Preparation and characterization of H4SiW12O40@MIL-100(Fe)and its catalytic performance for synthesis of 4,4′-MDA☆
Yunhuan Kong,Xiaomeng Cheng,Hualiang An,Xinqiang Zhao*,Yanji Wang
Hebei Provincial Key Lab of Green Chemical Technology and Efficient Energy Saving,School of Chemical Engineering and Technology,Hebei University of Technology,Tianjin 300130,China
1.Introduction
4,4′-Methylenedianiline(4,4′-MDA)is not only a key intermediate for the manufacture of methylene diphenyl diisocyanate(MDI),but also extensively applied in the production of epoxy resin tough curing agent,chain extender,organic dyes and so on[1].The synthesis of 4,4′-MDA from aniline and formaldehyde is a typical acid-catalyzed reaction.At present,the industrial manufacture of 4,4′-MDA usually utilizes hydrochloric acid as catalyst,suffering from some drawbacks,e.g.,corrosiveness of equipment and emission of a lot of acid waste water[2].In order to overcome these drawbacks,a lot of efforts have been made in the research on solid acid catalysts[3-8]mainly including acidic clay,molecular sieves,ion exchange resin,metal compound and so on.Some good results have been achieved but there still exists poor catalyst stability and low selectivity.Nafziger et al.[9]selected acid ion exchange resins to catalyze the synthesis of 4,4′-MDA and got a 4,4′-MDA yield of 75%with a selectivity of 85%at 120 °C.However,acid ion exchange resins had poor thermal stability.Bahulayan et al.[10]developed an efficient method for the synthesis of 4,4′-MDA catalyzed by kaolinite clay.The conversion of aniline was significantly high with a selectivity in the range of 68%-98%,butthe properties of kaolinite clay would severely affect its catalytic activity.Our group studied the synthesis of 4,4′-MDA using[HSO3-bmim]CF3SO3as catalyst.Under the suitable reaction condition of reaction temperature=80°C and reaction time=8 h,the yield and selectivity of 4,4′-MDA were 79.4%and 87.9%,respectively[11].However,the high price of ionic liquids caused high production cost and ionic liquids could be reused only after it was re-acidized by CF3SO3H because there was a chemical interaction between aniline and the sulfonic acid group of[HSO3-bmim]CF3SO3.So it was necessary to explore an environmentally friendly and highly effective catalyst.
As a solid acid,heteropolyacid has some advantages[12-16],e.g.,no equipment corrosion and no emission of acidic waste water.What's more,heteropolyacid has been widely used in acid-catalyzed reactions and good results have been achieved.Based on the advantages of high activity and high selectivity in condensation reactions,heteropolyacid is chosen as a catalyst in this paper.However,heteropolyacid can dissolve in the reaction system of 4,4′-MDA synthesis,which leads to the difficult recycle.Metal-organic frameworks(MOFs)encapsulated heteropolyacid usually shows not only high catalytic activity but also excellent reusability[17,18].Therefore,we prepared MIL-100(Fe)encapsulated heteropolyacid,characterized its structure,and finally investigated its catalytic performance for 4,4′-MDA synthesis.
2.Experimental
2.1.Materials and reagents
Aniline(AR,Sinopharm Chemical Reagent Co.,Ltd.,China),silicotungstic acid,phosphotungstic acid and phosphomolybdic acid(AR,Tianjin Kemiou Chemical Reagent Co.,Ltd.,China),trimesitinic acid(AR,Tianjin Heowns Biochem LLC,China),ferric nitrate(AR,Tianjin Damao Chemical Reagent Factory,China),formaldehyde(37 wt%aqueous solution)and N,N-dimethylformamide(AR,Tianjin Fengchuan Chemical Reagent Science and Technology Co.,Ltd.,China),and 4,4′-methylenedianiline(AR,J&K Chemical Technology Co.,Ltd.,China)were used.
2.2.Catalyst preparation
8.08 g of Fe(NO3)3·9H2O,2.8 g of trimesic acid,8 g of H4SiW12O40and 12 ml of water were charged into a three-necked flask equipped with a stirrer.Afterstirred atroom temperature for 30 min,the mixture was heated to 95°C and then re fluxed with stirring for another 12 h.The resultant solid-liquid mixture was filtered and the cake was washed with water and then dried in vacuum for 5 h.The dried cake was washed by three steps:(1)mixed with water and stirred at 80°C for 5 h in water bath, filtered and then dried in vacuum at 80°C for 3 h;(2)then mixed with DMF and stirred for 2 h, filtered and then dried in vacuum at 80°C for 3 h;(3) finally mixed with ethanol and stirred at 60 °C for 3 h, filtered and then dried in vacuum at 150 °C for 6 h.The above puri fication procedure was repeated twice.
2.3.Catalyst characterization
The textural properties of the catalysts were measured using an ASAP 2020 specific surface area and porosity analyzer.Prior to the test,0.2 g ofsample was degassed at150°C for4 h in vacuumto remove the impurities adsorbed on the sample surface.Then N2adsorptiondesorption test was performed at-195.8°C.The specific surface area was calculated by the Brunauer-Emmett-Teller(BET)method while the pore volume and pore diameter were calculated by the Barrett-Joyner-Halenda(BJH)method.
The X-ray diffraction(XRD)analysis was performed on a Rigaku D/max-2500 X-ray diffractometer using Cu Kαradiation and a graphite monochromator at 40 kV and 100 mA.The scan range covered from 3 to 30°at a rate of 8(°)·min-1.
The FT-IR spectra of H4SiW12O40@MIL-100(Fe)were recorded on a Bruker VECTOR 22 infrared spectroscopy.The instrument resolution was 4 cm-1and scanning rate was 0.2 cm·s-1in the wavenumber range of 400 to 4000 cm-1.
