Cobalt catalysts for Fischer–Tropsch synthesis:The effect of support,precipitant and pH value☆
Zhenhua Li,Mengyao Si,Li Xin,Renjie Liu,Runxue Liu,Jing Lü*
Key Lab for Green Chemical Technology of Ministry of Education,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
1.Introduction
Fischer–Tropsch synthesis(FTS)is a promising alternative route to convert hydrogen and carbon monoxide to liquid fuels and chemicals withoutsulfur,nitrogen and aromatic hydrocarbon,and itis polymerized to a myriad of hydrocarbon products with--CH2--being considered as the basic monomer[1–3].Compared to iron-based catalysts,cobaltbased catalysts for FTS are in focus of current interest on account of their high CO conversion,high selectivity to linear C5+,and low activity for water–gas shift reaction[4].With the purpose of gaining high metal dispersion and increasing the specific surface area of catalysts,various supports for cobalt catalysts have been used for FTS,including SiO2,Al2O3,TiO2,carbon nanotubes,zeolite,etc.[5–7].Because ofthe strong interactions between cobaltparticles and common oxide supports like SiO2and Al2O3,the cobaltspecies forms a spinelstructure such as Co2SiO4and CoAl2O4,which are hardly reducible under the reduction treatment[8].
ZrO2,a transition metal oxide with the excellent ion exchange performance and surface enrichment of oxygen vacancies,has been receiving considerable attention as the support for Co-based catalysts in Fischer–Tropsch synthesis.Zhang etal.[9]investigated thatan appropriate amount of CeO2promoter added to Co/ZrO2catalyst could increase the active sites of the catalysts due to the increase of reducibility and dispersion.Notably,some research efforts have been devoted to the preparation of Co/ZrO2catalysts by co-precipitation method.Chen and Sun[10]compared the various molar ratios of Co/ZrO2catalysts and found that the CO conversion increased monotonously with the increment of Co content.Subsequently,the effect of reduction atmosphere on the reaction performance of Co-ZrO2co-precipitation catalysts was investigated by Jia et al.[11].
It is well-known that the support plays a crucial role on the key performance indicators,including activity,selectivity and stability[12].Nevertheless,for cobalt-based catalysts prepared by co-precipitation,the comparison between the effect of typical oxides and ZrO2as supports on the FTS performance has not been found in the literature.Here,we choose three different metal oxides,ZnO,Al2O3and ZrO2,as the supports for Co-based catalysts to study the effect of different supports on their supported Co catalysts for FTS performance.
As reported,different precipitating agents often play crucial role on the final catalytic performance.Liu et al.[13]found that the Ni-Mg/Al2O3catalysts prepared by co-precipitation by using NH4OH,NaOH or Na2CO3as the precipitant have varied interaction between NiO and oxide support.Their syngas methanation performance decreased in the order NaOH>NH4OH>Na2CO3.Thus,the effects ofdifferentprecipitants including NH4OH,Na2CO3and NaOHwere investigated on the Cobased catalystfor FTS performance.In addition,the catalysts with different pH values were also prepared and evaluated in a fixed-bed reactor.
2.Experimental
2.1.Preparation of catalysts
All chemicals with analytical grade including cobalt(II)nitrate hexahydrate(Co(NO3)2·6H2O),zirconium(IV)nitrate pentahydrate(Zr(NO3)4·5H2O),aluminum(III)nitrate nonahydrate(Al(NO3)3·9H2O),zinc(II)nitrate hexahydrate(Zn(NO3)2·6H2O),sodium hydroxide(NaOH),sodium carbonate anhydrous(Na2CO3)and 25 wt%–28 wt%ammonia solution(NH4OH)were purchased from Tianjin Kermel Co.,LTD of China and used without any further puri fication.
Catalysts atdifferentsupports:the catalysts containing about20 wt%cobalt on differentsupports were prepared by co-precipitation method.The corresponding precusor of ZrO2,Al2O3or ZnO was precipitated by precipitant Na2CO3to prepare 20 wt%Co/ZrO2,Co/Al2O3or Co/ZnO,respectively.Here Na2CO3instead of NaOH was used as the precipitant since the Al(OH)3produced by the reaction ofNaOHand Al(NO3)3is soluble in NaOH solution.Take the preparation of 20 wt%Co/ZrO2catalyst as an example,a certain amountofCo(NO3)2·6H2Oand Zr(NO3)4·5H2O were dissolved in 50-ml deionized water to form solution A,wherein the concentration of Zr4+was 0.5 mol·L-1.Another solution B was obtained by dissolving some amounts of Na2CO3in 50-ml deionized water to form 1.47 mol∙L-1solution.The above two solutions of A and B were simultaneously added dropwise into a three mouths roundbottomed flask containing 200-ml deionized water at 60°C under vigorous stirring.Then,the pH value was adjusted to 9.0,being aged for 12 h at room temperature.The obtained products were filtered and washed thoroughly with deionized water until pH value of 7,dried overnight at 110 °C in an oven and calcined at 400 °C for 4 h at a heating ramp of 3 °C·min-1.
Catalysts at different precipitants:The 20-wt%Co/ZrO2catalysts were prepared using NH4OH,Na2CO3or NaOH as the precipitant at pH value 9 during aging process by adding some amount of 2.0 mol·L-1nitric acid.
Catalysts at different pH values:The 20-wt%Co/ZrO2catalysts were prepared using NaOH as the precipitant at different pH values(9,11 or 13)during aging process by adding some amount of 2.0 mol·L-1nitric acid.
The sample was denoted as Co/x-y-z,in which “x”represents the support;“y”stands for the precipitant used,and “z”represents pH value.
