A new nanocluster polyoxomolybdate[Mo36O110(NO)4(H2O)14]·52H2O:Synthesis,characterization and application in oxidative degradation of common organic dyes☆
Mojtaba Amini*,Mostafa Khaksar Arkady Ellern ,L.Keith Woo
1 Department of Chemistry,Faculty of Science,University of Maragheh,Maragheh 55181-83111,Iran
2 Chemistry Department,Iowa State University,Ames,IA 50011-3111,USA
1.Introduction
Recently,oxidative degradation of methylene blue(MB)and rhodamine B(RB)has attracted significant attention due to its promising application in the decrease of environmental pollutants[1-6].Up to now,a variety of methods,including adsorption,photocatalytic degradation,ozone oxidation,electrocatalytic oxidation,oxidative degradation,etc.,have been developed and extensively studied[7-13].However,exploring simpler,effective,lower cost and safer methods is still needed for practical applications.
Layered double hydroxides(LDHs)as a class of synthetic twodimensional nanostructured anionic clays with positively charged layers and parallely charge-balancing anions between them are a family of materials which have anionic exchange capacity and capability to capture inorganic anions in the interlayer spaces for the design of a large number of practicalapplications in catalysis,adsorption,pharmaceutics,photochemistry,electrochemistry and other areas[14-20].
Polyoxomolybdates as one kind of well-defined polyoxometalate clusters with abundant building blocks and potential applications in biology and medicine,photochemistry,magnetism,electrochemistry and catalysis are widely used as an embedding anion in LDHs to construct novel materials of LDHs with desired properties[21-26].Therefore in the presentwork we reportthe synthesis and characterization of a polyoxomolybdate[Mo36O110(NO)4(H2O)14]·52H2O(1).Then polyoxomolybdate 1 was pillared with MgAl-LDH-Nitrate by direct ion exchange to create a heterogeneous catalyst system.Further application of these compounds for degradation of dyes including methylene blue(MB)and rhodamine B(RB)has been carried out.This is the first report on the catalytic activity of a layered double hydroxide pillared by polyoxomolybdate in the oxidative degradation of MB and RB in the presence of H2O2as a green oxidant.
2.Experimental
2.1.Materials
MgAl-LDH-Nitrate was prepared according to the literature method[27].
2.2.Synthesis of[Mo36O110(NO)4(H2O)14]·52H2O(1)
In a 100 ml Erlenmeyer flask,to a H2O solution(30 ml)of Na2MoO4·2H2O(1.21 g,5 mmol)and NH2OH·HCl(0.83 g,1.2 mmol),2 ml HCl(3.5%)was added in a single portion.The mixture was allowed to stir slowly for 20 h in an oil bath at re flux temperature.The hot solution was filtrated and the cooled red filtrate was stored for crystallization at room temperature.
2.3.Preparation of MgAl-LDH–1
The intercalation of polyoxomolybdate 1 was done by directly adding a two-fold excess of1 solution to the MgAl-LDH-NO3suspension under N2with rapid stirring for 6 h at 70°C.Finally,MgAl-LDH-1 catalyst was obtained after being washed with deionized water several times and dried at 50°C.
2.4.Oxidative degradation of dyes
The catalytic activity of the catalyst was demonstrated by degrading MB and RB in aqueous solution.A round-bottom flask was charged with 50 ml of dye solution(10 mg·L-1)and 10 mg of MgAl-LDH-1 was added.After addition of H2O230%,(1 ml),the mixture was stirred under ambient conditions.For a given time interval,a small quantity of the mixture solution was pipetted into a quartz cell,and its absorption spectrum was measured using a UV-Visible spectrophotometer.
3.Results and Discussion
3.1.Material characterization
Polyoxomolybdate 1 was synthesized by a simple one-pot procedure through reducing an acidified mixture of Na2MoO4·2H2O and NH2OH·HCl in pH=3 at re flux temperature.The IR spectrum of 1 exhibits two strong bands at 875 cm-1and 619 cm-1which are attributed to νMo=Oand νMo--O--Movibrations,and a band at of 1615 cm-1is assigned to the stretching frequency associated with the N--O bond for the linear{MoNO}moiety[26].
