Carbon foams prepared from coal tar pitch for building thermal insulation material with low cost☆
Xiang Liu ,Yanli Wang ,Liang Zhan ,4,*
1 State Key Laboratory of Chemical Engineering,East China University of Science and Technology,Shanghai 200237,China
2 Key Laboratory for Specially Functional Polymers and Related Technology of Ministry of Education,East China University of Science and Technology,Shanghai 200237,China
3 Shanghai Key Laboratory of Multiphase Materials Chemical Engineering,East China University of Science and Technology,Shanghai 200237,China
4 CAS Key Laboratory of Carbon Materials,Institute of Coal Chemistry,Chinese Academy of Sciences,Taiyuan 030001,China
1.Introduction
Carbon foam,a kind of three-dimensional structural material,exhibits many excellent performance,such as low density,high mechanic strength,thermal shock resistance,low[1]or high[2]thermal conductivity,owing to its special interconnected pore structures[3].In the last decades,researchers focused on investigating the foaming methods(self-bubble foaming[4],template[5]and supercritical foaming[6]),carbonaceous precursors(meso phase pitch[2],phenol-formaldehyde resin[7],olive stone[8],coal[9])and potential applications(heat exchanger[10],absorbing material[11],biological material[12],electrode material[13])for carbon foams.To obtain a high compressive strength and an uniform pore structure with small pore size,carbon foams are generally prepared under rigorous foaming conditions of high temperature (>600 °C)/pressure (>10 MPa),which restricts their production in large scale as well as extensive applications.Therefore,it is still challengeable to prepare carbon foam at the foaming pressure of normal pressure.Additionally,mesophase pitch and phenol-formaldehyde resin are generally used as carbonaceous precursors for carbon foam,while they are too expensive to realize large-scale applications.Thus,it is also quite important to investigate a novel carbonaceous precursor with low cost for preparation of carbon foams.
Coal tar pitch,a kind of abundant industrial by-product,is well known as an excellent carbonaceous precursor for carbon materials due to its high carbon content and low price.Unfortunately,just a small part of coal tar pitch has been applied in industry as commercial impregnating pitch,but most of them have been become solid waste.So it is quite meaningful to develop an approach to transform coal tar pitch into a new material,whose market capacity is huge enough that can consume most of the abandoned coal tar pitch.
As far as we know,carbon foam has never been reported as a building thermal insulation material applied in energy saving building.So far,the commercial building thermal insulation materials are mainly the polymeric foams.However,the polymeric foams are well known to be easy to catch fire accompanied with a vast amount of toxic gases[14].Compared with polymeric foams,carbon foam not only cannot be fired at high flame temperature,but also exhibits other excellent performance,such as low thermal conductivity,low thermal expansion coefficient,acid and alkali resistance,hydrophobic,high strength and good machinability.
Herein,coal tar pitch was applied as a raw material for the preparation of carbon foam by self-bubble foaming at the foaming pressure of normal pressure.Moreover,the resultant carbon foam may be a novel potential candidate for building thermal insulation material applied in energy saving building.Notably,this kind of carbon based thermal insulation material can also be applied in other fields,for example,pipe insulation,security door,rocket nozzle and other porous carbon composites.
2.Experimental
2.1.Preparation of high softening point pitch
Coal tar pitch was initially oxidized into high softening point pitch(HSPP)as literature reported[15].
2.2.Modi fication of high softening point pitch
15 g HSPP was extracted by toluene(500 ml)in Soxhlet extractor.The HSPP extracted by toluene were denoted as T-HSPP-x,where x corresponds to the extraction times.For example,T-HSPP-5 refers to HSPP extracted by toluene for five times.
100 g HSPP was put into a stainless steel reactor,then vacuumized at 380-420°C per 10 kPa for 2 h.The vacuumized HSPP was denoted as V-HSPP-y,where y means the treatment temperature.For example,V-HSPP-400 °C refers to HSPP vacuumized at 400 °C for 2 h.
2.3.Preparation of carbon foams
Carbon foams were prepared by self-foaming method under the conditions of normal pressure and 600°C,subsequently,carbonized at 800°C for 2 h[16].For description,carbon foams were denoted as same as their raw material.For example,the V-HSPP-380°C sample of carbon foam means it was derived from V-HSPP-380°C pitch.