The thermal stability analysis of H4SiW12O40@MIL-100(Fe)was performed on a SDT-2960 TG-DTA Thermal Analyzer.The furnace atmosphere was the dynamic air(30 ml·min-1)and the sample was heated from 25 °C to 600 °C at a ramping rate of 10 °C·min-1.
The loading of H4SiW12O40was determined by inductively coupled plasma atomic emission spectrometry(ICP-AES)on an Optima 7300 V.
2.4.Catalyst activity
4,4′-MDA synthesis from the condensation reaction of aniline with formaldehyde on H4SiW12O40@MIL-100(Fe)was conducted in a 100 ml stainless steel autoclave.A typical procedure was described as follows:30 g of aniline,8.715 g of formaldehyde aqueous solution and 3.225 g of H4SiW12O40@MIL-100(Fe)were added into the autoclave and then the air inside was replaced with nitrogen.The reaction was conducted at 120°C for 6 h while stirring.And then the mixture was cooled to room temperature after the completion of reaction.The product mixture was separated by centrifugal separation and quantitatively analyzed by a liquid chromatography.The reaction equation of aniline with formaldehyde to 4,4′-MDA is shown in Fig.1.
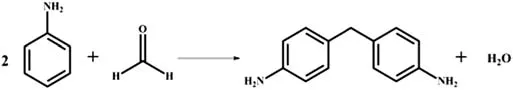
Fig.1.Reaction of aniline with formaldehyde to 4,4′-MDA.
2.5.Product analysis
4,4′-MDA was quantitatively analyzed by Waters HPLC with a Waters 1515 pump,a Kromasil C18 column(4.6 mm×150 mm),and a Waters 2489 UV-vis detector operated at 254 and 232 nm.Methanolwater with a volumetric ratio of 5/5 was used as the mobile phase whose flowrate was 0.4 ml·min-1.The yield of 4,4′-MDA was calculated on the basis of formaldehyde while the selectivity of 4,4′-MDA was calculated on the basis of aniline.

3.Results and Discussion
3.1.Catalytic performance of heteropolyacids
The catalytic performance of H3PW12O40,H4SiW12O40and H3PMo12O40was evaluated in the synthesis of 4,4′-MDA from aniline and formaldehyde,and the results were listed in Table 1.No reaction occurred and two phases in the reaction solution were observed in the absence of catalyst(aniline and formaldehyde aqueous solution were not dissolved with each other).There was no significant difference in the conversion of aniline over the three heteropolyacids and the reasons could be analyzed as follows.The condensation reaction of aniline with formaldehyde proceeds in two steps:(1)a rapid reaction of aniline with formaldehyde to for man intermediate,aminal,in a presence ofan acidic catalyst;(2)cleavage of C--N bond in the molecule of aminal and rearrangement to MDA over an acidic catalyst at high temperature[19].The conversion of aniline was determined by the first step,i.e.the condensation reaction of aniline with formaldehyde which would occur easily and quickly under acidic condition,so there was little difference in the conversion of aniline over the three heteropolyacids.But H4SiW12O40exhibited the highest catalytic performance;the yield and selectivity of 4,4′-MDA attained 62.2%and 80.4%,respectively.The acid strength of the common Keggin-type heteropolyacids decreased in the following order:H3PW12O40>H4SiW12O40>H3PMo12O40[13].H3PW12O40with much stronger acid strength would cause more side-reactions and decrease the selectivity of4,4′-MDAwhile H3PMo12O40with much weaker acid strength caused lower selectivity also.In conclusion,the catalytic performance of H4SiW12O40with a moderate acid strength was the highest.However,it is difficult for recovering and reusing H4SiW12O40because it was easily dissolved in aniline during the process of reaction.So H4SiW12O40@MIL-100(Fe)was prepared by encapsulating H4SiW12O40within the pores of MIL-100(Fe)according to the process described as 1.2 and good reusability will be expected.
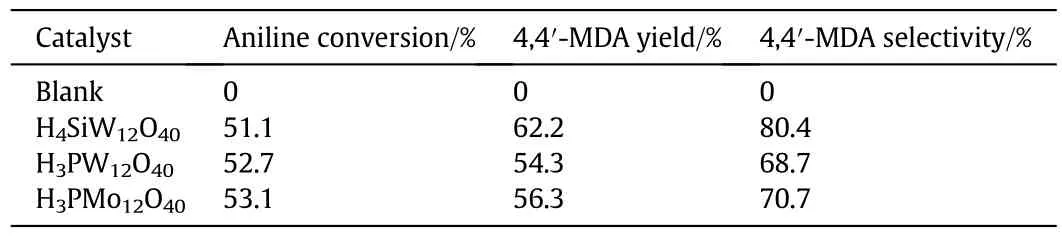
Table 1 Catalytic performance of different heteropolyacids
3.2.Characterization of H4SiW12O40@MIL-100(Fe)
3.2.1.Crystal structure
XRD patterns of MIL-100(Fe)and H4SiW12O40@MIL-100(Fe)with a H4SiW12O40loading of 43.2wt%were shown in Fig.2.The characteristic diffraction peaks of MIL-100(Fe)were consistent with those simulated by single crystal structure data[20],suggesting that MIL-100(Fe)was well-prepared.The characteristic diffraction peaks of H4SiW12O40@MIL-100(Fe)were similar with those of MIL-100(Fe)as a whole,but there was a slight change in the positions and relative intensities of the characteristic diffraction peaks at small angles owing to a significant change in the electronic density within the pores of MIL-100(Fe).X-rays atsmallangles were especially sensitive to the heterogeneity of the electron density of the sample because of the interaction of X-rays with the electron in the atoms of the sample.So,loading of H4SiW12O40would increase the electron density within the pores of MIL-100(Fe)and cause the change in the positions and relative intensities of peaks.Canioni[21]demonstrated a similar result in the characterization of H3PMo12O40@MIL-100(Fe).In Fig.2,no characteristic diffraction peak of H4SiW12O40could be detected,indicating that H4SiW12O40was highly dispersed in the pores of MIL-100(Fe).