The structure of compound 1 was determined by X-ray diffraction methods(Fig.1).According to elemental analysis and X-ray diffraction analyses,the formula of the title compound was af firmed to[MoVI36O110(NO)4(H2O)14]·52H2O.The structure of 1 shows a large cluster with a{Mo36}skeleton,which consists of two 18-molybdate{Mo18O55}subunits related to each other by an inversion center.Each{Mo18O55}unit has twelve(MoO6)octahedral,two(MoO6NO)pentagonal, five(MoO5(H2O))and one(MoO4(H2O)2)octahedron forming a large ‘ring’in the center of the polyoxomolybdate.The cage has crystallographic twofold symmetry with five formula units per unit cell as crimson-red crystals(Fig.2).All molybdenum atoms are bound to μ2and μ3framework oxygen atoms with Mo-O distances ranging from 0.1890(5)to 0.2295(5)nm and terminal oxygen atom[Mo-O=0.1698(7)-0.1709(8)nm],which are comparable with those found in other polyoxomolybdate[26].
As is common in polyoxomolybdate cages,each of the sixcoordinate molybdenum atoms for all(MoO6)octahedral subunits has two terminal oxygen atoms,with its remaining four oxygens participating in the cage framework.All pentagonal molybdenum atoms are bound to six μ2framework oxygen atoms with Mo-O distances ranging from 0.2039(6)to 0.2269(6)nm and one terminal nitrogen atom of nitrosyl ligand[Mo-N=0.1726(7)-0.1729(7)nm].Selected crystal data and details of the structure determination are summarized in Table 1.
Ion exchange of the 1 with MgAl-LDH-NO3under N2conditions results in the formation of new intercalated assembly of MgAl-LDH-1 as a heterogeneous catalyst system.The as-prepared nanocomposite material of MgAl-LDH-1 was characterized by powder X-ray diffraction(XRD),IR spectroscopy,transmission electron microscopy(TEM)and elemental analysis.Powder XRD patterns of MgAl-LDH-NO3in Fig.3 showed characteristic re flections of MgAl LDHs[28].Compared with the XRD pattern of the MgAl-LDH-NO3,some re flections of MgAl-LDH-1 shift to lower 2θ,indicating the successful intercalation of the 1 into the MgAl-LDH-NO3[29].
Fig.4 shows the IRspectra ofthe MgAl-LDH-NO3,polyoxomolybdate 1 and MgAl-LDH-1 compounds.Absorption at 1389 cm-1in the MgAl-LDH-NO3can be assigned to the ν3vibration of NO3[27].The presence of N--O bond vibration frequency at 1621 cm-1due to the linear{MoNO}moiety of 1[26]in the spectra of MgAl-LDH-1 indicates that the intercalation process was done.
TEM images of MgAl-LDH-1 show slightly irregular plate-like LDH crystallites(Fig.5).The plate sizes of MgAl-LDH-1 are in the range of 100-200 nm.Elemental analysis of MgAl-LDH-1 demonstrates that all of the elements of LDHs and the POM anions exist in the sample,which implies that the POM anions have been intercalated into the interlayer of LDHs.
3.2.Catalytic performance
The extentof dye degradation was monitored using UV-Vis spectroscopic techniques.To explore the potentialcapability of MgAl-LDH-1 to remove contaminants from waste water,catalytic activity ofMgAl-LDHNO3and MgAl-LDH-1 nanoparticles was evaluated in the oxidative degradation of methylene blue(MB)and rhodamine B(RB)in the presence of hydrogen peroxide as an oxidant.Experiments were carried out both in the presence and in the absence of catalyst.In the absence of a catalyst or H2O2and/or in the presence of MgAl-LDH-NO3without polyoxomolybdate 1,the oxidative degradation of MB and RhB was very slow,and almost no degradation of the both dyes was observed(Fig.6).
