Characteristics of oil shale pyrolysis in a two-stage fluidized bed☆
Yong Tian ,Mengya Li,Dengguo Lai,Zhaohui Chen ,Shiqiu Gao ,Guangwen Xu ,*
1 State Key Laboratory of Multi-phase Complex Systems,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
3 Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
1.Introduction
Oil shale is an important unconventional fossil fuel with large amount of reserve whose extractable shale oil is estimated to be over four times of crude oil in the world[1].Oil shale pyrolysis can be sorted into slow and rapid pyrolysis based on the heating rate[2].Fluidized bed pyrolysis can realize rapid pyrolysis and it is favorable for volatile's formation and oil vapor release which would lead to achieve a high shale oil yield[3,4].But meantime,shale oil products from fluidized bed pyrolysis suffer from poor quality with high content of heavy fraction(boiling point> 350 °C),which is less valuable than light fraction(boiling point< 350 °C),hard to process and detrimental to apparatus for the tendency of condensation and blockage in pipelines[5,6].Gasoline and diesel as light fraction in shale oil can be easily separated for direct use,and meanwhile heavy fraction rich in paraf fin-based oil is hard to transport in pipeline and needs to be re fined in downstream plant[7,8].Thus,it's meaningful and necessary to upgrade shale oil produced from rapid pyrolysis in order to convert heavy fraction into light components to get shale oil with high quality.
Utilizations of oil shale char or spent shale are very attractive in industry,since they provide both process heat and reaction agent[9].Meanwhile oil shale char and shale ash from oil shale pyrolysis are reported to have upgrading effect by enhancing oil cracking to convert heavy components into light components[5,10,11].Metallic oxide of mineral and sufficient porous structures of oil shale char provide catalytic function on the secondary reactions and have a marked influence on product yields and compositions[12-14].Inorganic minerals in oil shale char have been proved to have catalytic effects on pyrolysis[15].Kaolinite in oil shale char can cause oil cracking[16],while alkali and alkaline earth metal can increase oil shale reactivity[17].Thus,catalytic cracking of volatile could take place over oil shale char.On the other hand,the porous structures inside the oil shale char will prolong volatile's residence time and thus enhance the cracking and upgrading.
A two-stage pyrolysis reactor provides ways to study oil vapor upgrading by dividing pyrolysis and secondary reactions into two separate stages.Lai et al.investigated the catalytic effect of shale ash as bed material on shale oil upgrading in a two-stage fixed bed by varying the volatile residence time and shale ash temperature,which could increase light fraction by 46.4%at the cost of 31%shale oil loss[11].Carter et al.examined secondary reactions ofshale oil vapor from pyrolysis of Kentucky oil shale in the presence of combusted shale in a twostage fixed bed reactor and found that heavy n-alkanes in oil largely decreased when contacting with combusted shale which contributed to lower-mole cular-weight products[12].Paul studied shale oilcatalytic upgrading by using zeolite ZSM-5 catalyst in a two-stage fixed bed reactor and found that after catalysis on second stage almost all long chain alkanes and alkenes were converted to low-molecular species[18].Most of these studies adopted two-stage fixed bed for the investigation of oil vapor's secondary reactions in pursuit of in-situ oil upgrading.However,two-stage fluidized bed(TSFB)pyrolysis and upgrading have not been studied before,where the first fluidized bed(FB)enables rapid pyrolysis to give high oil yield[3,19]and at the same time the second FB cracking allows good upgrading to produce shale oil with high content of light fraction.This study was devoted to investigating the fluidized-bed rapid pyrolysis of oil shale together with shale oil vapor upgrading over oil shale char in a two-stage fluidized bed reactor.
2.Experimental
2.1.Materials
Oil shale used in this study was from Huadian oil shale mine,Jilin Province,China.Oil shale was crushed and sieved to 0.2-0.4 mm and then dried in an oven of 110°C for 12 h.Oil shale char used in this study was obtained by pyrolyzing Huadian oil shale at 800°C with N2sweeping and also sieved to 0.2-0.4 mm.The pyrolysis temperature for preparing oil shale char was higher than those carried out in following experiments to ensure that it wouldn't release any extra volatiles during experiments.Table 1 shows the proximate and ultimate analyses of Huadian oil shale and the result of X-ray fluorescence(XRF)analysis of the prepared oil shale char.Oil shale used in this study had a high ash content of 65.6 wt%and a volatile content of30.2 wt%.The oilyield from Fischer Assay was 9.98 wt%on dry basis.For the oil shale char,the major metallic oxide minerals were SiO2,Al2O3,CaO and Fe2O3.

Table 1 Proximate and ultimate analyses of Huadian oil shale and XRF analysis result of oil shale char.Properties of oil shale.
