Ni nanoparticles supported on carbon as efficient catalysts for steam reforming of toluene(model tar)☆
Chun Shen ,Wuqing Zhou ,Hao Yu ,Le Du *
1 Beijing Key Laboratory of Bioprocess,College of Life Science and Technology,Beijing University of Chemical Technology,Beijing 100029,China
2 The State Key Laboratory of Chemical Resource Engineering,Beijing Key Laboratory of Membrane Science and Technology,Beijing University of Chemical Technology,Beijing 100029,China
1.Introduction
Compared with fossil fuels,biomass exhibits significant advantages:abundance,inexhaustibility,renewability,carbon-neutrality,and low sulfur content as well.Therefore,biomass utilization has received increasing attention[1,2].Gasification for producing syngas is one of the most important technologies for biomass utilization[3-5].However,tar impurity,which would contaminate the downstream equipment,block system pipeline,and pollute the environment,is usually generated during the gasification process[6-9].Therefore,tar removing has become one of the major concerns in recent years.
Catalytic reforming is considered as the most potential method for removing tar[10-14].Nickel nanoparticles have been widely used as the active componentfor the removaland transformation of tar because of their high activity and low cost[15-20].Compared with homogeneous catalysts,Ni-based catalysts in the immobilization mode are more popular because of the good stability,easy separation and feasibility for continuous operation.Gong and coworkers[21-25]have devoted great efforts in catalytic reforming of oxygenates using Ni-based heterogeneous catalysts,providing deep insight into the structure-activity relationship and strategies for improving the catalytic performance.It is well demonstrated that decreasing the mean particle size of Ni nanoparticles and increasing the surface oxygen mobility would be bene fit for improvement of catalytic activity and stability.
Because of the high specific surface area,developed pore structure and low cost,activated carbon(AC)are extensively explored as the supporting material for metal nanoparticles[26-32].However,control over the mean diameter of Ni nanoparticles supported on carbon material remains a serious challenge until today.Hou et al.[30]prepared Nicarbon nanotubes through homogeneous deposition-precipitation method.The average particle sizes of nickel nanoparticles are 6.1,11.2 and 19.4 nm,respectively for the catalyst with the Ni loading amount of 5,15 and 35 wt%.In the work of Rashidi[31],the mean particle size of Ni nanoparticles(loading amount is 5 wt%)is 11.3 nm.It is reported that the particle size of Ni nanoparticles has greateffectboth on the catalytic activity and stability[33,34].Therefore,how to prepare Ni nanoparticles supported on AC with small particle size and narrow distribution has become a hot topic in this field.
It has been well established thatthe nature of nickelprecursors used during the catalyst preparation process and surface property of the supporting material are important parameters affecting the catalytic performance[1,35].However,there have been few studies discussing in fluences of precursors and surface properties of carbon material on nanoparticle morphology and catalytic activity until now.
Herein,in this work,we attempted to investigate effects of surface properties of the support and nickel precursors on morphology of Ni nanoparticles,and their catalytic performances for steam reforming of toluene.A series of Ni-AC catalysts were prepared using nickel nitrate,nickelchloride,and nickelacetate as the precursors,which were characterized by Fourier-transform infrared(FTIR),transmission electron microscopy(TEM),X-ray diffraction(XRD),Brunauer-Emmett-Teller(BET),and temperature programmed reduction(TPR)under hydrogen flow.The catalytic activities for steam reforming of toluene were investigated as well as the changes in morphologies of Ni nanoparticles.Effects of residence time,S/C ratio(molar ratio of steam to carbon in toluene),and nickelloading amounton conversion have been systematically investigated.The stability test of the catalyst was carried out as well.
2.Experimental
2.1.Materials and chemicals
2.2.Preparation and characteristic of AC supported with Ni nanoparticles
Charcoal active granular pretreated with concentrated HNO3was selected as the support.The char particles were sieved before pretreatment,and char particles with the size ranged from 590 to 840 μm were treated with concentrated HNO3for 2 h at room temperature.Then they were washed with deionized water for several times to remove the remaining nitric acid and dried at 378 K overnight before use.
A series of Ni-AC is synthesized using three different precursors,nickel nitrate,nickel chloride and nickel acetate by impregnation method and they are labeled as Ni-AC(N),Ni-AC(C)and Ni-AC(AC),respectively.A certain amount of precursors was dissolved in deionized water,followed by addition of the acid-treated char particles.After 5 h mixing at room temperature,the mixture was heated to evaporate the solution.The obtained solid was dried at 373 K for 12 h,and reduced by H2at 923 K.
The prepared catalysts were characterized by XRD on a Rigaku D/max-2500 diffractometer,using the CuKαradiation (λ =0.15406 nm)over the 2θ range from 10°to 90°.The micro structure of the catalyst was examined with transmission electron microscopy(TEM,JEOL JEM-2011,JEOL Ltd.,Japan).Analysis of BET surface area and pore diameter and volume was done using a surface area analyzer(Quanta chrome model 1-C,Boynton Beach,FL).The Fourier transformed infrared spectrums in the range 4000-400 cm-1were recorded using a Thermo Nicolet FTIR spectrophotometer.H2temperature programmed reduction(TPR)measurements for fresh catalysts were performed on a Micromeritics Autochem II equipped with a thermal conductivity detector(TCD).The temperature increased to 1050 K with a speed of 10 K·min-1.
2.3.Steam reforming of toluene
Catalytic tests were carried out in a continuous flow system,using a quartz fixed-bed reactor under atmospheric pressure.The internal diameter and length of the reactor were 8 and 550 mm,respectively.The temperature of the bed was monitored using a K-type thermocouple.0.2 g of catalyst was used in each test and held by the quartz wool placed in the middle of the reactor.Toluene and water were fed into the reactor using micro-injection pumps(Model:TS2-60,Longer Precision Pump)at a specific S/C ratio.They were firstly vaporized and then mixed with N2with the flow rate of 30 ml·min-1before entering the catalyst bed.The reaction products flowed through a cold trap maintaining 268 K to condense the unreacted toluene and water.Liquid product was analyzed using a gas chromatograph(GC)equipped with a flame ionization detector(FID)with a Porapak-Q column and the gas product was analyzed using a gas chromatograph(HP 6890)equipped with a thermal conductivity detector.The total flow rate of the produced gases was measured by a wet gas flow meter.Conversion of toluene was defined as Eq.(1),whereandwere molar flow rates of toluene at the inlet and outlet,respectively.Gas product composition was calculated using Eq.(2).