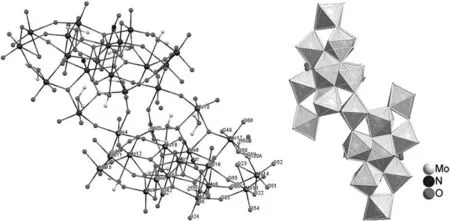
Fig.1.Ball-and-stick and polyhedral representation of 1 showing the atom labeling scheme.
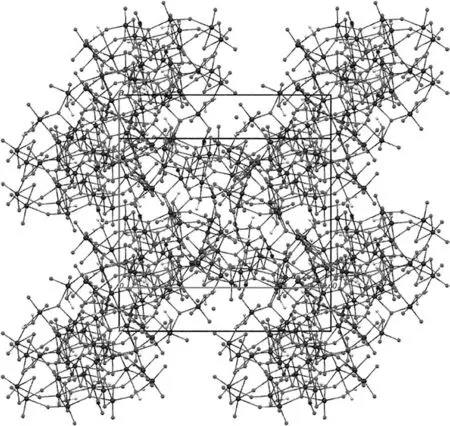
Fig.2.View of the unit cell content in the crystal structure of[Mo36O110(NO)4(H2O)14]·52H2O,along the c-axis.1 Å =0.1 nm.
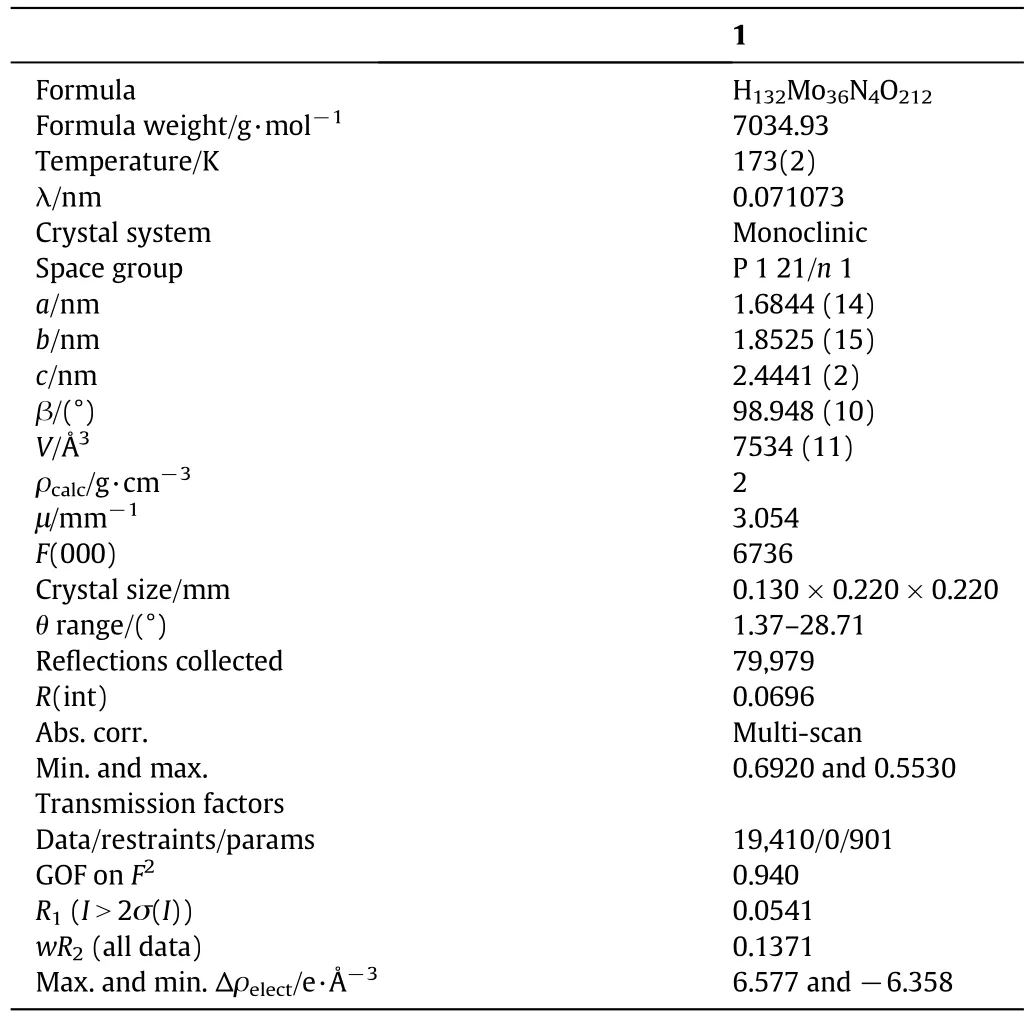
Table 1 Selected X-ray data for[MoVI36O110(NO)4(H2O)14]·52H2O
Butwhen the MgAl-LDH-1 catalyst was added to the MB or RB solution,the oxidative degradation of MB and RB wascomplete after60 min and 80 min respectively,showing that MgAl-LDH-1 facilitated the degradation of the MB and RB(Fig.7).