Fig.2.XRD patterns of MIL-100(Fe)and H4SiW12O40@MIL-100(Fe).(a)MIL-100(Fe)simulated by crystal structure data;(b)MIL-100(Fe);(c)H4SiW12O40@MIL-100(Fe);(d)H4SiW12O40.
3.2.2.FT-IR analysis
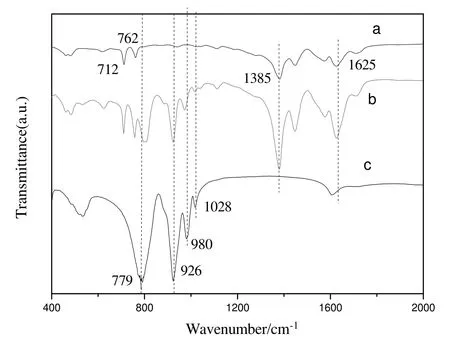
Fig.3.FT-IR spectra of MIL-100(Fe),H4SiW12O40@MIL-100(Fe)and H4SiW12O40.(a)MIL-100(Fe);(b)H4SiW12O40@MIL-100(Fe);(c)H4SiW12O40.
Fig.3 presented the FT-IR spectra of MIL-100(Fe),H4SiW12O40@MIL-100(Fe)and H4SiW12O40.In the FT-IR spectra of MIL-100(Fe),the peaks observed at 1385 cm-1and 1625 cm-1could be assigned to νs(COO-)and νas(COO-)of trimesic acid,and the peaks in 712-762 cm-1region could be assigned to ν(C--C)of benzene.In the FT-IR spectra of H4SiW12O40,the peaks at 980 cm-1,926 cm-1,1028 cm-1and 779 cm-1could be assigned to ν(W=O),ν(Si--O)and ν(W--O--W)[22].The FT-IR spectra of H4SiW12O40@MIL-100(Fe)combined the peaks of MIL-100(Fe)and H4SiW12O40.Therefore,H4SiW12O40molecules were dispersed in the pores of MIL-100(Fe)and the encapsulated H4SiW12O40still maintained its Keggin structure.
3.2.3.Thermal gravimetric analysis
TG curves of MIL-100(Fe)and H4SiW12O40@MIL-100(Fe)were displayed in Fig.4.Three mass losses occurred between 0°C and 600°C for MIL-100(Fe)and H4SiW12O40@MIL-100(Fe).In the first and second stages,their mass losses were similar.The first mass loss stage started from room temperature to 90°C,accounting for about 8%owing to the removal of surface free water;the second stage started from 90 °C to 280 °C,accounting for about 5%because of the removal of the bound water in pores and MOF skeleton.In the third stage,the mass loss of MIL-100(Fe)started from 280 °C to 370 °C,accounting for about 56%owing to the decomposition of trimesitinic acid in the skeleton.The mass would not change after 380°C and finally Fe2O3was obtained.The mass loss of H4SiW12O40@MIL-100(Fe)started from 280°C to 400°C accounting for about 20%,exhibiting a lower loss than MIL-100(Fe)because H4SiW12O40was decomposed into oxides such as WO3.And the weight would not change after 400°C.So,the thermal stability of MIL-100(Fe)would not decrease after H4SiW12O40was encapsulated within the pores of MIL-100(Fe).
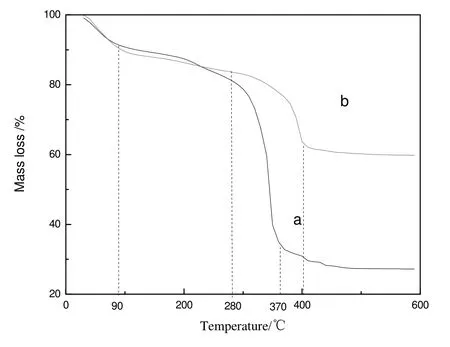
Fig.4.TG curve of MIL-100(Fe)and H4SiW12O40@MIL-100(Fe).(a)MIL-100(Fe);(b)H4SiW12O40@MIL-100(Fe).
3.2.4.Speci fic surface area and pore structure
Table 2 showed the textural properties of MIL-100(Fe)and H4SiW12O40@MIL-100(Fe)with a H4SiW12O40loading of 43.2 wt%.As for MIL-100(Fe),the specific surface area,pore volume,and the average pore size were 1352 m2·g-1,0.125 cm3·g-1and 3.86 nm,respectively.After H4SiW12O40was encapsulated within the pores of MIL-100(Fe),the specific surface area,pore volume and average pore size decreased to 402 m2·g-1,0.096 cm3·g-1and 2.87 nm,respectively,demonstrating further that H4SiW12O40was encapsulated within the pores of MIL-100(Fe).
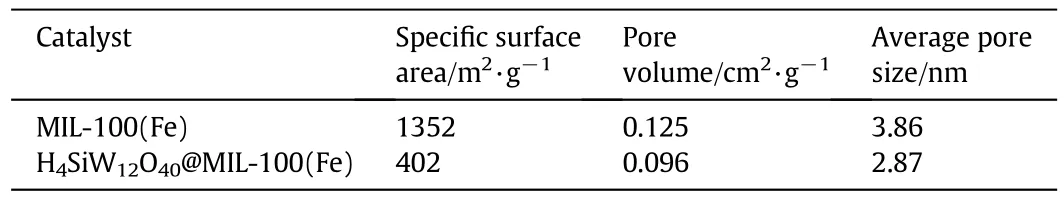
Table 2 Textural properties of H4SiW12O40@MIL-100(Fe)and MIL-100(Fe)
3.3.Catalytic performance of H4SiW12O40@MIL-100(Fe)
3.3.1.Effect of H4SiW12O40 loading
MIL-100(Fe)showed a certain catalytic activity for 4,4′-MDA synthesis because of its Lewis acidity of the available unsaturated metal sites[20];the yield and selectivity of 4,4′-MDA could reach 46.2%and 48.0%,respectively.The catalytic activity of H4SiW12O40@MIL-100(Fe)would be promoted with the increase of the loading of H4SiW12O40.The catalytic activity of H4SiW12O40@MIL-100(Fe)reached the highest when the loading of H4SiW12O40was 43.2%;the yield and selectivity of 4,4′-MDA could reach 66.6%and 69.8%,respectively.When the molar ratio of tungsten to iron(W/Fe)increased to 0.243,the yield and selectivity of 4,4′-MDA decreased to 65.5%and 67.1%,respectively.The specific surface area decreased from 402 m2·g-1to 308 m2·g-1while the W/Fe increased from 0.035 to 0.243,which directly led to a poor dispersion of H4SiW12O40and low active surface area,then affected the yield and selectivity of 4,4′-MDA(Table 3).