2.2.Catalyst characterization
2.2.1.N2 adsorption–desorption
N2adsorption–desorption experiments were conducted at-196 °C using a Micromeritics Tristar-3000 analyzer.Prior to an experiment,each catalyst was degassed at 90 °C for 1 h and 300 °C for 3 h to remove the moisture adsorbed at surface and internal pore.The specific surface areas of the samples were calculated by the BET method.The pore size distributions were evaluated from the desorption branches of the isotherms using the Barrett–Joyner–Halenda(BJH)method.
2.2.2.X-ray powder diffraction(XRD)
X-ray powder diffraction spectra for the calcined catalysts were recorded on a RigakuD/max-2500 diffractometer using Cu Kα radiation(40 kV,200 mA).The scan range was from 2θ=5°to 80°with scanning rate 8(°)·min-1.Crystallite phases were determined by comparing the diffraction patterns with those in the standard powder XRD files(JCPDS)published by the International Center for Diffraction Data.The average Co3O4size was calculated using the Scherrer equation.
2.2.3.X-ray fluorescence(XRF)
XRF measurements were performed on S4 Pioneer X-ray fluorescence spectrometer.The obtained data were used to determine the amount of Co in the catalyst.
2.2.4.Transmission electron microscopy(TEM)
The TEM analysis was performed on a JEM-2100F transmission electron microscope at 200 kV.The catalysts were dispersed in ethanol in a sonicator and then loaded onto a carbon-coated copper grid.
2.2.5.H2 temperature programmed reduction(H2-TPR)
The TPR experimentwas carried outwith a Micromeritics AutoChem 2910 equipped with a TCD detector to investigate the reduction behavior of the catalyst.The catalyst(0.1 g)was placed in a U-type quartz tubular reactor, fitted with a thermocouple for continuous temperature measurement.Before the TPR measurement,the calcined catalysts were purged with Ar( flow rate=30 ml·min-1)at 200 °C for 2 h to remove waterorimpurities,and then cooled down to 50°C.Then,10%H2/Ar was switched on,and the temperature was raised at a rate of 10 °C·min-1from 50 to 780 °C using a flow rate of 30 ml·min-1.
2.2.6.H2 temperature programmed desorption(H2-TPD)
The H2-TPD was used for estimating the Co dispersion ofthe catalyst using a Micromeritics AutoChem 2910 system.The sample weight used was 0.05 g.The catalyst was first reduced at 400°C for 5 h under a flow of high-purity hydrogen( flow rate=30 ml·min-1),then the sample was cooled to 50°C and saturated with H2.After removing the physically adsorbed H2by flush with Ar,the catalyst was heated to 780°C at 10 °C·min-1in an Ar flow(30 ml·min-1).The number of surface Co sites per unit mass of catalyst was determined by means of H2-TPD assuming the adsorption stoichiometry of H/Co=1:1.The dispersion of Co was calculated based on the volume of chemisorbed H2using the following simpli fied equation:

2.3.Catalytic evaluation
The Fischer–Tropsch synthesis was evaluated in a fixed-bed microreactor.One gram of catalyst sample(20–40 mesh)diluted with 2.6 g of quartz sands(40–60 mesh)was uploaded in a stainless steel reaction tube with an inner diameter of 8 mm.The addition of quartz sands was to avoid the generation of hotspot in the catalyst bed.Prior to the reaction,the catalyst was reduced at 400 °C in pure H2(100 ml·min-1)for 5 h and then the reactor temperature was decreased to 180°C under flowing hydrogen.Subsequently,A gaseous mixture of H2and CO,as well as Ar(as an internal standard)at a molar ratio of H2/CO/Ar=6/3/1was introduced to supply the reactant gas stream to the catalyst with a weight hourly space velocity(WHSV)of 5400 ml·(h·g)-1,and the pressure was increased gradually to 2.0 MPa.The average temperature of the catalyst bed was controlled at 230°C.
The products were continuously removed from the reactor and passed through two traps,one maintained at170°C(hot trap)to collect wax,and the other at-4°C(cold trap)to collectthe oil and water mixture.The uncondensed vapor stream was reduced to atmospheric pressure using a pressure reducing valve,and then analyzed online using an Agilent 6820 GC equipped with a thermal conductivity detector(TCD)and a flame ionization detector(FID).The composition of the liquid oil and solid wax was analyzed by a SP-3420 GC equipped with an FID.The catalytic activity was characterized by determining the CO conversion and the selectivity of C5+,which was measured at the steady state and calculated based on the carbon basis[14].
3.Results and Discussion
3.1.Characterization of catalysts
3.1.1.XRF analysis
To determine the amount of Co loading on catalysts prepared by the co-precipitation process,the samples were subjected to XRF analysis and the results are listed in Table 1.It is clear that the actual content of Co over allcatalysts is close to the intended value in allcatalysts,implying thatthere is almostno cobaltspecies lost during co-precipitation process.