The effect of H2O2amounton the percentage degradation of MB and RB is also studied.By varying the amount of H2O2from 0 to 10 mmol,the percentage degradation of MB and RB increased from 0 to 99%and 97%respectively(Fig.8).
Oxidation degradation of dyes in the presence of H2O2usually follows a radical oxidation pathway that may be done due to·OH radical formation when hydrogen peroxide decomposed homolytically[13].To prove the role of·OH radicals in the oxidation degradation of MB in the presence of MgAl-LDH-1,the effects of DMSO as an·OH-radical scavenging agent were checked in this process.With addition of DMSO(0.1 mmol)to dye solutions,the removal of MB and RB was decreased from 99%and 97%to 57%and 65%respectively,which confirmed the presence of·OH radicals in the oxidation process of dyes.H2SO4and HCl as degradation products of MB were identified gravimetrically by precipitation of BaSO4and AgCl by adding BaCl2and AgNO3as precipitants.
The effect of the solution pH on the MB adsorption rate of MgAl-LDH-1 was investigated in a pH range of 3-9.Results showed that the adsorbed percentage of MB at neutral and basic pH is almost complete,but at acidic pH,the amount of adsorbed MB is very poor,mainly due to the fact that MB as a cationic dye would prefer to adsorb on the negative surface and not the positive surface(Fig.9).

Fig.3.XRD pattern of a)CIF file of polyoxomolybdate 1;b)MgAl-LDH-NO3;c)polyoxomolybdate 1;d)MgAl-LDH-1 nanoparticles.

Fig.4.Overlay of FT-IR spectra for the MgAl-LDH-NO3,polyoxomolybdate 1 and MgAl-LDH-1 compounds.
Since reusability ofa heterogeneous catalystis crucialforits practical application,it is essential to evaluate the recoverability of the MgAl-LDH-1.The MgAl-LDH-1 catalyst can be easily separated by centrifugation after completion of the oxidative degradation process,washed by deionized water,and dried before the next cycle.According to the results of the recycling test(Fig.10),the reused MgAl-LDH-1 can be used at least five times without significant decrease of the catalytic activity.Moreover,XRD patterns of the reused MgAl-LDH-1 are in agreement with the one of freshly prepared MgAl-LDH-1,indicating the high stability of this material during the dye degradation.
To verify the nature of heterogeneity ofthe catalyst,we carry out hot filtration test based leaching for the oxidative degradation of MB.For this aim,after half of reaction time(30 min),the MgAl-LDH-1 was separated by filtration and the filtrate allowed to stir for another period of half the reaction time.After catalyst separation only a trace amount of degradation was detected.The molybdenum content leaching after filtering the reaction mixture from catalyst was also checked by ICP analysis.The overall leakage of molybdenum from the catalyst was 0.06%.These observations indicated stability of the MgAl-LDH-1 in the catalytic process and also revealed that the leaching of the catalyst into the reaction mixture is negligible and the catalyst is truly heterogeneous.