2.4.Characterization
The morphology and microstructure of the samples were investigated by SEM measurement(FEI Nova Nano).The thermogravimetric analysis(SDT-Q600,TA company)was carried out in nitrogen atmosphere up to 800°C.The viscosity and element composition were measured by a MARS 3 senior rotary rheometer(Thermo Hakke company)and an element analyzer(Elementar Analysensysteme Gmbh company),respectively.The thermal conductivity was measured using flash diffusivity instrument(LFA447 NanoFlash).The compressive strength was measured by Instron5869 electronic universal testing machine.
3.Results and Discussions
3.1.Carbon foams derived from toluene extracted HSPP

Fig.1.SEM images of carbon foams derived from HSPP(a),T-HSPP-2(b),T-HSPP-5(c)and T-HSPP-8(d).
Fig.1(a)shows the SEM image of carbon foam derived from the initial HSPP,where no bubble can be observed,except for certain of large holes.Compared with HSPP,T-HSPP-2 can be foamed under the same foaming conditions(Fig.1(b)),but the pore size is very large and inhomogeneous.If HSPP was extracted by toluene for five times,not only T-HSPP-5 can be foamed,but also the pore size of the resultant carbon foam is relatively uniform distributed in a narrow range of 450-500 μm(Fig.1(c)).If the extraction times increase from 5 to 8 times,the THSPP-8 can still be foamed(Fig.1(d)),while a layer of pitch cannot be foamed existing on the surface of carbon foam as shown in the inserted SEM image in Fig.1(d).
Fig.1 indicates that the foaming behaviors of HSPP closely relates to its physical performance.As listed in Table 1,the softening point of synthesized initial HSPP is only 200°C,which is obviously lower than that of T-HSPP-2(292 °C),T-HSPP-5(311 °C)and T-HSPP-8(>319 °C).Additionally,after being extracted by toluene,the QI component of HSPP increases from 37.3%to 61.76%(T-HSPP-2),64.29%(T-HSPP-5)and 73.32%(T-HSPP-8),while the components of HS and HI-TS of HSPP correspondingly decreased.Especially,the weight ratio of HS in T-HSPP-8 sample is only 0.59%,while that of the initial HSPP is 24.9%.Combined with the results in Fig.1,the weight ratios of QI and HS are the main effect factors on the foaming behaviors of HSPP.
As listed in Table 1,the softening point of HSPP seems to be a key effect factor on the foaming efficiency of HSPP,so viscositytemperature and TG-DTG curves are further carried out.As shown in Fig.2(a),the mass loss of initial HSPP during the foaming temperature of 300-550°C is 33%,while that of T-HSPP-2,T-HSPP-5 and T-HSPP-8 are only 16.8%,10.5%and 10.3%,respectively.Obviously,the more light component and/or pyrolysis gases are produced in the foaming process,the higher the foaming pressure is required to preventthe gases from escaping the molten pitch.Interestingly,Fig.2(b)indicates thatmostofthe mass loss for initial HSPP attributes to the lightcomponents produced in the range of 300-450 °C,resulting in a low viscosity(about 1000 Pa·s,Fig.3)during the foaming process.When the viscosity is low enough during the foaming process,the light components will directly escape from the molten pitch and cannot act as the foaming agent,agreeing well with the result in Fig.1(a).Compared with initial HSPP,the massloss of T-HSPP-5(10.5%,Fig.2a)mainly attributes to the pyrolysis gases produced in the range of 450-600°C.So the viscosity of T-HSPP-5 during the foaming temperature of 300-550°C is highly enough that the pyrolysis gases can act as a foaming agent(Fig.3),resulting in uniform pore structure(Fig.1(c).Additionally,although the weight loss of T-HSPP-8 is similar with that of T-HSSP-5,its viscosity during the foaming process is so high that certain of pitch cannot be foamed,forming a solid carbon layer(insert in Fig.1(d).

Table 1Properties of the high softening point pitches

Fig.3.Viscosity-temperature curves of HSPP samples.
3.2.Carbon foams derived from vacuumized HSPP
Considering the high cost and toxicity of toluene,the resultant HSPP was further modi fied by vacuumizing.As shown in Fig.4(a),although a few pores can be observed on the V-HSPP-380°C sample of carbon foam,the pore density is very low.If further increasing the vacuumizing temperature from 380 to 400°C,carbon foam with uniform pore size and high pore density was given(Fig.4(b)),whose pore size is distributed in a narrow range of 300-500 μm.Moreover,as shown in Fig.4(c),the compressive strength of V-HSPP-400°C based carbon foam is 6.3 MPa,which is higher than that of T-HSPP-5 based carbon foam(5.1 MPa).Unfortunately,if HSPP was vacuumized at a higher temperature of 420 °C,V-HSPP-420 °C pitch cannot be foamed(Fig.4(d)).These results suggest that the performance of HSPP has a significant effect on its foaming behaviors,agreeing well with the above discussions about the toluene extracted HSPP.