2.2.Apparatus and methods
Fig.1 shows a schematic diagram of the two-stage fluidized bed(TSFB)reactor applied in this article.The reactor was made of quartz glass with a total length of 450 mm and inner diameter of 30 mm.Two porous sintering quartz plates were fixed in the tube and located at 150 mm from the top and 150 mm from the bottom,respectively.The thicknesses of the plates were all 3 mm.Oil shale char was preloaded on the upper plate through an inlet on the top of the reactor.Oil shale was loaded on the lower plate through a feeder with two valves which formed an isolation chamber to avoid any gas leak when loading oil shale to hot zone in the reactor.The tube connected with oil shale feeder was embedded in the upper plate and ensured oil shale char would not fall onto lower plate.Bed temperatures on two plates were measured by two K-type thermocouples which were inserted into the reactor and immersed in their particle beds,they were calibrated by inserting another thermocouple before experiments and ensure that the indication error was within 5°C.High purity N2with a flow rate of 2 L·min-1was applied as fluidizing gas and it flew from the bottom to top.The superficial gas velocity was nearly 3 times of minimum fluidization velocity of oil shale particles and ensured full fluidized state.The minimum fluidization velocity(Umf)is calculated from:

Fig.1.Schematic diagram of experimental apparatus.1—nitrogen cylinder;2—gas mass- flow controller;3—valves;4—oil shale feeder;5—oil shale char inlet;6—thermocouples;7—upper plate;8—lower plate;9—alumina balls;10—condenser;11—collection bottle;12—acetone trap;13—gas flow meter;14—sodium carbonate washing bottle;15—dry silica gel bottle.
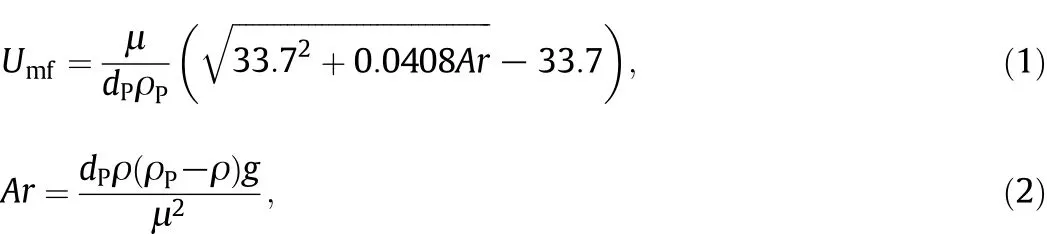
where dPand ρPare the mean diameter and density of oil shale particle,ρ and μ are gas density and viscosity of nitrogen gas.Since fluidizing gas is preheated,its density and viscosity change according to temperature in calculation,as:

Taking 450 °C for instance,Umfwas calculated as 0.0395 m·s-1.When super ficial gas velocity of nitrogen was set as 2 L·min-1,the practical flow rate after preheating was 4.851 L·min-1and velocity was 0.1144 m·s-1in the reactor,being nearly 3 times of Umfand enabling a full fluidization.Increasing temperature would lead to better fluidization.So the super ficial gas velocity of nitrogen in each experiment was set 2 L·min-1to ensure full fluidization in every experiment.
Alumina balls were filled in the lower section at the bottom of the reactor and heated by the furnace.This ensured that nitrogen would be preheated to the pyrolysis temperature when flowing through this section.The two plates were located in the constant temperature zone of the furnace,and the reactor was fully covered with silica wool to isolate heat loss and ensured that temperatures on two plates were the same.
In each experiment,the reactor was preheated to a specific temperature and held for 30 min to stabilize temperatures on both plates and meantime let gas sweep the reactor and eliminate air.About 10 g oil shale was pre-loaded in the small chamber of the feeder and a specific amount of oil shale char was preloaded on the upper plate.Fluidizing gas was cut off for a few seconds to quickly load the oil shale and then quickly turned on the gas.Pyrolysis was immediately started after loading oil shale.Primary volatile generated on the lower plate,which afterward passed through the oil shale char layer on the upper plate to undergo secondary reactions.
Pyrolysis products flowed out of the reactor and were immediately cooled down in the condenser,then further wentthrough three acetone washing bottles immersed in an ice bath to absorb and recover shale oil thoroughly.Uncondensed and unabsorbed gas passed through a gas flow meter to measure gas volume and then through sodium carbonate solution and dry silica gel to eliminate SO2and water,respectively.Finally,the gas was collected in gas bags for gas composition analysis in a micro-GC.Shale oil product was collected by washing condenser and pipes with acetone and further evaporated in a rotary evaporator.MgSO4was added to remove water in oil product and in turn filtered.Acetone was evaporated under 6°C and negative pressure(-0.08 MPa)to get pure shale oil,which was weighed to calculate shale oil yield and used for further analysis.The oil shale char on two plates was collected separately for analysis and weighed to calculate yield.
Oilshale rapid pyrolysis was firstcarried out in the TSFB withoutany oil shale char on the upper plate.Then the upgrading of shale oil from rapid pyrolysis over oil shale char was conducted.The reaction time was confirmed to be 10 min through observing no release of volatile from oil shale.Thus all subsequent experiments were performed in 10 min.
2.3.Analysis and characterization
After removal of water and dust,the pure shale oil was analyzed by a simulated distillation GC(Agilent 7890-B)to provide distillation fraction distribution according to the ASTM D2887 method.In this study,shale oil components were categorized into two kinds:light fraction(boiling point < 350 °C)which consisted of gasoline(IBP-180 °C)and diesel(180-350 °C),and heavy fraction(boiling point> 350 °C)which consisted of VGO(vacuum gas oil,350-500°C)and heavy oil(>500 °C).Shale oil was also tested in a gas chromatograph-mass spectrometer(GC-MS QP2010 Ultra,SHIMADZU)to get its chemical compositions.
