He,G.Chen,Y.S.Zhou,Y.G.Yu,Y.Zheng,S.Hao,The facile synthesis of mesoporous g-C3N4with highly enhanced photocatalytic H2evolution performance,Chem.Commun.51(2015)16244–16246.
[6]X.Y.Yuan,C.Zhou,Y.R.Jin,Q.Y.Jing,Y.L.Yang,X.Shen,Q.Tang,Y.H.Mu,A.K.Du,Facile synthesis of 3D porous thermally exfoliated g-C3N4nanosheet with enhanced photocatalytic degradation of organic dye,J.Colloid Interf.Sci.468(2016)211–219.
[7]Y.Z.Yu,J.G.Wang,Direct microwave synthesis of graphitic C3N4with improved visible-light photocatalytic activity,Ceram.Int.42(2016)4063–4071.
[8]M.Zhang,W.J.Jiang,D.Liu,J.Wang,Y.F.Liu,Y.Y.Zhu,Y.F.Zhu,Photodegradation of phenol via C3N4-agar hybrid hydrogel 3D photocatalysts with free separation,Appl.Catal.B-Environ.183(2016)263–268.
[9]X.L.Yang,F.F.Qian,G.J.Zou,M.L.Li,J.R.Lu,Y.M.Li,M.T.Bao,Facile fabrication of acidi fied g-C3N4/g-C3N4hybrids with enhanced photocatalysis performance under visible light irradiation,Appl.Catal.B-Environ.193(2016)22–35.
[10]Y.J.Cui,Y.B.Tang,X.C.Wang,Template-free synthesis of graphitic carbon nitride hollow spheres for photocatalytic degradation of organic pollutants,Mater.Lett.161(2015)197–200.
[11]P.F.Xia,B.C.Zhu,J.G.Yu,S.W.Cao,M.Jaroniec,Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2reduction,J.Mater.Chem.A 5(2017)3230–3238.
[12]G.H.Dong,W.K.Ho,Y.H.Li,L.Z.Zhang,Facile synthesis of porous graphene-like carbon nitride(C6N9H3)with excellent photocatalytic activity for NO removal,Appl.Catal.B-Environ.174(2015)477–485.
[13]F.Dong,Y.H.Li,Z.Y.Wang,W.K.Ho,Enhanced visible light photocatalytic activity and oxidation ability of porous graphene-like g-C3N4nanosheets via thermal exfoliation,Appl.Surf.Sci.358(2015)393–403.
[14]W.K.Ho,Z.Z.Zhang,M.K.Xu,X.W.Zhang,X.X.Wang,Y.Huang,Enhanced visiblelight-driven photocatalytic removal of NO:Effect on layer distortion on g-C3N4by H2heating,Appl.Catal.B-Environ.179(2015)106–112.
[15]X.F.Chen,J.S.Zhang,X.Z.Fu,M.Antonietti,X.C.Wang,Fe-g-C3N4-catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light,J.Am.Chem.Soc.131(2009)11658–11659.
[16]L.Ge,C.C.Han,X.L.Xiao,L.L.Guo,Y.J.Li,Enhanced visible light photocatalytic hydrogen evolution of sulfur-doped polymeric g-C3N4photocatalysts,Mater.Res.Bull.48(2013)3919–3925.
[17]K.Wang,Q.Li,B.S.Liu,B.Cheng,W.K.Ho,J.G.Yu,Sulfur-doped g-C3N4with enhanced photocatalytic CO2reduction performance,Appl.Catal.B-Environ.176(2015)44–52.
[18]J.W.Fu,B.C.Zhu,C.J.Jiang,B.Cheng,Hierarchical porous O-doped g-C3N4with enhanced photocatalytic CO2reduction activity,Small 13(2017),1603938.(9 pages).
[19]X.J She,J.J.Wu,J.Zhong,H.Xu,Y.C.Yang,R.Vajtai,J.Lou,Y.Liu,D.L.Du,H.M.Li,P.M.Ajayan,Oxygenated monolayer carbon nitride for excellent photocatalytic hydrogen evolution and external quantum efficiency,Nano Energy 27(2016)138–146.