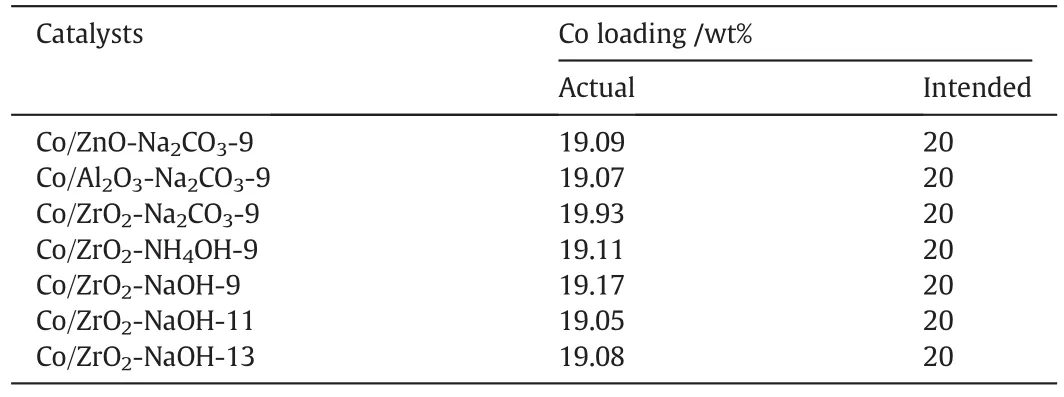
Table 1 XRF measurement results of different catalysts
3.1.2.Nitrogen adsorption analysis
The N2adsorption–desorption isothermals and the pore size distributions of the samples are displayed in Fig.1.As shown in Fig.1a,all catalysts show the type IV adsorption isothermals,which is the signi ficant feature of the mesoporous materials.For Co/ZnO-Na2CO3-9 and Co/Al2O3-Na2CO3-9,the isothermals belong to type IV with H1-type hysteresis loop.However,for the series ofCo/ZrO2catalysts,the isothermals attribute to type IV with H2-type hysteresis loop.This indicates that the mesoporous structure of ZrO2is different from other supports.And as shown in Fig.1b,the series ofCo/ZrO2catalysts have very narrow pore size distribution(PSD)curves around 3.0–4.0 nm,while the PSD curves of Co/ZnO-Na2CO3-9 and Co/Al2O3-Na2CO3-9 are broad.Table 2 lists the surface area,pore volume,and average pore diameterofthe calcined samples.As can be seen in Table 2,the Co/ZnO-Na2CO3-9 catalyst has a lower specific surface area,than both Co/Al2O3-Na2CO3-9 and Co/ZrO2-Na2CO3-9.It is noteworthy that the specific surface area of Co/Al2O3-Na2CO3-9 is 278.9 m2·g-1,which is higher than those of the Co/ZrO2catalysts.When it comes to the series of Co/ZrO2catalysts prepared by different precipitants and different pH values,the order of the specific surface area is Co/ZrO2-NaOH-11>Co/ZrO2-NaOH-13>Co/ZrO2-NaOH-9>Co/ZrO2-Na2CO3-9>Co/ZrO2-NH4OH-9.
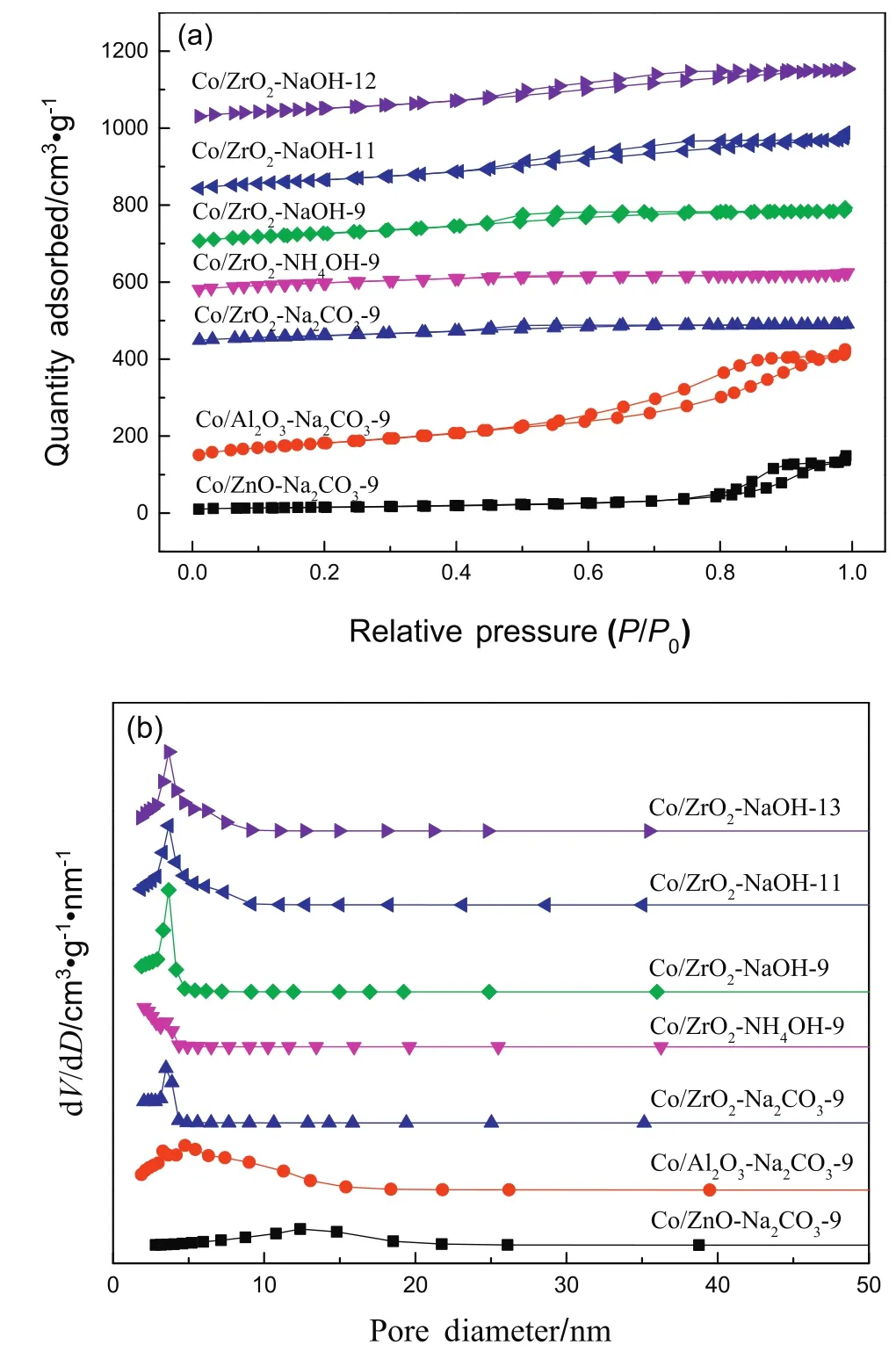
Fig.1.N2 adsorption isothermals(a)and pore size distribution profiles(b)ofthe catalysts.