3.3.2.Effect of molar ratio of aniline to formaldehyde
To avoid generating polynuclear condensation products and improve the selectivity of desired product,formaldehyde was chosen as the limiting reactant[23].The effect of molar ratio of aniline to formaldehyde was studied and the result was exhibited in Fig.5.With the increase of molar ratio of aniline to formaldehyde,the conversion of aniline decreased while the yield and selectivity of 4,4′-MDA increased first and then decreased.When the molar ratio of aniline to formaldehyde was 5,the yield and selectivity of 4,4′-MDA reached the highest,81.6%and 79.2%,respectively.When the molar ratio was lower than 5,the yield and selectivity of 4,4′-MDA decreased because high concentration of formaldehyde caused deep condensation to polynuclear products.On the contrary,when the molar ratio was higher than 5,the concentration of formaldehyde was so low that the reaction rate decreased.So the suitable molar ratio of aniline to formaldehyde was 5.
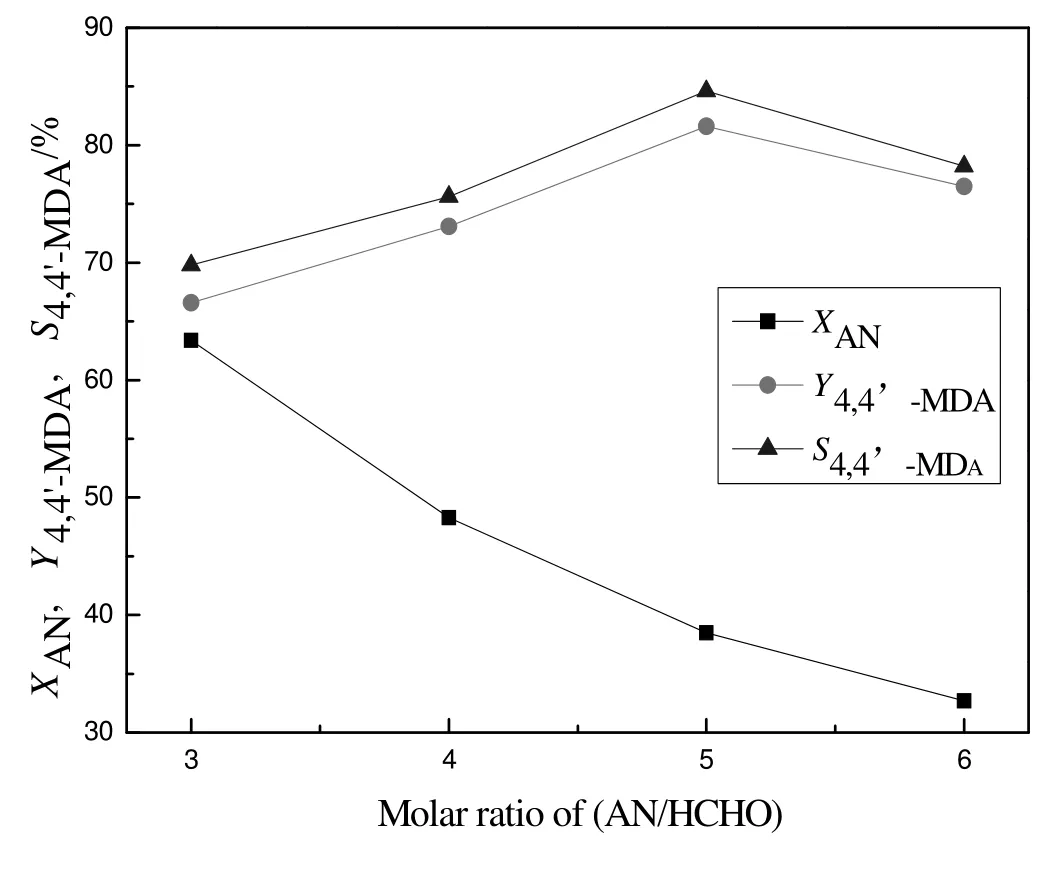
Fig.5.Effect of molar ratio of aniline to formaldehyde for synthesis 4,4′-MDA.Reaction conditions:a mass ratio of catalyst to formaldehyde=1.2,a reaction temperature of 120°C and a reaction time of 6 h.X:conversion;Y:yield;S:selectivity;AN:aniline.
3.3.3.Effect of reaction time
The influence of reaction time on the synthesis of 4,4′-MDA was displayed in Fig.6.With the extension of reaction time,the conversion of aniline remained stable while the yield and selectivity of4,4′-MDA increased first and then unchanged.When reaction time was 6 h,the yield and selectivity of 4,4′-MDA reached the highest,81.6%and 79.2%,respectively.When the reaction time was less than 6 h,the yield and selectivity of 4,4′-MDA decreased because insufficient of reaction time would confine the conversion of intermediate,aminal.On the contrary,when the reaction time was longer than 6 h,the yield and selectivity of 4,4′-MDA would also decrease because long reaction time resulted in deep condensation of 4,4′-MDA to polynuclear products.So the appropriate reaction time was 6 h.