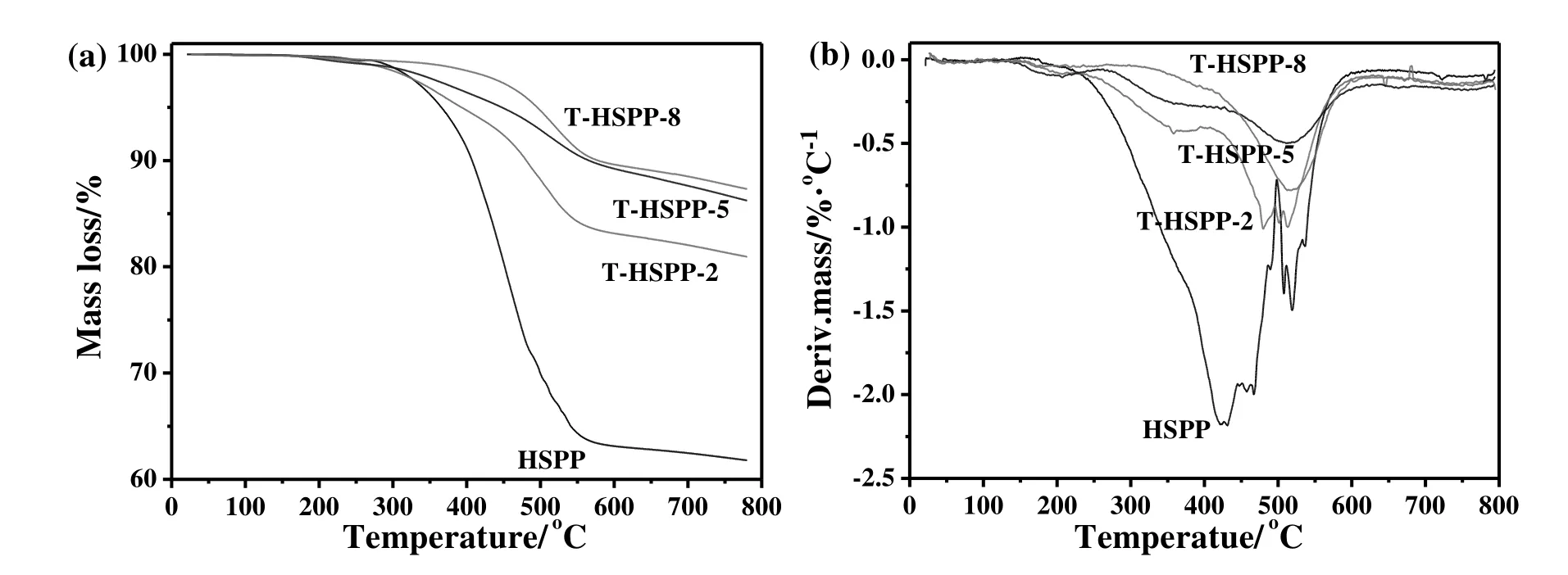
Fig.2.TG(a)and DTG(b)curves of HSPP samples.
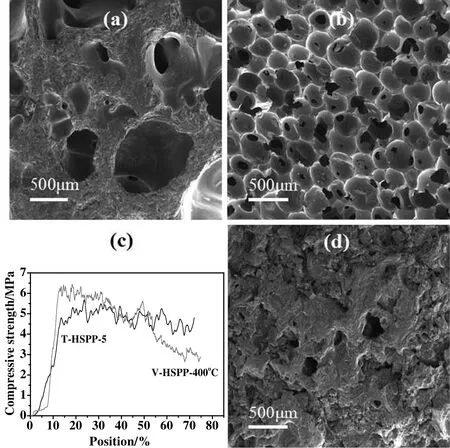
Fig.4.SEM images of carbon foams derived from V-HSPP-380 °C(a),V-HSPP-400 °C(b),V-HSPP-420 °C(d)pitches.Stress-strain curves of carbon foams(c).