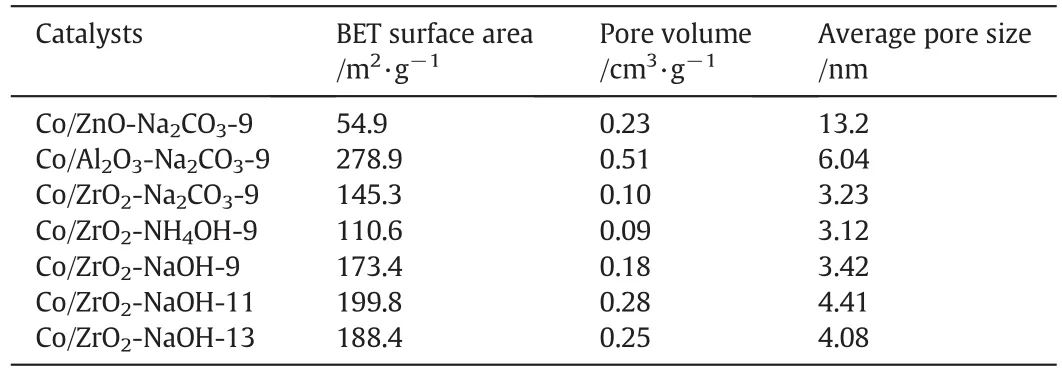
Table 2 Textural properties of various catalysts
3.1.3.XRD analysis
The crystal structure of the Co/ZnO-Na2CO3-9,Co/Al2O3-Na2CO3-9 and a series ofCo/ZrO2catalystswere conducted by XRD,and the results are displayed in Fig.2.For Co/ZnO-Na2CO3-9,the strong diffraction peaks at positions of 31.8°,34.4°,36.3°,47.5°,56.6°,62.7°,67.9°,69.0°and 76.8°of ZnO crystals were detected according to the JCPDS card no.05-0664[15].However,the XRD peak of Al2O3at 36.8°[16]is less apparent on Co/Al2O3-Na2CO3-9 due to the strong intensity of Co3O4peaks,which disturbs the observation of the Al2O3peak.Clearly,no XRD pattern for ZrO2was observed in a series of Co/ZrO2catalysts.Therefore,it is believed that the crystallinity of the ZnO and Al2O3carriers obtained by this preparation method is better,butthe ZrO2support is relatively poor and present in an amorphous state.In addition,Co/ZrO2-NaOH-13 catalyst displays diffraction peaks at 31.3°,36.8°,38.5°,44.7°,59.4°and 65.2°,assigned to different crystal panes of the Co3O4phase[17].However,there is no diffraction peak for Co3O4on Co/ZrO2-Na2CO3-9 catalyst,and this is probably because of a high dispersion of Co3O4over the Co/ZrO2-Na2CO3-9 catalyst.
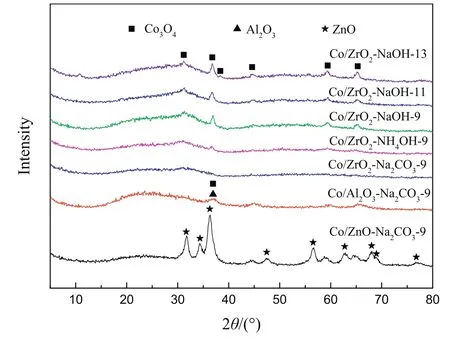
Fig.2.XRD patterns of calcined catalysts.
According to the XRD half-width of the diffraction peak,the average Co3O4crystalline sizes for all the fresh catalysts except Co/ZrO2-Na2CO3-9 estimated from the Scherrer equation were listed in Table 3.Depending on the results,it can be inferred that the Co dispersion in the samples supported by Al2O3is higher than that of by ZnO,which is also applicable to the series of Co/ZrO2samples with different precipitants and differentpHvalues.Furthermore,the expected cobaltparticle size determined by the molar volume correction of corresponding Co3O4size of unreduced catalysts using the equation d(Co)=0.75d(Co3O4)[8]was also obtained and shown in Table 3.
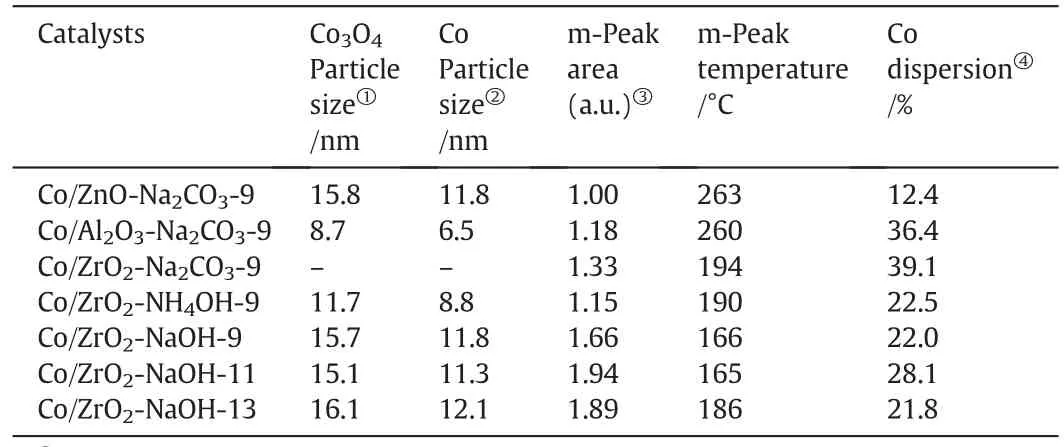
Table 3 Cobalt particle size and H2-TPD results of catalysts
To determine the valence state of cobalt species and whether ZrO2has a crystal transition during the reduction and reaction processes,the XRD analysis of reduced samples(reduction in H2)and the spent catalysts are also performed and shown in Fig.3.Interestingly,the slightly strong peak at 30.3°,50.5°and 60.4°corresponding to t-ZrO2can be detected over Co/ZrO2-NH4OH-9,Co/ZrO2-NaOH-11 and Co/ZrO2-NH4OH-13[18],indicating that the reduction process further crystallizes and then transforms the zirconia crystal from amorphous to tetragonal.For the reduced and used catalysts,the peaks at 44.3°and 75.8°attribute to the metallic Co(JCPDS card no.01-1255)[11],moreover,the degree of crystallization of the supports and metallic Co is further improved for all catalysts except Co/ZrO2-Na2CO3-9 after reaction(Fig.3b).For the Co/ZrO2-Na2CO3-9,no apparent ZrO2and Co species diffraction peaks can be found.