In order to show the removal efficiency of the present catalytic system in comparison with recently reported protocols,we compared the results of degradation of MB in the presence of various catalysts.As shown in Table 2,MgAl-LDH-1 is preferable to some of the previously reported catalysts in terms of reaction conditions and the presented catalytic system does not suffer from the harsh reaction conditions,such as using a source of visible light(Entries 2,3),using hazardous oxidant(Entry 4)and long degradation time(Entries 2-4).In addition,the attractive features of MgAl-LDH-1 catalyst,such as low cost,and ease of use,make it particularly suitable for the degradation of dyes.

Fig.5.TEM images of MgAl-LDH-1 nanoparticles.
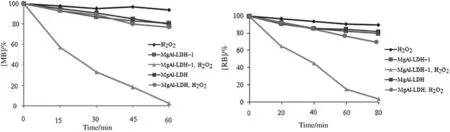
Fig.6.Percentage degradation of MB and RB with different conditions.
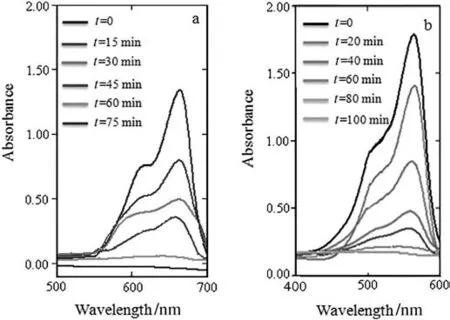
Fig.7.Changes of UV-Vis spectra during the oxidative degradation of(a)MB;(b)RB in the presence of the MgAl-LDH-1.
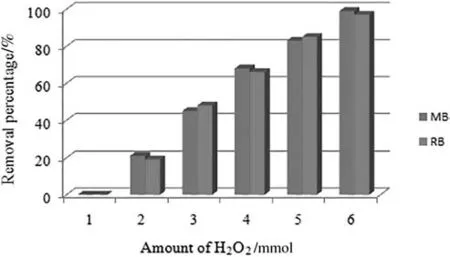
Fig.8.Effects of the amount of H2O2 on the percentage decomposition of MB and RB.
4.Conclusions
In summary,a polyoxomolybdate[Mo36O110(NO)4(H2O)14]·52H2O was synthesized and in order to create a heterogeneous catalyst system,this polyoxo molybdate was pillared with MgAl-LDH-NO3by direct ion exchange.The present study illustrates the feasibility and applicability of utilizing the MgAl-LDH-1 material to catalyze the oxidative degradation of methylene blue(MB)and rhodamine B(RB)as common dyes used in textile industry.Furthermore,the recyclability and reusability of catalyst for up to five consecutive cycles in the oxidative degradation of MB and RB without a significant loss of efficiency indicate the potential of the MgAl-LDH-1 as an efficient catalyst.

Fig.9.Effects of pH on the degradation of MB in the presence of MgAl-LDH-1.

Fig.10.Recycling studies of the MgAl-LDH-1 catalyst in the oxidative degradation of MB and RB.
Supplementary Material
The CIF file of crystal structure nanocluster,[Mo36O110(NO)4(H2O)14]·52H2O has been deposited with the CCDC,No.1471123.This data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html,or from the Cambridge Crystallographic Data Centre,12 Union Road,Cambridge CB2 1EZ,UK;fax:(+44)1223-336-033;or e-mail:deposit@ccdc.cam.ac.uk.Supplementary data associated with this article can be found in the online version,at doi:10.1016/j.cjche.2017.03.031.

Table 2 Recently reported catalytic systems for degradation of MB
[1]M.M.Alnuaimi,M.A.Rauf,S.S.Ashraf,Comparative decoloration study of Neutral Red by different oxidative processes,Dyes Pigments 72(2007)367-371.