Owing to the cooperation of vacuumizing and polycondensation,the chemical(Table 2)and physical(Fig.5)performances of original HSPP have changed significantly after being vacuumized.As listed in Table 2,V-HSPP-400°C pitch has a similar softening pointand componentdistribution as T-HSPP-5 sample(Table 1),resulting in the similar foaming efficiency(Figs.1(c)and 4(b)).These results also suggest that the optimum properties of HSPP for carbon foam are as follows:HS,3.76%;HI-PS,9.04%;TI-PS,9.88%;PI-QS,11.56%;and QI,65.76%,and the softening point is about 292°C.Additionally,the mass ratio of QI and the viscosity of HSPP are also quite important for its foaming behaviors.As shown in Fig.4(d),the foaming efficiency of VHSPP-420°C sample is very poor,because its mass ratio of QI is so high(78.47%,Table 2)that the amount of pyrolysis gases below 600°C is just about 5%(Fig.5(a).Especially,the viscosity of V-HSPP-420 °C pitch in the foaming temperature range of 450 °C-550 °C distributes in the range of 1 × 105-2.5 × 105Pa·s(Fig.5(d)),which is hundred times higher than that of V-HSPP-400°C and THSPP-5 pitches.These results suggest that the optimum viscosity of HSPP for foaming should be lower than 10000 Pa·s.
The above results indicate that the vacuumizing method not only can regulate the physical and chemical performance of HSPP for foaming,but also is a green and low cost approach.Additionally,the main reasons that both T-HSPP-5 and V-HSPP-400°C pitches can be foamed under normal pressure and have uniform pore structures should attribute to the narrower composition distribution,suitable viscosity and narrower viscosity-temperature change section.
3.3.Foaming mechanisms of high softening point pitch
To investigate the foaming mechanisms of HSPP based carbon foam,series of intermediates are given using V-HSPP-400 °C pitch as carbonaceous precursors by controlling the foaming temperature(350-470°C,Fig.6).As shown in Fig.6(a),many nuclei are formed irregularly under the foaming temperature of 350°C,which should attribute to the volatilization of the light components(the mass loss about 2%,Fig.5(a).When the foaming temperature increases to 400°C,the formed nuclei self-assemble into a circular underthe thermodynamics force of molten pitch(Fig.6(b)).If the foaming temperature was further increased to 450 °C-470 °C,the V-HSPP-400 °C pitch would decompose or pyrolysize more gases(the weight loss about 5%-8%,Fig.5(a),then the gases diffuse and aggregate around the formed circular nuclei(Fig.6(c)),forming bubbles(Fig.6(d)).When the temperature is up to 450-550 °C,the viscosity of V-HSPP-400 °C increases rapidly(Fig.5(d)),thus the formed bubbles are fixed,forming porous carbon foam.The detail foaming mechanisms of HSPP based carbon foam is illustrated in Fig.6(e).
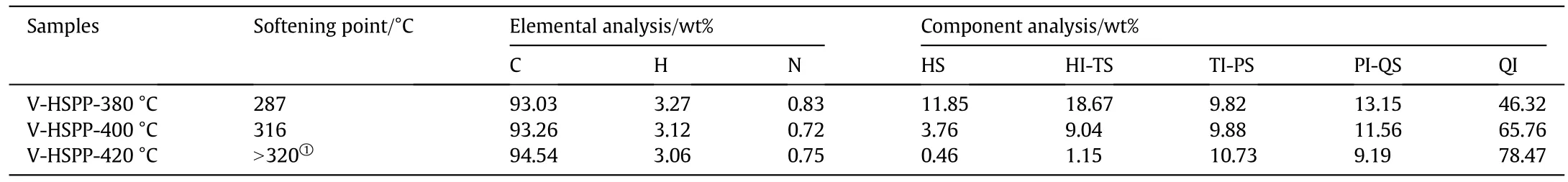
Table 2Properties of vacuum extracted high softening point pitches

Fig.5.TG(a),DTG(b)and viscosity-temperature(c,d)curves of vacuumized HSPP samples.

Fig.6.SEM images of carbon foams at the foaming temperature of 350 °C(a),400 °C(b),450 °C(c)and 470 °C(d).Foaming mechanisms of HSPP based carbon foam(e).