The non-condensable gases were analyzed by using a micro-GC(Agilent 3000A)to determine the gas composition including H2,CO,CO2,CH4,C2H4,C2H6,C3H6and C3H8.Hydrocarbon components of C2H4,C2H6,C3H6,and C3H8were sorted as C2+C3 in this study.Proximate analysis of oil shale char was conducted in a muffle furnace according to the national standard GBT212-2008 and ultimate analysis was conducted by using an elemental analyzer(vario MACRO cube).Pyrolysis product yield(Yp)is calculated as:

where M is the mass of oil shale on dry basis,and Mprefers to mass of pyrolysis products including shale oil,oil shale char and gas.The mass of gas is calculated from the gas volume of each gas species at 25°C,0.1 MPa according to the ideal gas equation of state.
Shale oilfraction yield Yfraction(lightfraction yield and heavy fraction yield)is calculated as:

where Cfractionrefers to the content of light fraction and heavy fraction which are alldetermined by simulated distillation GC.When comparing FB and TSFB pyrolysis,the shale oil yield decrease(Yoildecrease)and gas yield increase(Ygasincrease)are calculated as:

where Yoil(FB)and Yoil(TSFB)are shale oil yields in FB and TSFB respectively,Ygas(FB)and Ygas(TSFB)are gas yields in FB and TSFB respectively.The rates of shale oil decrease rate(RShaleoildecrease)and light fraction increase(RLightfractionincrease)are further calculated by.

where YFBshaleoiland YTSFBshaleoilare shale oil yields at different temperatures in FB and TSFB respectively,YFBlightfractionand YTSFBlightfractionare light fraction yield at different temperatures.
3.Results and Discussion
3.1.FB rapid pyrolysis
Rapid pyrolysis of oil shale in FB was conducted at temperatures from 450 °C to 700 °C to evaluate the yield and quality of shale oil.Fast heating rate of oil shale particle in fluidized bed was tested,the average heating rate was 127.5 °C·min-1and the maximum heating rate was 300 °C·min-1.The product distribution was shown in Fig.2.Shale oil yield increased as pyrolysis temperature rose and reached the maximum of 12.7 wt%at 500°C which was 127%of Fischer Assay yield.Further increasing pyrolysis temperature caused the loss of shale oil yield due to secondary reactions.Shale oil yield depended mainly on heating rate and final temperature of oil shale pyrolysis[20,21].Increasing temperature favored heat exchange from heating environment to oil shale particles and raised the heating rate,leading to promotion of volatile release from particles and produced high content of liquid product.Meantime secondary reactions caused shale oil loss and were enhanced by temperature elevation,resulting in shale oil yield drop at higher temperatures.Shale oil yields from 500 °C to 600 °C were higher than Fischer Assay yield,reflecting the advantage of FB rapid pyrolysis on enhancing shale oil production.The gas/oil mass ratio increased slowly from 450 °C to 550 °C and quickly increased with temperature further increasing.This indicated that higher temperature promoted bond cleavages in oil shale macromolecular structure in forms of oil cracking,leading to the conversion of liquid products into gas phase,thus corresponding gas yield increased with temperature increasing.

Fig.2.Shale oiland gas yields of oil shale pyrolysis in FB reactor at different temperatures.
FB pyrolysis showed superiority in the aspect of higher shale oilyield when compared with traditional fixed bed pyrolysis.High heating rate in FB pyrolysis accelerated bond cleavage in kerogen organic structure and favored more radical production which was the precondition for high shale oil yield.Also autogenous intra-particle gas sweep during fast heating was more intensive and led to less oil degradation[22].On the other hand,gas swept around oil shale particle and caused lower oil vapor partial pressure,thus initial oil products were quickly discharged with limited secondary reactions in FB free board to retain large portion of primary pyrolysis products,resulting in higher shale oil yield which could exceed 100%Fischer Assay yield.By contrast,the fixed bed reactor had low heating rate and long residence time of oil vapor,primary pyrolysis product was lower in amount and took more severe secondary reactions,causing low shale oil yield which could hardly reach Fischer Assay yield.
Fig.3(a)illustrates simulated distillation GC results in terms of shale oil fraction distribution as temperature increased.The major fractions in shale oil were diesel and VGO,gasoline and heavy oil contents were very small.Content of diesel showed increase trend and VGO changed slightly,heavy oil content gradually decreased with the increase of temperature.Fig.3(b)shows corresponding oil fraction yields with the increase of temperature,VGO and heavy oil yield first increased then decreased,and reached maximum of 7.43 wt%and 1.88 wt%respectively at 500°C,diesel fraction yield increased gradually from 2.64 wt%to 4.11 wt%,gasoline yield was very small and changed slightly,indicating partial conversion of heavy fraction into light fraction through cracking.It should be noted that heavy fraction(VGO and heavy oil)in all cases was over 50 wt%without exception and their corresponding yields were much higher than corresponding light fraction yield.High heating rate in FB pyrolysis accelerated bond cleavage and favored the formation of initial pyrolysis products with high molecular weight and was dominantly responsible for heavy fraction.On the other hand,oil vapor was fast released and quickly condensed with suppressed secondary reactions which led to low cracking extent and retained most of the heavy fraction,resulting in large portion of heavy fraction.