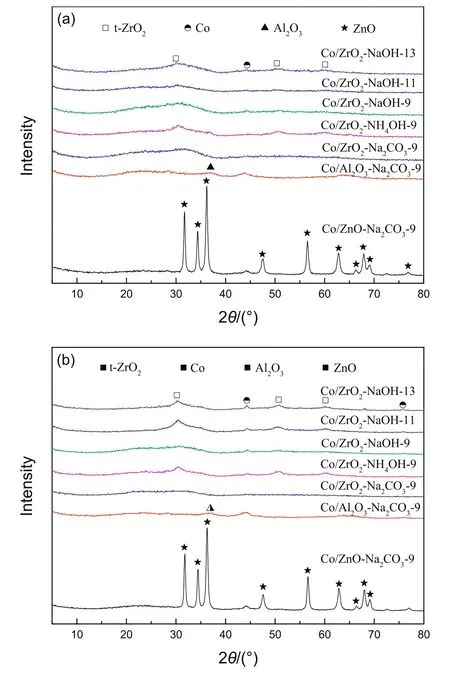
Fig.3.XRD patterns of reduced catalysts(a)and used catalysts(b).
3.1.4.H2-TPR analysis
The reduction behavior of catalysts was monitored by H2-TPR in Fig.4.Obviously,all the catalysts except Co/ZrO2-NH4OH-9 have three reduction peaks,which means that there are several forms of cobalt species in the catalyst.For Co/ZrO2-NH4OH-9,the peak at temperature of 334°C is attributed to the reduction of Co3O4to Co0[19],and as to other catalysts,the TPR peaks at around 200-450°C are assigned to the two-step reduction of Co3O4to Co0via CoO as the intermediate species[20].While the reduction peak located higher than 500°C is due to the strong interaction between cobalt particles and the support[15,21,22].Compared with Co/ZnO-Na2CO3-9 and Co/ZrO2-Na2CO3-9 catalysts,the third peak of Co/Al2O3-Na2CO3-9 is significantly shifted to a higher temperature,indicating that the Co–ZnO and Co–ZrO2interactions are pretty mild and a more hardly reducible cobalt species exists in Co/Al2O3-Na2CO3-9.Similarly,the third reduction peak temperature of Co/ZrO2-NaOH-9 is lower than that of Co/ZrO2-Na2CO3-9 and Co/ZrO2-NH4OH-9,which suggests that the cobalt species in Co/ZrO2-NaOH-9 has a weak interaction with the ZrO2support.In addition,as displayed in Fig.4,the reduction peaks at 200-450°C of Co/ZrO2-NaOH-11 and Co/ZrO2-NaOH-13 shift to lower temperatures when compared to Co/ZrO2-NaOH-9,suggesting that the Co/ZrO2-NaOH-11 and Co/ZrO2-NaOH-13 has a slightly higher reducibility.

Fig.4.H2-TPR profiles of fresh catalysts.
3.1.5.H2-TPD analysis
H2-TPD experiments can provide valuable quantitative information about amount of active sites and cobalt dispersion.Fig.5 shows the H2-TPD profiles of the catalysts.For all catalysts,there are two main H2desorption peaks at around 200-300 and 350-500°C.The first peak(peak m)at low temperature is attributed to the chemisorbed hydrogen on the metal Co with a high density of defects,which often serves as capture traps for hydrogen that are able to reduce the activation energy of hydrogen dissociation[23].The second peak located at 350-500°C is due to the adsorption of H2from the metal Co formed by the interaction between cobalt and the support[23].It is reported[24]that the hydrogen adsorption sites located at before 300°C had an important impact on the Fischer–Tropsch synthesis reaction,while the high temperature adsorption of H2is without Fischer–Tropsch synthesis reactivity.In consequence,the main research on the low temperature adsorption peak(peak m)is carried out.
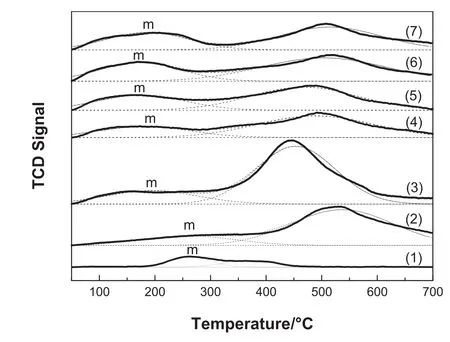
Fig.5.H2-TPDprofiles ofcalcined catalysts:(1)Co/ZnO-Na2CO3-9;(2)Co/Al2O3-Na2CO3-9;(3)Co/ZrO2-Na2CO3-9;(4)Co/ZrO2-NH4OH-9;(5)Co/ZrO2-NaOH-9;(6)Co/ZrO2-NaOH-11;(7)Co/ZrO2-NaOH-13.