[20]Z.F.Huang,J.J.Song,L.Pan,Z.M.Wang,X.Q.Zhang,J.J.Zou,W.B.Mi,X.W.Zhang,L.Wang,Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution,Nano Energy 12(2015)646–656.
[21]S.C.Yan,Z.S.Li,Z.G.Zou,Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4under visible light irradiation,Langmuir 26(2010)3894–3901.
[22]J.Liu,Y.Liu,N.Y.Liu,Y.Z.Han,X.Zhang,H.Huang,Y.Lifshitz,S.T.Lee,J.Zhong,Z.H.Kang,Metal-free efficient photocatalyst for stable visible water splitting via a twoelectron pathway,Science 347(2015)970–974.
[23]Y.P.Zhu,T.Z.Ren,Z.Y.Yuana,Mesoporous phosphorus-doped g-C3N4nanostructured flowers with superior photocatalytic hydrogen evolution performance,ACS Appl.Mater.Inter.7(2015)16850–16856.
[24]J.R.Ran,T.Y.Ma,G.P.Gao,X.W.Du,S.Z.Qiao,Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-lightphotocatalytic H2production,Energy Environ.Sci.8(2015)3708–3717.
[25]Z.A.Lan,G.G.Zhang,X.C.Wang,A facile synthesis of Br-modi fied g-C3N4semiconductors for photoredox water splitting,Appl.Catal.B-Environ.192(2016)116–125.
[26]Y.Shiraishi,Y.Kofuji,S.Kanazawa,H.Sakamoto,S.Ichikawa,S.Tanaka,T.Hirai,Platinum nanoparticles strongly associated with graphitic carbon nitride as efficient cocatalysts for photocatalytic hydrogen evolution under visible light,Chem.Commun.50(2014)15255–15258.
[27]S.Samanta,S.Martha,K.Parida,Facile synthesis of Au/g-C3N4nanocomposites:An inorganic/organic hybrid plasmonic photocatalyst with enhanced hydrogen gas evolution under visible-light irradiation,ChemCatChem 6(2014)1453–1462.
[28]Y.S.Fu,T.Huang,L.L.Zhang,J.W.Zhu,X.Wang,Ag/g-C3N4catalyst with superior catalytic performance for the degradation of dyes:A borohydride-generated superoxide radical approach,Nanoscale 7(2015)13723–13733.
[29]F.Dong,Z.W.Zhao,Y.J.Sun,Y.X.Zhang,S.Yan,Z.B.Wu,An advanced semimetal-organic Bi spheres-g-C3N4nanohybrid with SPR-enhanced visible-light photocatalytic performance for NO puri fication,Environ.Sci.Technol.49(2015)12432–12440.
[30]Z.W.Zhao,Y.J.Sun,F.Dong,Graphitic carbon nitride based nanocomposites:A review,Nanoscale 7(2015)15–37.
[31]G.Mamba,A.K.Mishra,Graphitic carbon nitride(g-C3N4)nanocomposites:A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation,Appl.Catal.B-Environ.198(2016)347–377.
[32]J.L.Zhang,Z.Ma,Novel β-Ag2MoO4/g-C3N4heterojunction catalysts with highly enhanced visible-light-driven photocatalytic activity,RSC Adv.7(2017)2163–2171.
[33]J.L.Zhang,Z.Ma,Ag6Mo10O33/g-C3N41D-2D hybridized heterojunction as an ef ficient visible-light-driven photocatalyst,Mol.Catal.432(2017)285–291.
[34]M.Groenewolt,M.Antonietti,Synthesis of g-C3N4nanoparticles in mesoporous silica host matrices,Adv.Mater.17(2005)1789–1792.
[35]X.Jin,V.V.Balasubramanian,S.T.Selvan,D.P.Sawant,M.A.Chari,G.Q.Lu,A.Vinu,Highly ordered mesoporous carbon nitride nanoparticles with high nitrogen content:A metal-free basic catalyst,Angew.Chem.Int.Ed.48(2009)7884–7887.