3.Results and Discussion
3.1.Characteristics of AC supported with Ni nanoparticles
In order to obtain Ni nanoparticles highly dispersed on AC,two groups of experiments were carried out.
In the first group of experiment,effect of the surface property was investigated,and AC before and after the treatment with nitric acid were used as the support for Ni nanoparticles(the loading amount of Ni was kept as 10 wt%).Because of the hydrophobic nature of carbon materials and their poor wettability in aqueous solutions,the adsorption and transportation of metal ions along the channel of carbon material are difficult[36-41].As a result,the target metal ions which are just adsorbed on the outer surface of carbon material would be impregnated onto carbon support via drying and reduction steps,resulting in poor dispersion and severe aggregation of metal species.In order to get the carbon material hydrophilic,pretreatment with concentrated HNO3(16 mol·L-1)was carried out.The acid treatment would increase the amount of oxygen-containing functional groups on the surface of the carbon material(such as C=O,COOH and OH)[36,38],and it has also been proved by FTIR characterization.As shown in Fig.1,peaks at 1711 cm-1and 1529 cm-1assigned to the C=O stretching vibration and lactone structure,respectively were detected in AC support treated with nitric acid.The presence of the functional groups could help to get the metal ions adsorbed on the surface more homogeneously by making the support more hydrophilic[26].TEM images of Ni nanoparticles supported on AC before and after the treatment are shown in Fig.2(a)and(b),respectively.And the corresponding histograms ofparticle size distribution are given in Fig.2(c)and(d),respectively.The average Niparticle size was quantified based on a number-weighted diameteris the number of counted Ni particles with a diameter of di).As expected,Ni nanoparticles supported on raw AC exhibited poor dispersion and severe aggregation of metal species.While Ni nanoparticles showed much smaller mean particle size and narrow distribution if the support was treated with concentrated nitric acid.