3.3.4.Effect of catalyst dosage
The effect of catalyst dosage on the synthesis of 4,4′-MDA was studied and the result was shown in Fig.7.With the increase of catalyst dosage,the yield and selectivity of 4,4′-MDA increased first and then unchanged.When the mass ratio of catalyst to formaldehyde was less than 1.2,the yield and selectivity of 4,4′-MDA decreased because of the insufficient number of catalytic active center.When the mass ratio of catalyst to formaldehyde was more than 1.2,the yield and selectivity of 4,4′-MDA would remain unchanged.So the appropriate mass ratio of catalyst to formaldehyde was 1.2.
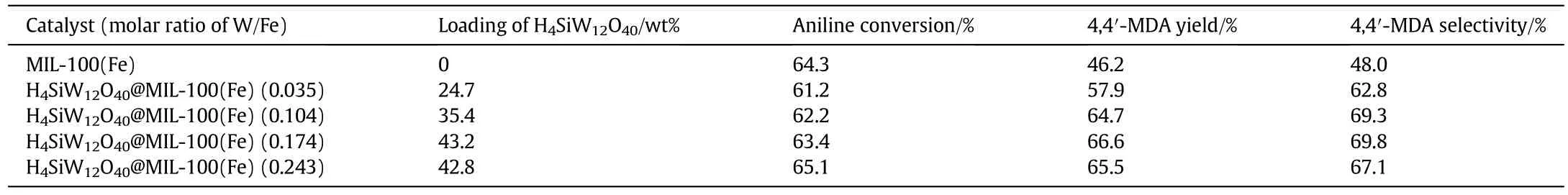
Table 3 Effect of H4SiW12O40 loading on the catalytic performance of H4SiW12O40@MIL-100(Fe)
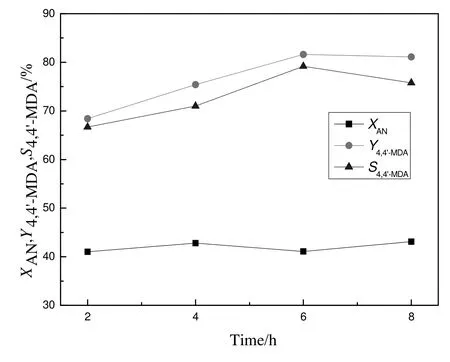
Fig.6.Effect of reaction time for synthesis of4,4′-MDA.Reaction conditions:a mass ratio of catalyst to formaldehyde=1.2,a mole ratio of aniline to formaldehyde=5 and a reaction temperature of 120°C.X:conversion;Y:yield;S:selectivity;AN:aniline.
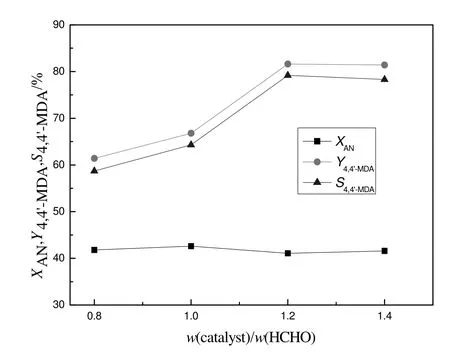
Fig.7.Effect of cataly stdosage for synthesis of 4,4′-MDA.Reaction conditions:a mole ratio of aniline to formaldehyde=5,a reaction temperature of120°C and a reaction time was 6 h.X:conversion;Y:yield;S:selectivity;AN:aniline.
3.3.5.Effect of reaction temperature
Fig.8 showed the effect of reaction temperature on the synthesis of 4,4′-MDA.With the increase of reaction temperature,the conversion of aniline remained unchanged while the yield and selectivity of4,4′-MDA decreased first and then increased.When the temperature increased to 120 °C,the yield and selectivity of 4,4′-MDA reached the highest,81.6%and 79.2%,respectively.At a lower temperature,animal was not rapidly converted to the desired product because the reaction rate was slow.However,at a much higher temperature,the yield and selectivity of 4,4′-MDA decreased because polynuclear condensation products were easily generated.So the appropriate reaction temperature was 120°C.