Fig.4 shows the variation of pyrolysis gas yield at different temperatures.By elevating pyrolysis temperature,the yields of all gas species increased.Obvious increase of the yield of C2+C3was derived from long-chain alkane's breakage in secondary cracking reactions.The formation of CH4was related to hydrogenation and chain breakage of methyl in oil cracking[23]and H2was derived from C--H breakage.These indicated that thermal cracking caused oilyield loss when increasing pyrolysis temperature.A slightly increase of CO2yield indicated enhanced decomposition of carboxyl groups and inorganic minerals,which contributed mainly to the formation of CO2.
In general,oil shale FB rapid pyrolysis had potentials in producing shale oil with high yield.However,undesirably high content of heavy components in shale oil reduced its quality,thus leaving the necessity to further regulate oil vapor by upgrading in a secondary FB.The trend of upgrading effects through oil shale char as a function of char amount and pyrolysis temperature was then systematically analyzed in the following TSFB pyrolysis experiments.

Fig.4.Yields of major gas species per gram of oil shale varied with pyrolysis temperature in FB reactor.
3.2.Oil vapor upgrading over char particles
In the TSFB reactor,oil shale was rapidly pyrolyzed in the lower section and the produced volatile went through the upper plate packed with oil shale char to cause secondary cracking at the same temperature as that of oil shale pyrolysis in lower FB.The effect of contact time between oil vapor and oil shale char was investigated by adjusting oil shale char amount(0 g,2 g,6 g,10 g,14 g)in the upper FB.Table 2 presents the variation of the yield and quality of shale oil with the variation of oil shale char amount at 500 °C.The temperature of 500 °C was chosen based on the fact that the shale oil yield was found to be highest in our previous FB pyrolysis experiments,which could better compare the catalytic cracking effect from oil shale char with differentamounts and explore the optimal value.Shale oil yield decreased from 12.74 wt%to 6.25 wt%with increasing oil shale char amount from 0 to 14 g.More oil shale char on the upper plate physically prolonged contact time of oil vapor by forming longer gas flowing path,which made oil vapor undergo more secondary reactions.On the other hand,more catalytic sites for cracking were brought by increasing oil shale char,especially with the function of CaO and Fe2O3in oil shale char which had been proved to provide catalytic effects on shale oil cracking to give reasonable high light fraction yield[11].Light fraction content in shale oil had an increasing trend with increasing oil shale char amount and light fraction yields first increased from 3.43 wt%to 5.65 wt%when adding oil shale char from 0 g to 10 g and then decreased slightly,revealing the conversion trend from heavy fraction to light fraction through catalytic cracking over oil shale char.Maximum light fraction yield was obtained by adding 10 g oil shale char which was in a mass ratio of 1:1 with oil shale,at which condition light fraction content was elevated by 130%and light fraction yield was elevated by 64.7%.Further increasing oil shale char brought deeper cracking of shale oil from extended contacting time and over-sufficient catalytic effects,and the generation rate of light fraction was lower than losing rate,resulting in drop of light fraction yield.Thus the following tests used 10 g of oil shale char(mass ratio with oil shale 1:1)to get the highest light fraction yield.
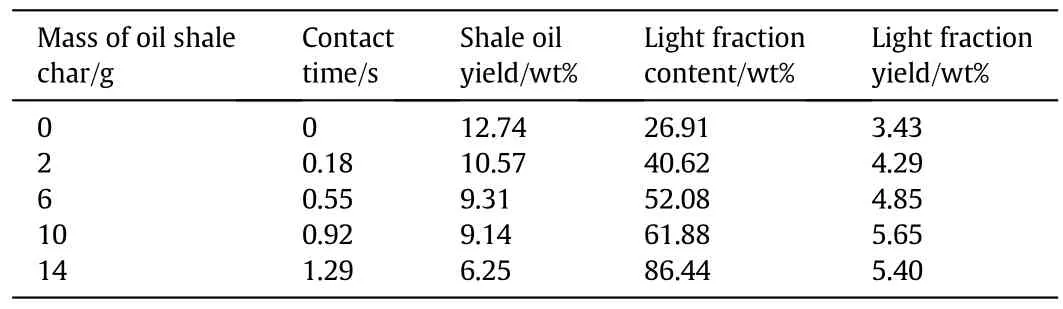
Table 2 Yield of shale oil and light fraction varied by oil shale char mass in TSFB pyrolysis at 500°C

Fig.3.Shale oil fraction content(a)and yield(b)varying by pyrolysis temperature in FB reactor.
The effect of temperature on secondary cracking was tested in TSFB with 10 g char on the upper plate.Fig.5(a)shows the effects of pyrolysis and cracking temperatures on product yield in TSFB.Shale oil yield had a slight decrease from 9.14 wt%to 8.3 wt%as temperature increased from 500 °C to 600 °C and then decreased rapidly to 5.91 wt%when increasing temperature to 700°C,indicating severe cracking reactions over oil shale char after 600°C.Gas yield had a steady increasing trend as temperature increased due to the promotion of cracking reactions.Fig.5(b)compares oil yields and gas yields between FB and TSFB.Shale oil yields decreased in the presence of oil shale char in TSFB in contrast with the corresponding cases in FB,and the value of shale oil decrease(Yoildecrease)between FB and TSFB dropped first and then increased as temperature increased,reaching a minimum of 1.79 wt%at600°C.Gas yields increased distinctly in TSFB and the difference of gas yield between FB and TSFB increased with the increase of temperature.