Fig.1.FTIR spectra of raw and nitric acid treated AC.
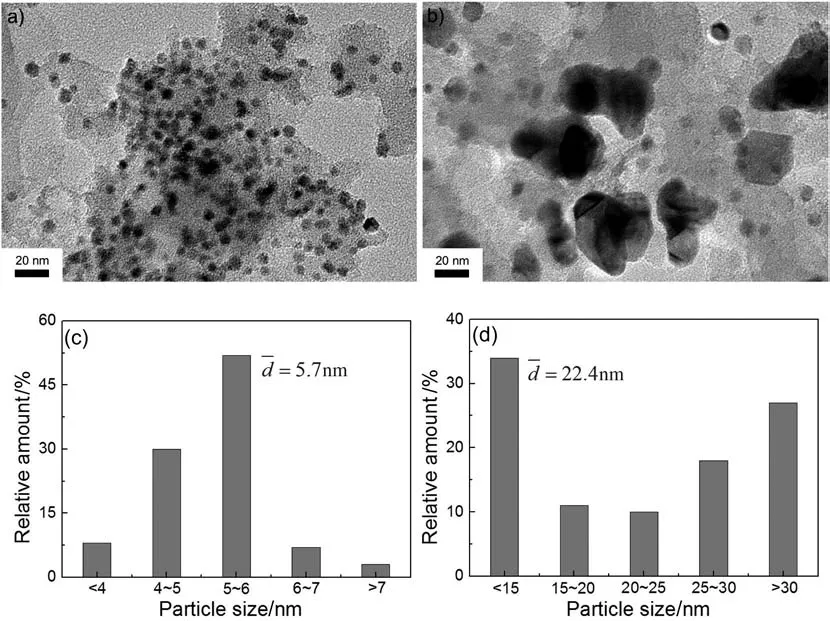
Fig.2.(a)and(b):TEM images of Ni nanoparticles supported on raw AC and acid treated AC,respectively;(c)and(d):histograms of particle size distribution for Ni nanoparticles supported on raw AC and acid treated AC,respectively.(Ni loading amount:10 wt%).
In the second group of experiment,the effect of nickel precursors was investigated.TEM images of immobilized Ni nanoparticles synthesized with different precursors(the loading amount of Ni was kept as 6 wt%)are presented in Fig.3(a)to(c),and the corresponding particle size distributions are shown as Fig.3(d)to(f),respectively.As for the Ni-AC(N)catalyst,Ni nanoparticles were uniformly dispersed with a narrow size distribution.While Ni-AC(AC)and Ni-AC(C)catalysts showed much larger mean particle sizes of Ni nanoparticles.The mean diameter of Ni nanoparticles followed the order Ni-AC(N)(4.5 nm)<Ni-AC(AC)(36.5 nm)<Ni-AC(C)(41.5 nm).When nickel acetate was used,CH4was formed during the thermal decomposition.As reported by Helveg[42],CH4decomposed over Ni nanocrystals forming carbon nano fibers.It suggested that Ni nanoparticles would sinter due to the cracking ofCH4.This explained the largermean diameterofNinanoparticles using nickel acetate as the precursor.Similar results were also reported by Sietsma etal.and Ren etal.[43-45].When nickelchloride was used,hydrogen chloride wasone ofthe productsduring the reduction of Ni2+ions;furthermore,nickel chloride was appreciable volatile in the presence of hydrogen and hydrogen chloride[46].As the reaction proceeded,nickel chloride crystals vaporized and tended to deposit on the formed Ni nanocrystals.It promoted the growth of Ni nanoparticles and resulted in the largest mean particle size as shown in Fig.3c.Therefore,nickel nitrate was chosen as the optimized precursor for the preparation of catalysts.
A series of Ni-AC(N)catalysts with different nickel loading amounts(varied from 6 wt%to 18 wt%)were synthesized and their XRD patterns are presented in Fig.4.The sharp and symmetric peaks are evidently observed,indicating the good crystallization of Ni nanoparticles.Peaks at 44.6°,51.3°,and 76.4°could be assigned to diffractions of the(111),(200),and(220)planes of metallic Ni,respectively.
腕表搭载Cal.3638自动上链机心,机心由镶有39枚红宝石轴承的464个部件组成,动力储存可达7天。12点钟位置的小表盘显示时辰数字及字符(24小时一周期);3点钟位置显示五行元素和十天干(10年一周期);9点钟位置显示农历月(12个月一周期)、农历日(30天一周期)及闰月;12点钟位置的年份视窗显示当年所属生肖(12年一周期),月相盈亏显示则位于表盘6点钟方位。2018为中国狗年,一只生肖狗昂首立于12点钟位置,寓意2018吉犬“旺”年,福禄呈祥。
TEM images of supported Ni nanoparticles with different nickel loading amounts are shown in Fig.5(a)to(c).The corresponding particle size distributions are shown in Fig.5(d)to(f).Ni nanoparticles are homogeneously immobilized on the surface of carbon.With the loading amount increased from 9 wt%to 18 wt%,the mean particle size of Ni nanoparticles increased steadily from 5.1 nm to 9.8 nm.
H2-TPR profiles of the as-prepared catalyst are shown in Fig.6.Two reduction peaks are distinguished,the one attemperature~586 K could be assigned to the reduction of NiO species,and the other at higher temperature~821 K corresponded to the reduction of larger NiO nanoparticles or nickel oxides with interaction with AC.
3.2.Effect of residence time on conversion
After the adjustment in the loading amount and particle size of supported Ni catalysts,their catalytic performances were further explored for steam reforming of toluene.The effects of several important parameters were investigated.
Residence time of the reactants in the catalyst bed directly affects the conversion of toluene.Longer residence time means longer reaction duration,as a result,the conversion is higher.Fig.7 illustrates the effect of residence time of the conversion of toluene with catalysts prepared with different Ni precursors.As expected,for all three catalysts,the toluene conversion increased by extending the residence time.And catalysts prepared based on different nickel precursors presented different catalytic performances.The conversion of toluene follows the order Ni-AC(N)<Ni-AC(AC)<Ni-AC(C),which matches with the order of the nickel particle sizes.As for Ni-AC(N),with the residence time increased from 0.15 s to 0.31 s,a sharp increase from 39.5%to 95.1%of toluene conversion was observed.The highest conversion of 97.4%was reached when the residence time was 0.48 s.