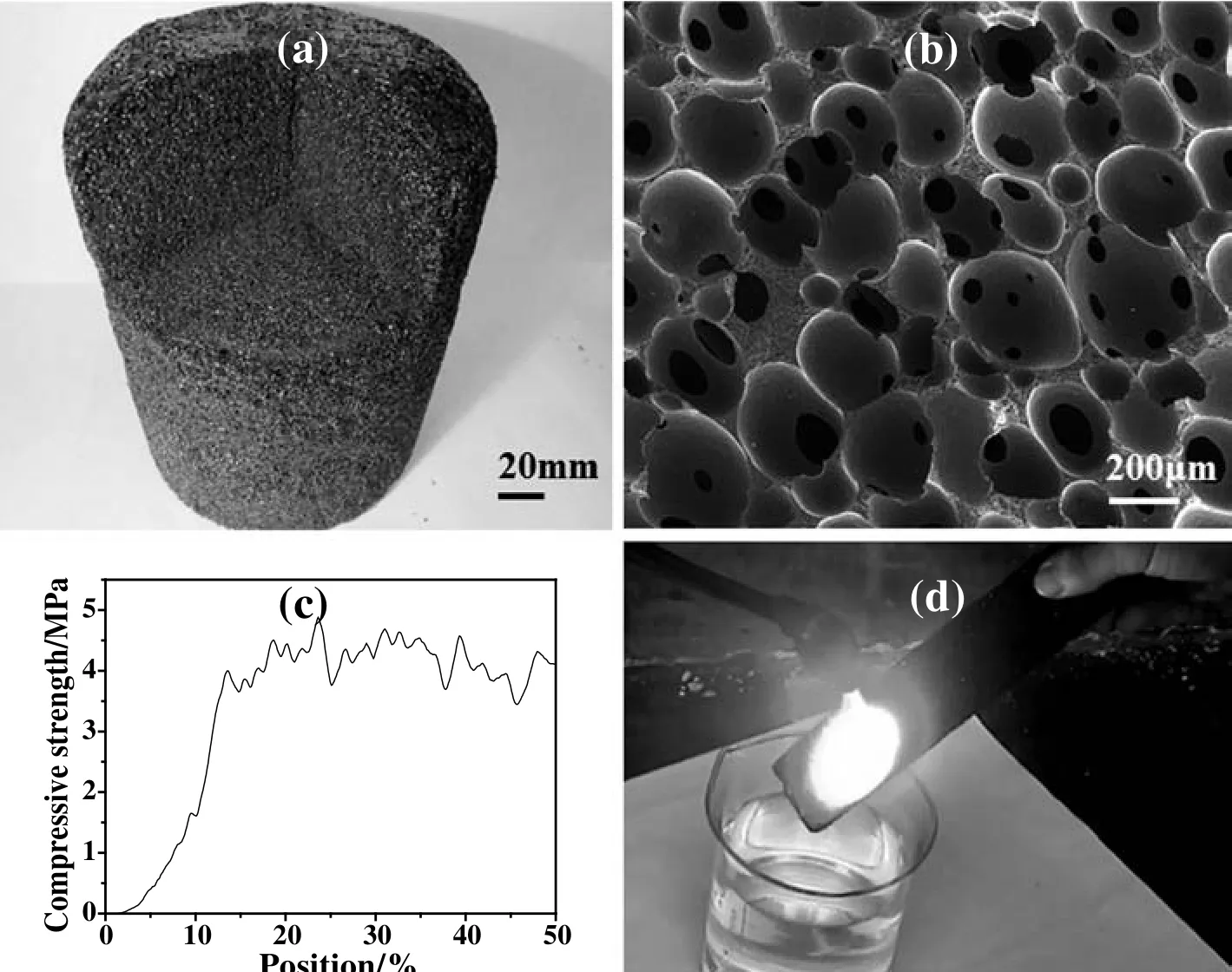
Fig.7.Photo(a),SEM image(b)and stress-strain curve(c)of HSPP based carbon foams with a size of Φ200 mm × 200 mm.Combustion performance of HSPP based carbon foam(d).
3.4.Carbon foam for building thermal insulation materials
According to above foaming mechanisms,carbon foam with a size of Φ200 mm × 200 mm was successfully obtained using V-HSPP-400°C as carbonaceous precursor.As shown in Fig.7(a),the resultant carbon foam has a homogenous pore structure both in the outside and inside.The pore size mainly distributes in a narrow range of 300-500 μm with a high ratio of open pores(Fig.7(b)),and its compressive strength,thermal conductivity and density are 4.7 MPa(Fig.7(c)),0.07 W·m-1·K-1and 0.31 g·cm3,respectively.Importantly,the resultant carbon foam not only cannot be fired under the acetylene flame temperature(about 1200°C),but also exhibits an excellent heat-insulation performance(Fig.7(d)).