[36]P.Niu,L.L.Zhang,G.Liu,H.M.Cheng,Graphene-like carbon nitride nanosheets for improved photocatalytic activities,Adv.Funct.Mater.22(2012)4763–4770.
[37]S.B.Yang,Y.J.Gong,J.S.Zhang,L.Zhan,L.L.Ma,Z.Y.Fang,R.Vajtai,X.C.Wang,P.M.Ajayan,Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light,Adv.Mater.25(2013)2452–2456.
[38]X.J.Bai,L.Wang,R.L.Zong,Y.F.Zhu,Photocatalytic activity enhanced via g-C3N4nanoplates to nanorods,J.Phys.Chem.C 117(2013)9952–9961.
[39]Y.Zhao,F.Zhao,X.P.Wang,C.Y.Xu,Z.P.Zhang,G.Q.Shi,L.T.Qu,Graphitic carbon nitride nanoribbons:Graphene-assisted formation and synergic function for highly efficient hydrogen evolution,Angew.Chem.Int.Ed.53(2014)13934–13939.
[40]Y.S.Jun,E.Z.Lee,X.C.Wang,W.H.Hong,G.D.Stucky,A.Thomas,From melaminecyanuric acid supramolecular aggregates to carbon nitride hollow spheres,Adv.Funct.Mater.23(2013)3661–3667.
[41]D.D.Zheng,C.J.Huang,X.C.Wang,Post-annealing reinforced hollow carbon nitride nanospheres for hydrogen photosynthesis,Nanoscale 7(2015)465–470.
[42]Q.Han,B.Wang,J.Gao,Z.H.Cheng,Y.Zhao,Z.P.Zhang,L.T.Qu,Atomically thin mesoporous nanomesh of graphitic C3N4for high-efficiency photocatalytic hydrogen evolution,ACS Nano 10(2016)2745–2751.
[43]S.N.Guo,Y.Zhu,Y.Y.Yan,Y.L.Min,J.C.Fan,Q.J.Xu,Holey structured graphitic carbon nitride thin sheets with edge oxygen doping via photo-Fenton reaction with enhanced photocatalytic activity,Appl.Catal.B-Environ.185(2016)315–321.
[44]Q.H.Liang,Z.Li,X.L.Yu,Z.H.Huang,F.Y.Kang,Q.H.Yang,Macroscopic 3D porous graphitic carbon nitride monolith for enhanced photocatalytic hydrogen evolution,Adv.Mater.27(2015)4634–4639.
[45]L.Q.Yang,J.F.Huang,L.Shi,L.Y.Cao,Q.Yu,Y.N.Jie,J.Fei,H.B.Ouyang,J.H.Ye,A surface modi fication resultant thermally oxidized porous g-C3N4with enhanced photocatalytic hydrogen production,Appl.Catal.B-Environ.204(2017)335–345.
[46]S.W.Cao,J.X.Low,J.G.Yu,M.Jaroniec,Polymeric photocatalysts based on graphitic carbon nitride,Adv.Mater.27(2015)2150–2176.
[47]J.L.Zhang,H.Liu,Z.Ma,Flower-like Ag2O/Bi2MoO6p-n heterojunction with enhanced photocatalytic activity under visible light irradiation,J.Mol.Catal.A Chem.424(2016)37–44.
[48]J.L.Zhang,Z.Ma,Flower-like Ag2MoO4/Bi2MoO6heterojunctions with enhanced photocatalytic activity under visible light irradiation,J.Taiwan Inst.Chem.Eng.71(2017)156–164.
[49]J.L.Zhang,Z.Ma,Flower-like Ag3VO4/BiOBr n-p heterojunction photocatalysts with enhanced visible-light-driven catalytic activity,Mol.Catal.436(2017)190–198.
[50]J.L.Zhang,Z.Ma,Enhanced visible-light photocatalytic performance of Ag3VO4/Bi2WO6heterojunctions in removing aqueous dyes and tetracycline hydrochloride,J.Taiwan Inst.Chem.Eng.78(2017)212–218.