Oilvapor from lower FB passed through char bed on upper FB,heavy fraction was more easily physically captured by char than light fraction.Meantime,heavy fraction yield from lower FB decreased with the increase of temperature due to thermal cracking as shown in Fig.3.Thus larger portion of heavy fraction was retained in oil shale char with moderate cracking in upper FB when temperature was lower,which was responsible for the big loss of shale oil at the temperature of 500 °C and 550 °C.On the other hand,the tendency of catalytic cracking of oil vapor in upper FB was enhanced by elevating particle bed temperature and the captured heavy fraction was gradually released through cracking,thus led to small oil yield difference and further temperature increase resulted in large oil loss.The effects of heavy fraction trapping and catalytic cracking were respectively the dominant reasons for shale oil yield loss before and after 600°C,and these two effects neutralized at 600°C,which resulted in the lowest shale oil yield difference.This further explained oil yield loss at both low and high temperatures and emphasized the necessity of temperature regulation of oil vapor from rapid pyrolysis.Similar mechanism had been studied in two-stage fixed bed[24]to explain the match between temperature profile and secondary reactions for shale oil with both high yield and high quality.And the explanation in this paper could be a supplement in terms of two-stage fluidized bed.
Table 3 shows proximate and ultimate analyses of oil shale char from upper FB at different temperatures and unused oil shale char.Volatile contents in oil shale char from 500 °C to 600 °C were higher than that in unused oil shale char and decreased with the increase of temperature,indicating that shale oil was captured in char before 600°C and elevating temperature enhanced its release,which was in accordance with the explanation of shale oilloss in Fig.5.Carbon and fixed carbon of oilshale char increased slightly compared with unused oil shale char and didn't change obviously,which indicated only slight coking reactions occurred on oil shale char in upper FB.
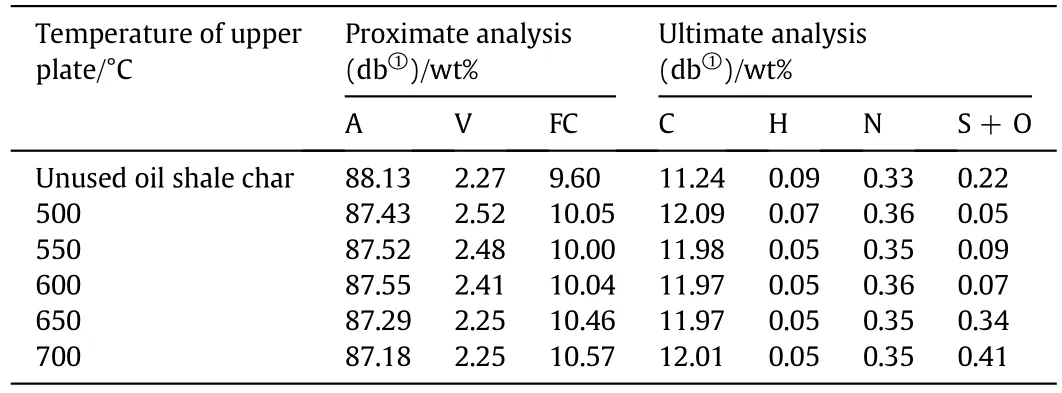
Table 3 Proximate and ultimate analyses of oil shale char varying with temperature of upper plate

Fig.5.Shale oil and gas yield from oil shale pyrolysis in TSFB with 10 g char on the upper plate at different temperatures(a)and comparison of product yields between FB and TSFB pyrolysis(b).
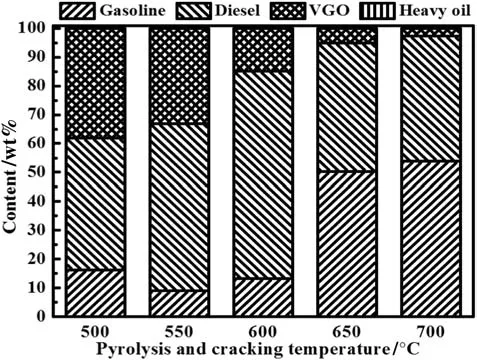
Fig.6.Shale oil composition from TSFB pyrolysis with 10 g char on upper plate at different temperatures.
Fig.6 shows oil fraction distribution in TSFB pyrolysis,light fraction as the dominant component in shale oil increased from 61.88 wt%to 97.24 wt%with temperature increasing and was much higher than FB pyrolysis at the same temperature.Heavy fraction content decreased significantly and almost disappeared at 700°C,indicating good catalytic activity of oil shale char for converting heavy fraction to light fraction.Fig.7(a)shows pyrolysis gas yields from TSFB pyrolysis at different temperatures.Yields of H2,CH4and C2+C3increased with the increase of temperature,indicating catalytic cracking over oil shale char to convert long-chain alkane into gas products.The increases of CO and CO2yields were attributed to the enhanced cracking reactions of oxygencontaining functional groups and decomposition of inorganic minerals[11].Fig.7(b)shows variation of ethene/ethane and propene/propane mass ratios,they all obviously increased as temperature rose.The mass ratio of alkene/alkane in pyrolysis gas can be used to indicate degree of cracking reactions[25,26],and the increase of ethene/ethane and propene/propane ratios revealed enhanced oilvapor cracking when passing oil shale char layer.