Table 3 lists the calculated relative m-peak area,the temperature of peak m and the Co dispersions of catalysts based on the H2-TPD results.To have a better understanding of the amount of adsorption sites on catalysts,the m peak area of Co/ZnO-Na2CO3–9 is de fined as 1.Then the relative m peak area of other catalysts can also be obtained.As seen in Table 3,Co/ZrO2-NaOH-11 has the most low-temperature active adsorption sites,followed by Co/ZrO2-NaOH-13,Co/ZrO2-NaOH-9,Co/ZrO2-Na2CO3-9,Co/Al2O3-Na2CO3-9,Co/ZrO2-NH4OH-9,and Co/ZnONa2CO3-9 has the lowest.The interaction between Co and the adsorbed H2could also be investigated on the basis ofH2desorption peak positions.From the Fig.5 and Table 3,it can be observed that the Tmof the first H2desorption peaks follow an order of Co/ZnO-Na2CO3-9>Co/Al2O3-Na2CO3-9>Co/ZrO2-Na2CO3-9>Co/ZrO2-NH4OH-9>Co/ZrO2-NaOH-13>Co/ZrO2-NaOH-9>Co/ZrO2-NaOH-11.This suggests that when the temperature ofthe first H2desorption peak is higher,the interaction between active Co and adsorbed H2is stronger,which may strengthen the chain growth and thus increase the C5+selectivity.
Table 3 shows that among all the catalysts,Co/ZrO2-Na2CO3-9 has the highest Co dispersion of 39.1%,which is consistent with the conclusion from XRD analysis(Fig.3a).In addition,the Co dispersion of Co/ZnO-Na2CO3-9 is almost one-third of the Co/Al2O3-Na2CO3-9 catalyst,and the reason may be related to the weak metal-support interaction and small surface area of the catalyst.And as for the rest of catalysts,the cobalt dispersion of Co/ZrO2-NaOH-11 is larger than Co/ZrO2-NH4OH-9,Co/ZrO2-NaOH-9 and Co/ZrO2-NaOH-13,due to its larger numbers of H2adsorption sites.
3.1.6.TEM observations
The TEMimages and the corresponding energy dispersive X-ray spectroscopy(EDX)spectra of the reduced catalysts are shown in Fig.6.It is observed that the ZnO planes are obvious with the lattice fringes of 0.26 nm in Fig.6a′,which is in good agreement with the results of XRD analysis.Besides,the EDX spectra of the specified region in Co/ZrO2-NH4OH-9 and Co/ZrO2-NaOH-9 show clear peaks of Co,Zr and Cu,and the results show that the darker and larger particles mainly contain the cobalt element,while the smaller particles of light-colored material are assigned to ZrO2ignoring the Cu[25].So it can be judged that in Fig.6,the dark black particles are identified as metal Co,whereas the graycolored particles are recognized as supports.However,for Co/ZrO2-Na2CO3-9,it can't be observed the significant structure of ZrO2and cobalt particles from Fig.6c,which speculates that Co particles are homogeneously dispersed across the whole catalyst.In addition,the TEM images of Co/ZnO-Na2CO3-9,Co/ZrO2-NH4OH-9,Co/ZrO2-NaOH-9,Co/ZrO2-NaOH-11 and Co/ZrO2-NaOH-13(Fig.6a,d,e,f,g)exhibitan apparentagglomeration of Co species and can't be determined the dispersion of cobalt particles,but the Co particles in Co/Al2O3-Na2CO3-9 are uniformly dispersed on supports and because of the strong Co-Al2O3interaction,the average Co particle size is small,which exhibits the smaller Co particle size,the higher Co dispersion.
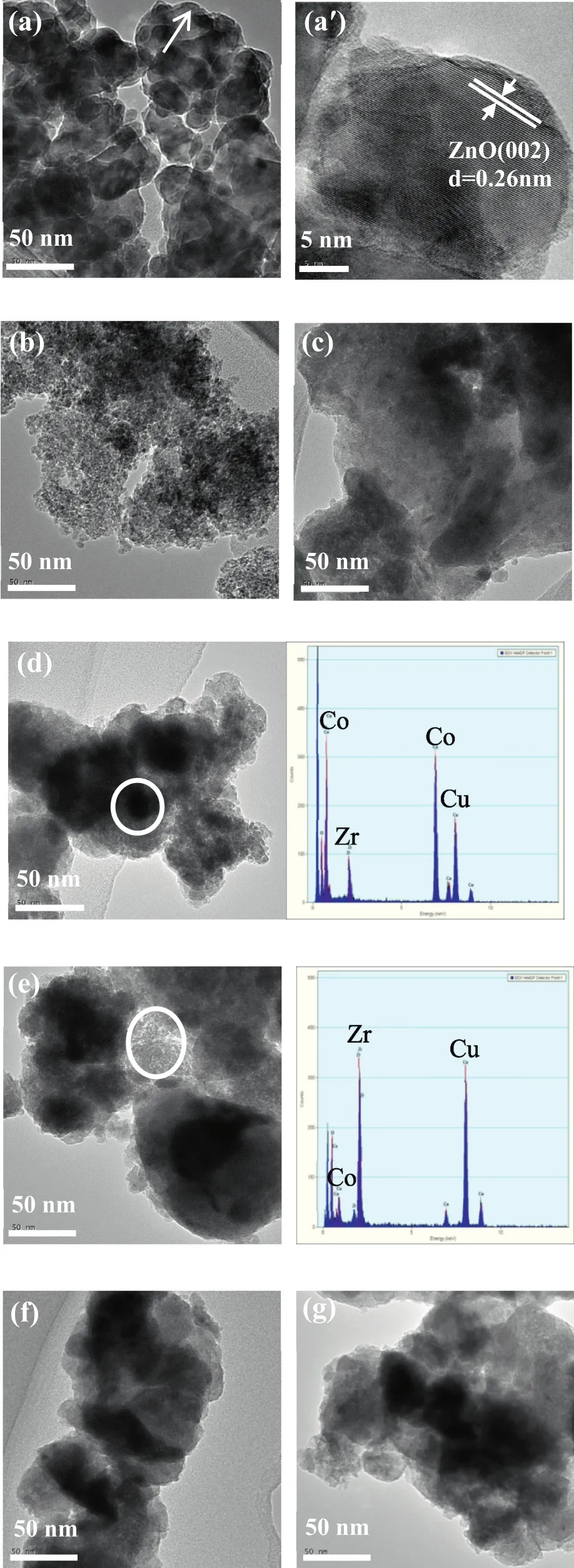
Fig.6.TEM images and the corresponding EDX spectra of the reduced catalysts:(a),(a′)Co/ZnO-Na2CO3-9;(b)Co/Al2O3-Na2CO3-9;(c)Co/ZrO2-Na2CO3-9;(d)Co/ZrO2-NH4OH-9;(e)Co/ZrO2-NaOH-9;(f)Co/ZrO2-NaOH-11;(g)Co/ZrO2-NaOH-13.