3.3.6.Reusability of catalyst
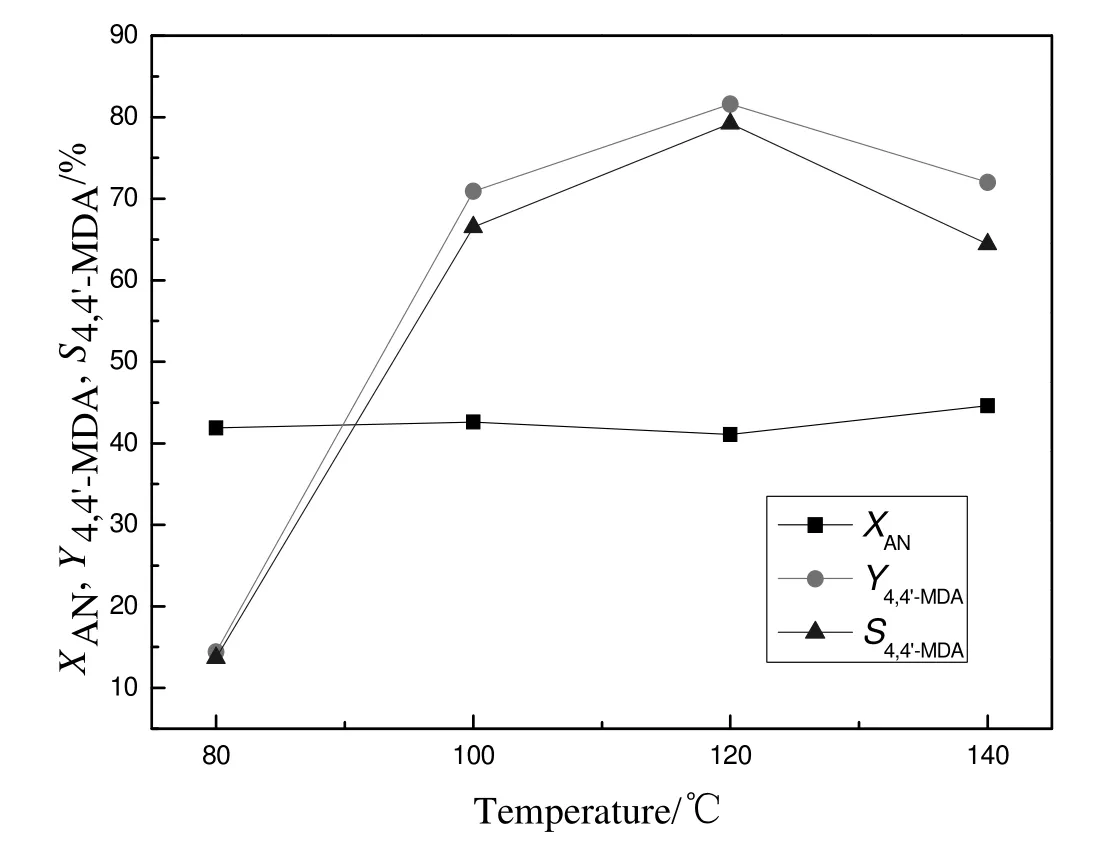
Fig.8.Effect of reaction temperature for synthesis of 4,4′-MDA.Reaction conditions:a mass ratio of catalyst to formaldehyde=1.2,a mole ratio of aniline to formaldehyde=5 and a reaction time of 6 h.X:conversion;Y:yield;S:selectivity;AN:aniline.
The biggest problem about the reusability of supported heteropolyacid catalyst was the dissolution of heteropolyacid.Fortunately,this problem could be efficiently solved by encapsulating heteropolyacid within the pores of MIL-100(Fe)[24].H4SiW12O40@MIL-100(Fe)was recovered after the completion of reaction,washed three times with methanol,dried in vacuum at 80°C for 8 h and then reused in the reaction for the synthesis of 4,4′-MDA.The result was shown in Table 4.Compared with the fresh H4SiW12O40@MIL-100(Fe),the catalytic activity of the recovered one decreased severely and the yield of 4,4′-MDA decreased about 26%when the catalyst was used in the second cycle.The dissolution of H4SiW12O40was not the reason for catalyst deactivation because the catalyst had been washed with water,DMF and ethanol during the preparation process.Based on the study on the causes for Cs2.5H1.5SiW12O40deactivation[25],it was speculated that the change of the catalyst acidity and pore structure caused the deactivation ofthe recovered H4SiW12O40@MIL-100(Fe).The recovered H4SiW12O40@MIL-100(Fe)adsorbed some alkaline organic compounds and these organic compounds could not be removed entirely by washing with methanol,causing the change of the catalyst acidity and pore structure.In order to further remove these alkaline organic compounds,the recovered H4SiW12O40@MIL-100(Fe)was washed twice with DMF after it was washed three times with methanol.After that,the color of DMF solution changed to dark red,illustrating that H4SiW12O40@MIL-100(Fe)washed with methanol still contained organics.H4SiW12O40@MIL-100(Fe)could be reused after it was washed three times with methanol,twice with DMF,then three times with methanol and finally dried.As a result,the catalytic performance of the recovered H4SiW12O40@MIL-100(Fe)remained essentially unchanged after H4SiW12O40@MIL-100(Fe)was reused for three runs.

Table 4 Reusability of H4SiW12O40@MIL-100(Fe)
In order to further explore the deactivation and activity recovery of H4SiW12O40@MIL-100(Fe),IR spectra of H4SiW12O40@MIL-100(Fe)washed with only methanol and with both methanol and DMF were compared and the results are showed in Fig.9.The infrared peaks at 980 cm-1,926 cm-1,1028 cm-1and 779 cm-1could be assigned to ν(W=O),ν(Si--O)and ν(W--O--W),illustrating that the recovered catalyst still contained H4SiW12O40.By comparing the infrared spectra of the fresh catalyst with the recovered one washed with methanol,the peaks of the recovered catalyst at 1190 cm-1and 1510 cm-1could be assigned to ν(C--N)and benzene,suggesting that the recovered H4SiW12O40@MIL-100(Fe)washed with methanol still absorbed alkaline organics such as aromatic amines.To further confirm the existence of the aromatic amines,the fresh and the recovered DMF which had been used for washing H4SiW12O40@MIL-100(Fe)were analyzed by HPLC and the result showed that there were aniline and 4,4′-MDA in the recovered DMF.Therefore,it was confirmed that the recovered H4SiW12O40@MIL-100(Fe)washed with methanol still absorbed aromatic amines,causing a decrease of catalytic activity.After H4SiW12O40@MIL-100(Fe)was washed with methanol and DMF,the FT-IR peaks vanished at 1190 cm-1and 1510 cm-1,illustrating that DMF could clean up alkaline organics such as aniline and 4,4′-MDA.Furthermore,the H4SiW12O40@MIL-100(Fe)was reused for three runs and its catalytic activity remained unchanged,suggesting that the catalytic activity was completely recovered.