3.3.Comparison between FB and TSFB pyrolysis
By adding upper FB and upgrading oil vapor in the presence of oil shale char,quality of shale oil was modified in terms of light fraction content as well as light fraction yield.Further comparisons between FB and TSFB were made in this section to illustrate the advantages of TSFB.
Shale oil quality was improved by oil shale char in terms of variation in light fraction content and yield.Table 4 compares mass ratio percentages of light fraction in both FB and TSFB pyrolysis.TSFB pyrolysis produced higher light fraction contents which were all over 2 times of FB at same pyrolysis temperatures.Heavy fraction was almost eliminated at 700°C which made high-quality shale oil.Fig.8 further compares light fraction yields between FB and TSFB.Light fraction yield of FBincreased from 3.42 wt%to 4.38 wt%as temperature increased due to thermal cracking reactions.Light fraction yields in TSFB were much higher than their counterparts in FB pyrolysis and meantime higher than Fischer Assay light fraction yield.Maximum value of 7.07 wt%at 600°C was 1.86 times of light fraction yield in FB and it was 32.3%higher than Fischer Assay light fraction yield,showing TSFB's advantage in favoring light fraction production.TSFB showed superiority in producing high-yield light fraction in shale oil.Lower FB provided rapid pyrolysis and favored the production of shale oil due to fas the a ting and short residence time of oil vapors.Further adding upper FB for secondary cracking over oil shale char brought in-situ upgrading of shale oil.Oil vapor from lower FB underwent secondary cracking reactions in the presence of minerals and the active sites of oil shale char,converting heavy fraction into light fraction as major end-products.Secondary cracking reactions led to reduction of oil yield,but quality of shale oil was improved,leading to promotion of light fraction products[27,28].Though overall oil yield in TSFB was lower than FB,a compromise was reached by converting heavy fraction into light fraction with high yield as well as high quality.

Table 4 Comparison of light fraction contents from FB and TSFB pyrolysis with 10 g char on upper plate

Fig.8.Comparison of light fraction yield in shale oil from FB and TSFB pyrolysis.
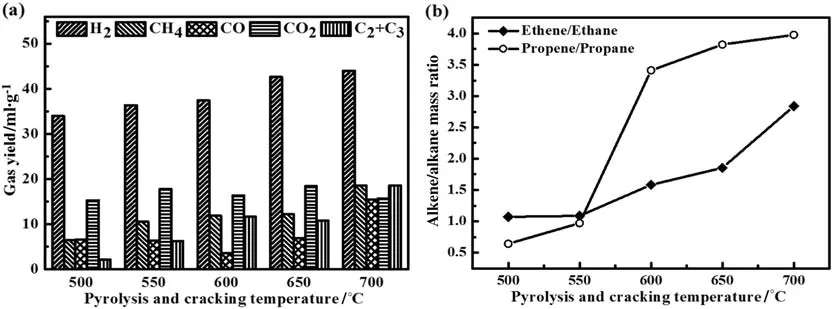
Fig.7.Pyrolysis gas yield(a)and mass ratio of alkene/alkane species in pyrolysis gas(b)varied by pyrolysis and cracking temperatures in TSFB pyrolysis.
Since light fraction yield's increase was at the cost of shale oil yield.Shale oil decrease rate and light fraction increase rate at different temperatures were calculated and shown in Fig.9.Decrease rate of shale oil yield first dropped then rose as temperature increased and got a minimum of 17.74%at 600°C.The increase rate of light fraction yield had an opposite trend which first rose then dropped,left a maximum rate of 86.11%at 600°C.These again strongly indicated that the optimal temperature for shale oil upgrading in the TSFB was 600°C,at which shale oil loss rate was minimum but the light fraction yield increase rate was maximum.This could be explained by the match between shale oil cracking and tendency of light fraction formation.Increasing temperature promoted shale oil cracking over oil shale char at different levels in which partial shale oil converted into light fraction and gas.The bond cleavage energy for gas production is higher than that of light fraction when shale oil cracked.Production tendency of light fraction was bigger than that of gas before 600°C,but further increasing temperature brought excessive cracking which tended to crack both light fraction and heavy fraction into gas and brought more oil loss.Thus the optimal temperatures were 600°C for pyrolysis and cracking in TSFB to provide best compromise between oil yield and oil quality.

Fig.9.Decrease rate of shale oil yield and corresponding increase rate of light fraction yield by comparison of FB and TSFB pyrolysis.
GC-MS analysis results of shale oil from FB and TSFB pyrolysis were listed in Table 5.Major components in shale oil for both cases were alkane and TSFB had higher alkane content than that in FB pyrolysis,which was derived from the scission of carbon-carbon bonds in long chain alkyl groups through cracking to form small molecular species like gaseous products and light fraction[29],indicating oil shale char'sfunction in converting long chain alkane to small molecular species.Aromatic species in TSFB pyrolysis were little higher than those in FB,possibly because of increased secondary reactions(i.e.,cyclization,dehydrogenation and the conversion of aliphatic chains to gaseous components).Contents of oxygen-containing components like ketone,alcohol and other heteroatom-containing components in TSFB were generally lower than those in FB due to deoxidization reaction by secondary cracking over shale oil char and their decrease was associated with the increase of CO and CO2yields in TSFB.