[51]J.H.Li,B.Shen,Z.H.Hong,B.Z.Lin,B.F.Gao,Y.L.Chen,A facile approach to synthesize novel oxygen-doped g-C3N4with superior visible-light photoreactivity,Chem.Commun.48(2012)12017–12019.
[52]L.Shi,K.Chang,H.B.Zhang,X.Hai,L.Q.Yang,T.Wang,J.H.Ye,Drastic enhancement of photocatalytic activities over phosphoric acid protonated porous g-C3N4nanosheets under visible light,Small 12(2016)4431–4439.
[53]P.Qiu,H.Chen,C.Xu,N.Zhou,F.Jiang,X.Wang,Y.Fu,Fabrication of an exfoliated graphitic carbon nitride as a highly active visible light photocatalyst,J.Mater.Chem.A 3(2015)24237–24244.
[54]W.J.Ong,L.L.Tan,Y.H.Ng,S.T.Yong,S.P.Chai,Graphitic carbon nitride(g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation:Are we a step closer to achieving sustainability?Chem.Rev.116(2016)7159–7329.
[55]J.L.Zhang,L.S.Zhang,N.Yu,K.B.Xu,S.J.Li,H.L.Wang,J.S.Liu,Flower-like Bi2S3/Bi2MoO6heterojunction superstructures with enhanced visible-light-driven photocatalytic activity,RSC Adv.5(2015)75081–75088.
[56]J.L.Zhang,L.S.Zhang,X.F.Shen,P.F.Xu,J.S.Liu,Synthesis of BiOBr/WO3p-n heterojunctions with enhanced visible light photocatalytic activity,CrystEngComm 18(2016)3856–3865.
[57]M.Yan,Y.L.Wu,F.F.Zhu,Y.Q.Hua,W.D.Shi,The fabrication of a novel Ag3VO4/WO3heterojunction with enhanced visible light efficiency in the photocatalytic degradation of TC,Phys.Chem.Chem.Phys.18(2016)3308–3315.
[58]L.F.Yue,S.F.Wang,G.Q.Shan,W.Wu,L.W.Qiang,L.Y.Zhu,Novel MWNTs-Bi2WO6composites with enhanced simulated solar photoactivity toward adsorbed and free tetracycline in water,Appl.Catal.B-Environ.176(2015)11–19.
[59]M.L.Li,L.X.Zhang,X.Q.Fan,Y.J.Zhou,M.Y.Wu,J.L.Shi,Highly selective CO2photoreduction to CO over g-C3N4/Bi2WO6composites under visible light,J.Mater.Chem.A 3(2015)5189–5196.
[60]S.S.Yi,J.M.Yan,B.R.Wulan,S.J.Li,K.H.Liu,Q.Jiang,Noble-metal-free cobalt phosphide modi fied carbon nitride:An efficient photocatalyst for hydrogen generation,Appl.Catal.B-Environ.200(2017)477–483.
[61]T.T.Zhu,L.Y.Huang,Y.H.Song,Z.G.Chen,H.Y.Ji,Y.P.Li,Y.G.Xu,Q.Zhang,H.Xu,H.M.Li,Modi fication of Ag3VO4with graphene-like MoS2for enhanced visible-light photocatalytic property and stability,New J.Chem.40(2016)2168–2177.
[62]Y.F.Li,R.X.Jin,X.Fang,Y.Yang,M.Yang,X.C.Liu,Y.Xing,S.Y.Song,In situ loading of Ag2WO4on ultrathin g-C3N4nanosheets with highly enhanced photocatalytic performance,J.Hazard.Mater.313(2016)219–228.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- CFD modeling of turbulent reacting flow in a semi-batch stirred-tank reactor☆
- Synergistic and interference effects in coaxial mixers:Numerical analysis of the power consumption☆
- Atomic layer deposition of Al2O3 on porous polypropylene hollow fibers for enhanced membrane performances☆
- Experimental investigation and cost assessment of the salt production by solar assisted evaporation of saturated brine☆
- Equilibrium of liquid-liquid extraction of 2-phenylbutyric acid enantiomers:Experiment and model☆