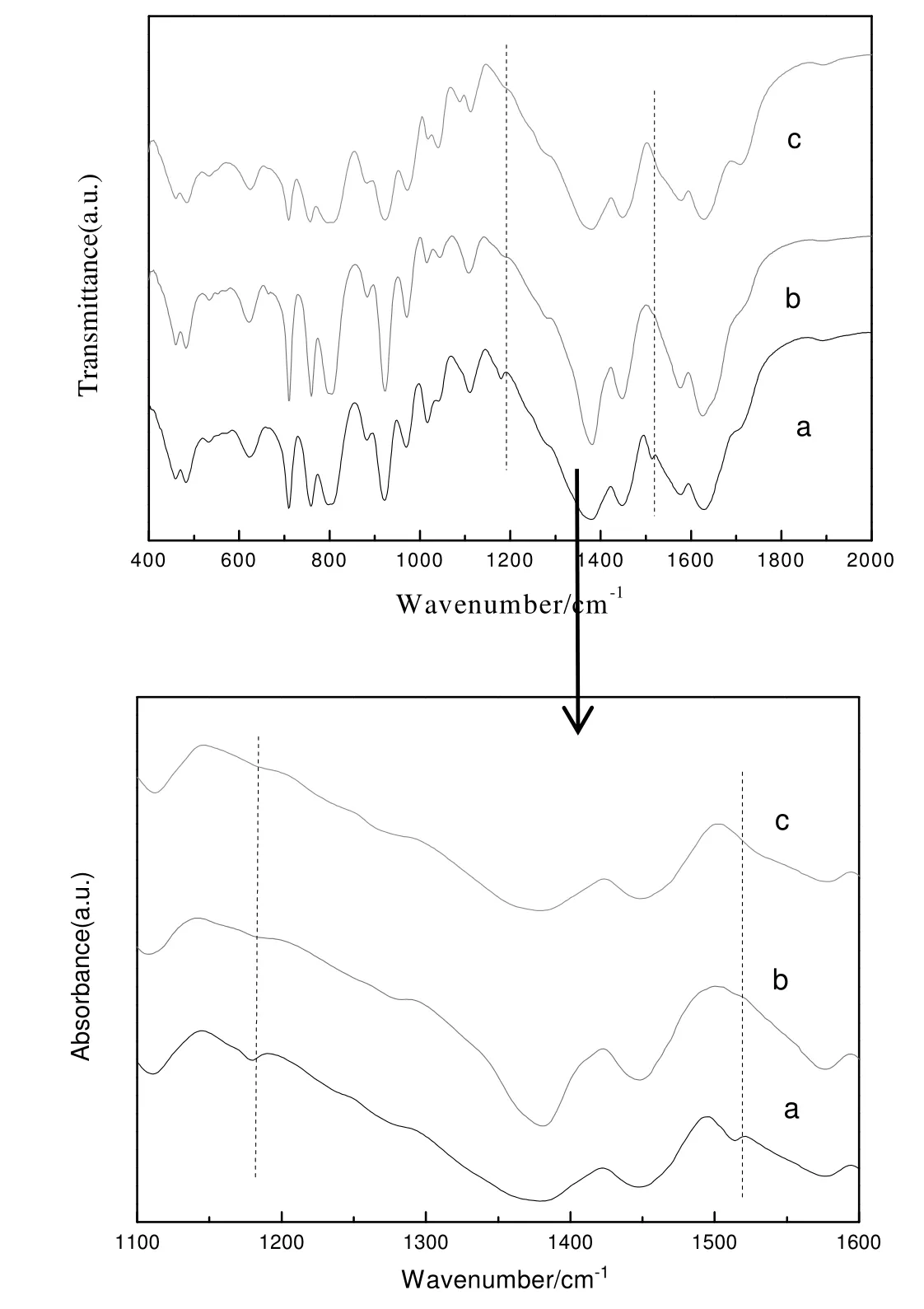
Fig.9.FT-IR spectra of H4SiW12O40@MIL-100(Fe).(a)The recovered H4SiW12O40@MIL-100(Fe)washed with methanol.(b)The recovered H4SiW12O40@MIL-100(Fe)washed with methanol and DMF.(c)The fresh H4SiW12O40@MIL-100(Fe).
4.Conclusions
(1)The catalytic performance of the three common heteropolyacids(H3PW12O40,H4SiW12O40and H3PMo12O40)for 4,4′-MDA synthesis from aniline and formaldehyde was investigated.The result showed that H4SiW12O40with moderate acid strength exhibited the best catalytic performance.
(2)H4SiW12O40@MIL-100(Fe)was prepared and characterized by means of FT-IR,N2adsorption-desorption and XRD.The H4SiW12O40@MIL-100(Fe)showed excellent catalytic performance for 4,4′-MDA synthesis;the conversion of aniline was 41.1%,the yield and selectivity of 4,4′-MDA were 81.6%and 79.2%respectively under the conditions ofa molarratio ofaniline to formaldehyde=5,a mass ratio of catalyst to formaldehyde=1.2,a reaction temperature of 120°C and a reaction time of 6 h.
(3)The change of the catalyst acidity and pore structure caused the catalytic activity decrease of the recovered H4SiW12O40@MIL-100(Fe)because the H4SiW12O40@MIL-100(Fe)adsorbed alkaline organic compounds.The adsorbed alkaline organics could be removed by washing with methanol followed by washing with DMF.The catalytic activity of the H4SiW12O40@MIL-100(Fe)was restored and no appreciable loss in the catalytic activity was observed after the H4SiW12O40@MIL-100(Fe)was reused for three runs.
[1]Y.F.Liu,C.Y.Liao,Z.Z.Hao,The polymerization behavior and thermal properties of benzoxazine based on o-allylphenol and 4,4′-diaminodiphenyl methane,React.Funct.Polym.75(2014)9-15.
[2]The Upjohn Company,Process for preparing methylene-di-anilines,U.S.Pat.,3857890(1974).
[3]R.S.Varma,Solvent-free organic syntheses using supported reagents and microwave irradiation,Green Chem.1(1999)43.
[4]Texaco Inc.,Tungsten catalyzed aniline-formaldehyde condensation,U.S.Pat.,4284815(1981).
[5]The Dow Chemical Company,Process for preparing polyamines with ion exchange resin catalysts,U.S.Pat.,4554378(1985).