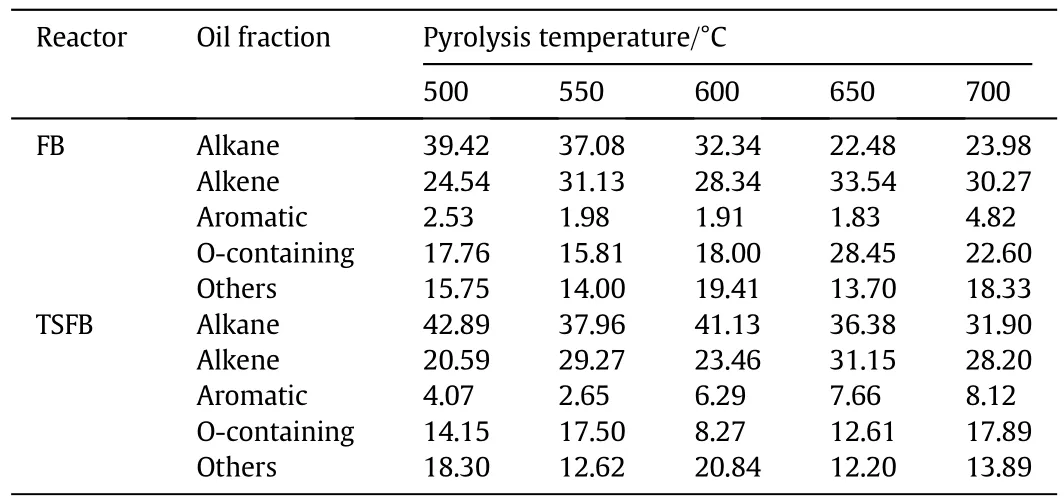
Table 5 GC-MS analysis results of shale oil from FB and TSFB pyrolysis with 10 g char on upper plate.
4.Conclusions
This article investigated oil shale rapid pyrolysis and in-situ upgrading of oil vapor over oil shale char in a two-stage fluidized bed(TSFB)reactor.Rapid pyrolysis in fluidized bed(FB)provided fast heating and quick volatile release to suppress secondary reactions and resulted in shale oil yield higher than the Fischer Assay yield in the temperature range of 500-600°C.The maximum shale oil yield was 12.7 wt%at 500°C which was 127%of the Fischer-Assay yield,but oil quality from FB fast pyrolysis faced disadvantages of high-content heavy fraction(VGO and heavy oil)over 50%at all tested temperatures,which put forward the necessity of oil vapor upgrading in order to get shale oil with high yield and high quality.
In the tested TSFB,oil vapor from lower FB rapid pyrolysis was upgraded through cracking over oil shale char on an upper FB.Effects of contact time between oil vapor and oil shale char were investigated by adding different amounts of oil shale char and the suitable mass ratio of oil shale to oil shale char was found to be 1:1 in terms of producing shale oil with the highestlight fraction yield.Effects of pyrolysis and cracking temperatures were studied in TSFB.Heavy fraction was greatly removed by secondary cracking over oil shale char and the light fraction content ranged from 61.9%to 97.24%,which was much higher than that in FB fast pyrolysis.The light fraction yield all exceeded the Fischer Assay light fraction yield,and the optimal temperatures for pyrolysis and secondary cracking were 600°C in TSFB,at which the light fraction yield increased by 86.11%at a cost of 17.74%shale oil yield loss in comparison with FB fast pyrolysis.Thus a compromise was reached between total shale oil yield and high oil quality in TSFB pyrolysis of oil shale.
References
[1]X.Li,H.Zhou,Y.Wang,Y.Qian,S.Yang,Thermoeconomic analysis of oil shale retorting processes with gas or solid heat carrier,Energy 87(2015)605-614.
[2]J.Desypris,P.Murdoch,A.Williams,Investigation of the flash pyrolysis of some coals,Fuel 61(1982)807-816.
[3]S.D.Carter,U.M.Graham,A.M.Rubel,T.L.Robl,Fluidized Bed Retorting of Oil Shale,Springer,Netherlands,1995.
[4]S.D.Carter,D.N.Taulbee,Fluidized bed steam retorting of Kentucky oil shale,Fuel Process.Technol.11(1985)251-272.
[5]J.H.Edward,K.Schluter,R.J.Tyler,Upgrading of flash pyrolysis tars to synthetic crude oil:1.First stage hydrotreatment using a disposable catalyst,Fuel 64(1985)594-599.
[6]P.T.Williams,J.M.Nazzal,Polycyclic aromatic compounds in oils derived from the fluidised bed pyrolysis of oil shale,J.Anal.Appl.Pyrolysis 35(1995)181-197.
[7]H.Qin,P.Y.Sun,Q.Wang,H.P.Liu,J.R.Bai,M.S.Chi,Effect of extracting wax from oil shale before retorting on shale oil,Sci.Technol.Eng.16(2014)49-54.
[8]H.Yu,S.Li,G.Jin,Reaction conditions for catalytic hydrotreating of diesel distillate from Fushun shale oil,Shiyou Xuebao Shiyou Jiagong/Acta Petrolei Sin.26(2010)403-406.