3.2.FTS performance of the catalysts
The variations ofCOconversion with the time on stream(TOS)for the catalysts are shown in Fig.7.Allcatalysts exhibita good stability over22-h test.Table 4 summarizes the FTS catalytic activities and product distributions by averaging the values after the reaction reaching a steady-state.

Fig.7.CO conversion of the catalysts with time on stream.

Table 4 The FTS performance on different catalysts
As seen in Table 4,the CO conversion of Co/ZrO2-Na2CO3-9 is higher than Co/ZnO-Na2CO3-9 and Co/Al2O3-Na2CO3-9 catalysts.Thus,in order to further optimize the Co/ZrO2catalyst,different Co/ZrO2catalysts were prepared by co-precipitation method using differentprecipitants atdifferentpHvalues.Itwasfound thatdifferentcatalysts exhibitdifferentFTSactivity,following an order of Co/ZrO2-NaOH-13>Co/ZrO2-NaOH-11>Co/ZrO2-NaOH-9>Co/ZrO2-Na2CO3-9>Co/ZrO2-NH4OH-9.Clearly,the catalyst prepared by NaOH as the precipitant is more active than that prepared by using NH4OH or Na2CO3and the best pH value is 13.
Combined with the TPD results as listed in Table 3,it can be deduced that CO conversion on the catalysts increases with the increase of the amounts ofthe weak adsorption sites,illuminating thatthe main factor affecting the COconversion ofcatalysts is the quantity oflow-temperature active adsorption sites.In addition,C5+selectivity is closely related to the peak temperature of the weak hydrogen adsorption sites,and higher peak temperature willlead to higher C5+selectivity due to the enhanced interaction between the active Co and the adsorbed H2.Moreover,no CO2is detected for Co/ZnO-Na2CO3-9,Co/ZrO2-NH4OH-9 and Co/ZrO2-NaOH-9,which implies a negligible WGS activity over these three catalysts.
4.Conclusions
The cobalt catalysts supported on ZnO,Al2O3and ZrO2with Na2CO3as precipitant were prepared by co-precipitation method and their FTS activity increased in the order of ZnO<Al2O3<ZrO2.Then a series of Co/ZrO2catalysts using different precipitants(NH4OH,Na2CO3and NaOH)and different aging pH values(pH=9,11,13)were investigated.It was clari fied that the amounts of low-temperature active adsorption sites played important roles in the CO conversion of all catalysts,and a positive relationship was observed between the weak adsorption sites and FTS performance.For this series ofcobaltcatalysts,C5+selectivity is increased with the increase of peak temperature of the weak hydrogen adsorption sites.
References
[1]Y.Chen,C.C.Liu,Y.H.Zhang,Y.X.Zhao,L.Wei,X.Wen,X.Zhao,J.L.Li,The in fluence of Fe,Ti,Ga and Zn on the Fischer-Tropsch synthesis catalytic performance of Cobased hierarchically porous ZSM-5 zeolite catalysts,Catal.Lett.147(2)(2017)502–508.
[2]J.Aluha,Y.F.Hu,N.Abatzoglou,Effect of CO concentration on the α-value of plasmasynthesized Co/C catalystin Fischer-Tropsch synthesis,Catalysts 7(3)(2017)502–508.
[3]V.R.R.Pendyala,W.D.Shafer,G.Jacobs,M.Martinelli,D.E.Sparks,B.H.Davis,Fischer-Tropsch synthesis:effect of ammonia on product selectivities for a Pt promoted Co/alumina catalyst,RSC Adv.7(13)(2017)7793–7800.
[4]A.P.Savost'yanov,R.E.Yakovenko,S.I.Sulima,V.G.Bakun,G.B.Narochnyi,V.M.Chernyshev,S.A.Mitchenko,The impact of Al2O3promoter on an efficiency of C5+hydrocarbons formation over Co/SiO2catalysts via Fischer-Tropsch synthesis,Catal.Today 279(2017)107–114.
[5]W.Chu,J.Q.Xu,J.P.Hong,T.Lin,A.Khodakov,Design of efficient Fischer Tropsch cobalt catalysts via plasma enhancement:reducibility and performance(review),Catal.Today 256(2015)41–48.
[6]S.A.Chernyak,G.E.Selyaev,E.V.Suslova,A.V.Egorov,K.I.Maslakov,A.N.Kharlanov,S.V.Savilov,V.V.Lunin,Effect of cobalt weight content on the structure and catalytic properties of Co/CNT catalysts in the Fischer-Tropsch synthesis,Kinet.Catal.57(5)(2016)640–646.