4.Conclusions
Anew approach was provided to resolve the large-scale applications of coal tar pitch.Carbon foams with uniform pore size have been prepared using coal tar pitch as raw materials.Considering the high cost and toxicity of toluene,vacuumizing method was adapted to regulate the physical and chemical performance of high softening point pitch(HSPP)because of its cooperation of vacuumizing and poly condensation.Ascon cluded from the pore structures of carbon foams,the optimums of tening point and compositions of HSPP should be as follows:about 292°C,HS 3.76%,HI-TS 9.04%,TI-PS 9.88%,PI-QS 11.56%and QI 65.76%,respectively;and the optimum viscosity of HSPP during foaming process should be in the range of 1000-10000 Pa·s.The resultant carbon foam exhibits excellent thermal insulation performance,so it is a potential candidate for thermal insulation material applied in energy saving building,pipe insulation,security door,rocket nozzle and other porous carbon composites.
[1]D.F.Walter,Method of making cellular refractory thermal insulating material,US Patent(1964),3121050.
[2]J.Klett,R.Hardy,E.Romine,et al.,High-thermal-conductivity,mesophase-pitchderived carbon foams:Effect of precursor on structure and properties,Carbon 38(7)(2000)953-973.
[3]B.Maruyama,J.E.Spowart,D.J.Hooper,et al.,A new technique for obtaining threed imensional structures in pitch-based carbon foams,Scr.Mater.54(9)(2006)1709-1713.
[4]I.Mochida,Y.Korai,C.H.Ku,et al.,Chemistry of synthesis,structure,preparation and application of aromatic-derived mesophase pitch,Carbon 38(2)(2000)305-308.
[5]Z.Min,C.Min,S.Zhang,et al.,Effect of precursor on the pore structure of carbon foams,New Carbon Mater.22(1)(2007)75-79.
[6]J.Li,C.Wang,L.Zhan,et al.,Carbon foams prepared by supercritical foaming method,Carbon 47(4)(2009)1204-1206.
[7]S.Lei,Q.Guo,J.Shi,et al.,Preparation of phenolic-based carbon foam with controllable pore structure and high compressive strength,Carbon 48(9)(2010)2644-2646.
[8]R.Rios,E.M.Martínez,M.Molina-Sabio,et al.,Carbon foam prepared by pyrolysis of olive stones under steam,Carbon 44(8)(2006)1448-1454.
[9]C.Chen,E.B.Kennel,A.H.Stiller,et al.,Carbon foam derived from various precursors,Carbon 44(8)(2006)1535-1543.
[10]A.M.Druma,M.K.Alam,C.Druma,Analysis of thermal conduction in carbon foams,Int.J.Therm.Sci.43(7)(2004)689-695.
[11]V.K.Saini,M.L.Pinto,J.Pires,Synthesis and adsorption properties of micro/mesoporous carbon-foams prepared from foam-shaped sacrificial templates,Mater.Chem.Phys.138(2-3)(2013)877-885.
[12]G.Turgut,A.Eksilioglu,N.Gencay,etal.,Pore structure engineering for carbon foams as possible bone implant material,J.Biomed.Mater.Res.A 85(3)(2008)588-596.
[13]E.Frackowiak,F.Beguin,Carbon materials for the electrochemical storage of energy in capacitors,Carbon 39(6)(2001)937-950.
[14]D.Price,Y.Liu,G.J.Milnes,et al.,An investigation into the mechanism of flame retardancy and smoke suppression by melamine in flexible polyurethane foam,Fire Mater.26(4-5)(2002)201-206.
[15]V.Prada,M.Granda,J.Bermejo,et al.,Preparation of novel pitches by tar airblowing,Carbon 37(1)(1999)97-106.
[16]Y.Bao,L.Zhan,C.Wang,et al.,Carbon foams used as packing media in a biological aerated filter system,Mater.Lett.65(19-20)(2011)3154-3156.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Surface chemical characterization of deactivated low-level mercury catalysts for acetylene hydrochlorination☆
- Insight into fouling behavior of poly(vinylidene fluoride)(PVDF)hollow fiber membranes caused by dextran with different pore
- Protein adsorption onto diethylaminoethyl dextran modi fied anion exchanger:Effect of ionic strength and column behavior☆
- Gas emission source term estimation with 1-step nonlinear partial swarm optimization-Tikhonov regularization hybrid method☆
- Dissolution of antibiotics mycelium in ionic liquids:Performance and mechanism☆
- Kinetic studies on extra heavy crude oilupgrading using nanocatalysts by applying CFD techniques☆