[9]A.M.Rubel,T.L.Robl,S.D.Carter,Fluidized bed gasification characteristics of Devonian oil shale char,Fuel 69(1990)992-998.
[10]A.M.Rubel,S.M.Rimmer,R.Keogh,T.L.Robl,S.D.Carter,F.J.Derbyshire,Effect of process solids on secondary reactions during oil shale retorting,Fuel 70(1991)1352-1356.
[11]D.Lai,Z.Chen,L.Lin,Y.Zhang,S.Gao,G.Xu,Secondary cracking and upgrading of shale oil from pyrolyzing oil shale over shale ash,Energy Fuel 29(2015)2219-2226.
[12]S.D.Carter,M.Citiroglu,J.Gallacher,C.E.Snape,S.Mitchell,C.J.Lafferty,Secondary coking and cracking of shale oil vapours from pyrolysis or hydropyrolysis of a Kentucky Cleveland oil shale in a two-stage reactor,Fuel 73(1994)1455-1458.
[13]W.J.Shi,Z.Wang,Y.Duan,S.G.Li,W.L.Song,In fluence of shale ash on pyrolytic behaviors of oil shale,Chin.J.Process.Eng.15(2015)266-271.
[14]Z.Wang,S.Deng,Q.Gu,Y.Zhang,Pyrolysis kinetic study of Huadian oil shale,spent oilshale and their mixtures by thermogravimetric analysis,Fuel Process.Technol.110(2013)103-108.
[15]J.Yan,X.Jiang,X.Han,J.Liu,A TG-FTIR investigation to the catalytic effect of mineral matrix in oil shale on the pyrolysis and combustion of kerogen,Fuel 104(2013)307-317.
[16]R.W.Taylor,K.Curry,M.S.Oh,T.Coburn,R.W.Taylor,K.Curry,M.S.Oh,T.Coburn,Clay-induced Oil Loss and Alkene Isomerization During Oil Shale Retorting,1987.
[17]L.Ballice,Effect of demineralization on yield and composition of the volatile products evolved from temperature-programmed pyrolysis of Beypazari(Turkey)Oil Shale,Fuel Process.Technol.86(2005)673-690.
[18]P.T.Williams,H.M.Chishti,Two stage pyrolysis of oil shale using a zeolite catalyst,J.Anal.Appl.Pyrolysis 55(2000)217-234.
[19]J.H.Richardson,E.B.Huss,L.L.Ott,J.E.Clarkson,M.O.Bishop,J.R.Taylor,L.J.Gregory,C.J.Morris,Fluidized-bed pyrolysis of oil shale:Oil yield,composition,and kinetics,NASA STI/Recon Technical Report N,83,1982.
[20]J.O.Jaber,S.D.Probert,P.T.Williams,Influence of particle size,grade and pyrolysis temperature on the oil yield from Jordanian oil shales,Oil Shale 16(1999)197-221.
[21]L.Lin,C.Zhang,H.Li,D.Lai,G.Xu,Pyrolysis in indirectly heated fixed bed with internals:The first application to oil shale,Fuel Process.Technol.138(2015)147-155.
[22]S.M.Shih,Y.S.Hong,A mathematical model for the retorting of a large block of oil shale:Effect of the internal temperature gradient,Fuel 57(1978)622-630.
[23]L.Lin,D.Lai,E.Guo,C.Zhang,G.Xu,Oil shale pyrolysis in indirectly heated fixed bed with metallic plates of heating enhancement,Fuel 163(2016)48-55.
[24]Y.Zhang,Z.Han,H.Wu,D.Lai,P.Glarborg,G.Xu,Interactive matching between temperature profile and secondary reactions of oil shale pyrolysis,Energy Fuel 30(2016)2865-2873.
[25]J.H.Campbell,G.J.Koskinas,G.Gallegos,M.Gregg,Gas evolution during oil shale pyrolysis.1.Nonisothermal rate measurements,Fuel 59(1980)718-726.
[26]S.Wang,J.Liu,X.Jiang,X.Han,J.Tong,Effect of heating rate on products yield and characteristics of non-condensable gases and shale oil obtained by retorting Dachengzi oil shale,Oil Shale 30(2013)27-47.
[27]N.V.Dung,A.J.Feltenstein,R.G.Benito,N.V.Dung,A.J.Feltenstein,Processing oil shales with heavy oil recycle,71(1992)1505-1510.
[28]N.V.Dung,Factors affecting product yields and oil quality during retorting of Stuart oil shale with recycled shale:a screening study,Fuel 74(1995)623-627.
[29]E.R.Bissell,A.K.Burnham,R.L.Braun,Shale oil cracking kinetics and diagnostics,Ind.Eng.Chem.Process Des.Dev.24(1985)381-386.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Transport hindrances with electrodialytic recovery of citric acid from solution of strong electrolytes
- Experimental investigation on CO2-light crude oil interfacial and swelling behavior
- Biosynthesis of 4-hydroxyphenylpyruvic acid from L-tyrosine using recombinant Escherichia coli cells expressing membrane bound L-amino acid deaminase☆
- Process development for producing a food-grade glucose solution from rice straws
- Carbon dioxide induced degradation of diethanolamine during absorption and desorption processes
- Biodegradation of natural and synthetic estrogens in moving bed bioreactor