[7]L.V.Sineva,E.V.Kulchakovskaya,E.Y.Asalieva,V.Z.Mordkovich,Effect of water on the secondary transformations of hydrocarbons in the Fischer-Tropsch synthesis on Co-zeolite catalysts,Mendeleev Commun.27(1)(2017)75–77.
[8]T.J.Fu,Y.H.Jiang,J.Lv,Z.H.Li,Effect of carbon support on Fischer-Tropsch synthesis activity and product distribution over Co-based catalysts,Fuel Process.Technol.110(2013)141–149.
[9]X.H.Zhang,H.Q.Su,Y.L.Zhang,X.J.Gu,Effect of CeO2promotion on the catalytic performance ofCo/ZrO2catalysts for Fischer-Tropsch synthesis,Fuel184(2016)162–168.
[10]J.G.Chen,Y.H.Sun,The structure and reactivity of coprecipitated CO-ZrO2catalysts for Fischer-Tropsch synthesis,Stud.Surf.Sci.Catal.147(2004)277–282.
[11]L.T.Jia,K.G.Fang,J.G.Chen,Y.H.Sun,Effects of reduction atmosphere on structure and catalytic activity of Co-ZrO2catalyst for Fischer-Tropsch synthesis,Chin.J.Catal.28(7)(2007)596–600.
[12]A.T.Najafabadi,A.A.Khodadadi,M.J.Parnian,Y.Mortazavi,Atomic layer deposited Co/γ-Al2O3catalyst with enhanced cobalt dispersion and Fischer-Tropsch synthesis activity and selectivity,Appl.Catal.A 511(2016)31–46.
[13]J.Liu,J.Yu,F.B.Su,G.W.Xu,Intercorrelation of structure and performance of Ni-Mg/Al2O3catalysts prepared with different methods for syngas methanation,Catal.Sci.Technol.4(2)(2014)472–481.
[14]T.J.Fu,J.Lv,Z.H.Li,Effect of carbon porosity and cobalt particle size on the catalytic performance of carbon supported cobalt Fischer-Tropsch catalysts,Ind.Eng.Chem.Res.53(4)(2014)1342–1350.
[15]X.Q.Wang,W.S.Ning,L.H.Hu,Y.L.Li,In fluences of Al2O3on the structure and reactive performance of Co/ZnO catalyst,Catal.Commun.24(2012)61–64.
[16]Z.D.Pan,M.Parvari,D.B.Bukur,Fischer-Tropsch synthesis on Co/Al2O3catalyst:effect of pretreatment procedure,Top.Catal.57(6–9)(2014)470–478.
[17]Z.Li,J.H.Wu,J.Q.Yu,D.Z.Han,L.Y.Wu,J.Q.Li,Effect of incorporation manner of Zr on the Co/SBA-15 catalyst for the Fischer-Tropsch synthesis,J.Mol.Catal.A Chem.424(2016)384–392.
[18]K.C.Zhao,W.H.Wang,Z.H.Li,Highly efficient Ni/ZrO2catalysts prepared via combustion method for CO2methanation,J.CO2Util.16(2016)236–244.
[19]Y.C.Liu,H.T.Wu,L.T.Jia,Z.H.Fu,J.G.Chen,D.B.Li,D.L.Yin,Y.H.Sun,Effect of the calcination temperature on the catalyst performance of ZrO2-supported cobalt for Fischer-Tropsch synthesis,Adv.Mater.Res.347-353(2011)3788–3793.
[20]T.J.Fu,R.J.Liu,J.Lv,Z.H.Li,In fluence of acid treatment on N-doped multi-walled carbon nanotube supports for Fischer-Tropsch performance on cobalt catalyst,Fuel Process.Technol.122(2014)49–57.
[21]M.N.Lu,N.Fatah,A.Y.Khodakov,Solvent-free synthesis of alumina supported cobalt catalysts for Fischer-Tropsch synthesis,J.Energy Chem.25(6)(2016)1001–1007.
[22]Y.C.Liu,K.G.Fang,J.G.Chen,Y.H.Sun,Effect of pore size on the performance of mesoporous zirconia-supported cobalt Fischer-Tropsch catalysts,Green Chem.9(6)(2007)611–615.
[23]Q.Liu,J.J.Gao,F.N.Gu,X.P.Lu,Y.J.Liu,H.F.Li,Z.Y.Zhong,B.Liu,G.W.Xu,F.B.Su,One-pot synthesis of ordered mesoporous Ni-V-Al catalysts for CO methanation,J.Catal.326(2015)127–138.
[24]L.T.Jia,D.B.Li,B.Hou,Z.Q.Sun,B.Liu,J.G.Guo,R.H.Ren,Y.H.Sun,In fluence ofreductionoxidation-reduction treatment on the structure and catalytic performance of Co-ZrO2for Fischer-Tropsch synthesis,J.Fuel Chem.Technol.38(6)(2010)710–715.
[25]P.Dumrongbunditkul,T.Witoon,M.Chareonpanich,T.Mungcharoen,Preparation and characterization of Co-Cu-ZrO2nanomaterials and their catalytic activity in CO2methanation,Ceram.Int.42(8)(2016)10444–10451.
 Chinese Journal of Chemical Engineering2018年4期
Chinese Journal of Chemical Engineering2018年4期
- Chinese Journal of Chemical Engineering的其它文章
- An innovative design of septic tank for wastewater treatment and its performance evaluation:An applicable model for developing countries
- CFD modeling of turbulent reacting flow in a semi-batch stirred-tank reactor☆
- Synergistic and interference effects in coaxial mixers:Numerical analysis of the power consumption☆
- Atomic layer deposition of Al2O3 on porous polypropylene hollow fibers for enhanced membrane performances☆
- Experimental investigation and cost assessment of the salt production by solar assisted evaporation of saturated brine☆
- Equilibrium of liquid-liquid extraction of 2-phenylbutyric acid enantiomers:Experiment and model☆