[2]A.Xu,X.Li,S.Ye,G.Yin,Q.Zeng,Catalyzed oxidative degradation of methylene blue by in situ generated cobalt(II)-bicarbonate complexes with hydrogen peroxide,Appl.Catal.B Environ.102(2011)37-43.
[3]W.Wei,P.Gao,J.Xie,S.Zong,H.Cui,X.Yue,Uniform Cu2Cl(OH)3hierarchical microspheres:A novel adsorbent for methylene blue adsorptive removal from aqueous solution,J.Solid State Chem.204(2013)305-313.
[4]S.Ameen,M.Shaheer Akhtar,Y.Soon Kim,O-Bong Yang,H.Shik Shin,An effective nanocomposite of polyaniline and ZnO:preparation,characterizations,and its photocatalytic activity,Colloid Polym.Sci.289(2011)415-421.
[5]S.Caudo,G.Centi,C.Genovese,S.Perathoner,Homogeneous versus heterogeneous catalytic reactions to eliminate organics from waste water using H2O2,Top.Catal.40(2006)207-219.
[6]P.Bautista,A.F.Mohedano,J.A.Casas,J.A.Zazo,J.J.Rodriguez,An overview of the application of Fenton oxidation to industrial wastewaters treatment,J.Chem.Technol.Biotechnol.83(2008)1323-1338.
[8]J.Chu,L.Zhong,Photocatalytic Degradation of Methylene Blue with Side-glowing Optical Fiber Deliverying Visible Light,Chin.J.Chem.Eng.20(2012)895-899.
[8]H.Habazaki,Y.Hayashi,H.Konno,Characterization of electrodeposited WO3films and its application to electrochemical wastewater treatment,Electrochim.Acta 47(2012)4181-4188.
[9]S.Thirumalairajan,K.Girija,V.R.Mastelaro,N.Ponpandian,Photocatalytic degradation of organic dyes under visible light irradiation by floral-like LaFeO3nanostructures comprised of nanosheet petals,New J.Chem.38(2014)5480-5490.
[10]M.Amini,B.Pourbadiei,T.Purnima,A.Ruberu,L.Keith Woo,Catalytic activity of MnOx/WO3nanoparticles:synthesis,structure characterization and oxidative degradation of methylene blue,New J.Chem.38(2014)1250-1255.
[11]S.T.Liu,A.B.Zhang,K.K.Yan,Y.Ye,X.G.Chen,Microwave-enhanced catalytic degradation of methylene blue by porous MFe2O4(M=Mn,Co)nanocomposites:Pathways and mechanisms,Sep.Purif.Technol.135(2014)35-41.
[12]P.Zhang,Y.Zhan,B.Cai,C.Hao,J.Wang,C.Liu,Z.Meng,Z.Yin,Q.Chen,Shapecontrolled synthesis of Mn3O4nanocrystals and their catalysis of the degradation of methylene blue,Nano Res.3(2010)235-243.
[13]M.Khaksar,M.Amini,D.M.Boghaei,K.H.Chae,S.Gautam,Mn-doped ZrO2nanoparticles as an efficient catalyst for green oxidative degradation of methylene blue,Catal.Commun.72(2015)1-5.
[14]Q.Wang,D.O'Hare,Recent advances in the synthesis and application of layered double hydroxide(LDH)nanosheets,Chem.Rev.112(2012)4124-4155.
[15]B.Velde,Clays and Clay Minerals in Natural and Synthetic Systems,Elsevier,Amsterdam,1977.
[16]A.Meunier,Clays,Springer,Berlin,2005.
[17]S.M.Auerbach,K.A.Carrado,P.K.Dutta(Eds.),Handbook of Layered Materials,2004.
[18]T.Selvam,A.Inayat,W.Schwieger,Reactivity and applications of layered silicates and layered double hydroxides,Dalton Trans.43(2014)10365-10387.
[19]L.Liu,W.Wang,Y.Hu,Layered double hydroxide-decorated flexible polyurethane foam:Significantly improved toxic effluent elimination,RSC Adv.5(2015)97458-97466.