[6]T.Kugita,S.Hirose,S.Namba,Catalytic activity of zeolites for synthesis reaction of methylenedianiline from aniline and formaldehyde,Catal.Today 111(2006)275-279.
[7]Chen LJ and Jimenez J,Process for making polyaminopolyphenyl methanes using a mixed solid acid catalyst system,U.S.Pat.,20110021741A1(2011).
[8]M.Salzinger,B.Fichtl,J.A.Lercher,On the influence of pore geometry and acidity on the activity of parent and modified zeolites in the synthesis of methylenedianiline,Appl.Catal.A Gen.393(1)(2011)189-194.
[9]Nafziger JL,Rader LA and Seward Jr I J,Process for preparing polyamines with ion exchange resin catalysts,U.S.Pat.,4554378(1985).
[10]D.Bahulayan,R.Sukumar,K.R.Sabu,M.Lalithambika,An easy synthesis of 4,4′-diaminodiphenyl methanes on natural kaolinites,Green Chem.1(1999)191-193.
[11]J.P.Tian,H.L.An,X.M.Cheng,X.Q.Zhao,Y.J.Wang,Synthesis of 4,4′-methylenedianiline catalyzed by SO3H-functionalized ionic liquids,Ind.Eng.Chem.Res.54(53)(2015)7571-7579.
[12]L.Y.Wen,E.Z.Min,The research progress of solid heteropoly acid catalyst,Petrochem.Technol.1(29)(2000)49-54.
[13]R.Tesser,M.D.Serio,M.Ambrosio,M.Santacesaria,Heterogeneous catalysts for the production of anthraquinone from 2-benzoylbenzoic acid,Chem.Eng.J.90(2002)195-201.
[14]M.M.Heravi,K.Bakhtiari,F.F.Bamoharram,12-Molybdophosphoric acid:A recyclable catalyst for the sythesis of biginelli-type 3,4-dihydropyrimidine-2(1H)-ones,Catal.Commun.7(6)(2006)373-376.
[15]X.Zeng,Q.L.Deng,X.Yuan,J.Wu,H.A.Luo,Catalytic activity of heteropoly acids(salts)for ammoximation of cyclohexanone,Acta Pet.Sin.(Pet.Process.Sect.)26(5)(2010)779-784.
[16]T.K.Huang,L.Shi,R.Wang,X.Z.Guo,X.X.Lu,Keggin type heteropolyacids catalyzed synthesis of quinoxaline derivatives in water,Chin.Chem.Lett.20(2)(2009)161-164.
[17]X.L.Yang,H.B.Li,Y.X.Miao,Preparation,characterization and catalytic performance of novel HPWs@MIL-101 catalyst,Chem.Res.Appl.27(2015)642-648.
[18]F.M.Zhang,Y.Jin,J.Shi,Y.J.Zhong,W.D.Zhu,M.Samy EI-Shall,Polyoxometalates confined in the mesoporous cages of metal-organic framework MIL-100(Fe):Efficient heterogeneous catalysts for esteri fication and acetalization reactions,Chem.Eng.J.269(2015)236-244.
[19]A.Corma,P.Botella,C.Mitchell,Replacing HCl by solid acids in industrial processes:Synthesis of diamino diphenyl methane(DADPM)for producing polyurethanes,Chem.Commun.17(2004)2008-2010.
[20]F.M.Zhang,Y.Jin,J.Shi,Y.H.Fu,Y.J.Zhong,W.D.Zhu,Facile synthesis of MIL-100(Fe)under HF-free conditions and its application in the acetalization of aldehydes with diols,Chem.Eng.J.259(2015)183-190.
[21]C.Romain,R.M.Catherine,S.Francis,H.Patricia,S.Christian,H.D.Menaschi,et al.,Stable polyoxometalate insertion within the mesoporous metal organic framework MIL-100(Fe),J.Mater.Chem.21(2010)1226-1233.
[22]Yu-ki Miura,Hiroyuki Imai,Toshiyuki Yokoi,T.Tatsumi,Y.Kamiya,Microporous cesium salts of tetravalent Keggin-type polyoxotungstates Cs4[SiW12O40],Cs4[PW11O39(Sn-n-C4H9)],and Cs4[PW11O39(Sn-OH)]and their adsorption properties,Microporous Mesoporous Mater.174(2013)34-43.
[23]C.Y.Wang,H.Q.Li,Y.Cao,H.T.Liu,X.J.Hou,Y.Zhang,Thermodynamic analysis on synthesis of 4,4-methylenedianiline by reaction of aniline and formaldehyde,CIESC J.63(8)(2012)2348-2355.
[24]J.Q.Yan,Y.Tan,Y.F.Li,Z.M.Wu,M.Chen,L.S.Pan,Y.J.Liu,Synthesis of bisphenol F catalyzed by phosphotungstic acid encapsulated in metal-organic frameworks MIL-100(Cr)and MIL-101(Cr),CIESC J.66(2015)576-582.
[25]X.M.Cheng,H.L.An,X.Q.Zhao,Y.J.Wang,In fluences of treatment with ethanol on structure and performances of Cs2.5H1.5SiW12O40catalyst for synthesis of 4,4′-diaminodiphenylmethanes,Petrochem.Technol.45(3)(2016)285-290.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Transport hindrances with electrodialytic recovery of citric acid from solution of strong electrolytes
- Experimental investigation on CO2-light crude oil interfacial and swelling behavior
- Biosynthesis of 4-hydroxyphenylpyruvic acid from L-tyrosine using recombinant Escherichia coli cells expressing membrane bound L-amino acid deaminase☆
- Process development for producing a food-grade glucose solution from rice straws
- Carbon dioxide induced degradation of diethanolamine during absorption and desorption processes
- Biodegradation of natural and synthetic estrogens in moving bed bioreactor