[20]C.Taviot-Guého,M.Halma,K.Charradi,C.Forano,C.Mousty,Structural and electrochemical characterization of metallo-porphyrins intercalated into ZnCr-layered double hydroxides:Some evidence of dimer formation,New J.Chem.35(2011)1898-1905.
[21]H.Lv,Y.V.Geletii,C.Zhao,J.W.Vickers,G.Zhu,Z.Luo,J.Song,T.Lian,D.G.Musaev,C.L.Hill,Polyoxometalate water oxidation catalysts and the production of green fuel,Chem.Soc.Rev.41(2012)7572-7589.
[22]S.Uchida,K.Kamata,Y.Ogasawara,M.Fujita,N.Mizuno,Structural and dynamical aspects of alkylammonium salts of a silicodecatungstate as heterogeneous epoxidation catalysts,Dalton Trans.41(2012)9979-9983.
[23]Y.Ding,W.Zhao,H.Hua,B.Ma,[π-C5H5N(CH2)15CH3]3[PW4O32]/H2O2/ethyl acetate/alkenes:A recyclable and environmentally benign alkenes epoxidation catalytic system,Green Chem.10(2008)910-913.
[24]Y.Nishiyama,Y.Nakagawa,N.Mizuno,High turnover numbers for the catalytic selective epoxidation of alkenes with 1 atm of molecular oxygen,Angew.Chem.Int.Ed.40(2001)3639-3641.
[25]N.V.Izarova,M.T.Pope,U.Kortz,Noble metals in polyoxometalates,Angew.Chem.Int.Ed.51(2012)9492-9510.
[26]M.Amini,H.Naslhajian,S.M.F.Farnia,M.Hołyńska,Selective oxidation of sul fides catalyzed by the nanocluster polyoxomolybdate(NH4)12[Mo36(NO)4O108(H2O)16],Eur.J.Inorg.Chem.2015(2015)3873-3878.
[27]P.Liu,C.Wang,C.Li,Epoxidation of allylic alcohols on self-assembled polyoxometalates hosted in layered double hydroxides with aqueous H2O2as oxidant,J.Catal.262(2009)159-168.
[28]P.Koilraj,K.Sasaki,Fe3O4/MgAl-NO3layered double hydroxide as a magnetically separable sorbent for the remediation of aqueous phosphate,J.Environ.Chem.Eng.4(2016)984.
[29]Y.Chen,Z.Yao,H.N.Miras,Y.F.Song,Modular polyoxometalate-layered double hydroxide composites as efficient oxidative catalysts,Chem.Eur.J.21(2015)10812-10820.
[30]Y.Zhou,W.Hu,J.Yu,F.Jiao,Effective photocatalytic degradation of methylene blue by Cu2O/MgAl layered double hydroxides,React.Kinet.Mech.Catal.115(2015)581-596.
[31]Shengjie Xia,Lianyang Zhang,Guoxiang Pan,Pingping Qian,Zheming Ni,Photocatalytic degradation of methylene blue with a nanocomposite system:Synthesis,photocatalysis and degradation pathways,Phys.Chem.Chem.Phys.17(2015)5345-5351.
[32]M.Khaksar,D.M.Boghaei,M.Amini,Synthesis,structural characterization and reactivity of manganese tungstate nanoparticles in the oxidative degradation of methylene blue,C.R.Chim.18(2015)199-203.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Surface chemical characterization of deactivated low-level mercury catalysts for acetylene hydrochlorination☆
- Insight into fouling behavior of poly(vinylidene fluoride)(PVDF)hollow fiber membranes caused by dextran with different pore
- Protein adsorption onto diethylaminoethyl dextran modi fied anion exchanger:Effect of ionic strength and column behavior☆
- Gas emission source term estimation with 1-step nonlinear partial swarm optimization-Tikhonov regularization hybrid method☆
- Dissolution of antibiotics mycelium in ionic liquids:Performance and mechanism☆
- Kinetic studies on extra heavy crude oilupgrading using nanocatalysts by applying CFD techniques☆