Fig.3.(a),(b)and(c):TEM images of Ni/ACcatalysts synthesized with nickelnitrate,nickelacetate and nickelchloride,respectively;(d),(e)and(f):histograms ofparticle size distribution prepared with nickel nitrate,nickel acetate and nickel chloride respectively.(Ni loading amount was 6 wt%).

Fig.4.XRD spectra of catalysts with different Ni loading amounts.
3.3.Effect of S/C ratio on conversion
Major reactions occur during toluene steam reforming are shown in Eqs.(3)to(9).As shown in Eqs.(3)and(4),water is one of the reactants,the steam reforming would be enhanced and CO2instead of CO would be the favorable product by increasing water content in the feed.It indicates that the S/C ratio plays an important role in determining the conversion of toluene.Conversions of toluene increased steadily from 70.1%to 93.3%with the S/C ratio increased from 1 to 4(Fig.8).Besides,the water content in the feed also has a major impact on the carbon formations.In the water-less environment,carbon and hydrogen would be produced according to Eq.(9).Therefore,layers of carbon would be accumulated in a relatively short time leading to catalyst deactivation.While in a water-rich environment,Eqs.(3)and(4)are favored and the carbon accumulating rate on the Ni surface is slow;at the same time,water may clean part of the formed carbon on the surface of catalysts by oxidizing it to CO or CO2[47].

Fig.5.(a)(b)and(c):TEM images of Ni nanoparticles supported on AC with Ni loading amount of 9 wt%,15 wt%and 18 wt%,respectively;(d),(e)and(f):histograms of particle size distribution for Ni content of 9 wt%,15 wt%and 18 wt%,respectively.

Fig.6.H2-TPR profile of 12 wt%Ni-AC(N)catalyst.
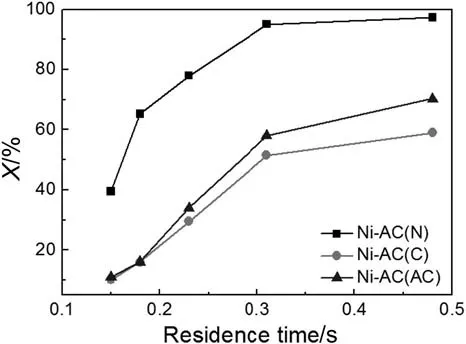
Fig.7.Effect of residence time on the conversion of toluene(reaction temperature:973 K;Ni content:12 wt%;S/C:1.3;toluene flow speed:1-5 ml·h-1).

Fig.8.Effect of S/C ratio on conversion(reaction temperature:973 K;Ni content:12 wt%;S/C:1-4;toluene flow speed:3 ml·h-1).
Steam reforming:

3.4.Effect of nickel loading amount on conversion
The effect of Ni loading amount was determined in relation to the changes in toluene conversion,as shown in Fig.9.The Ni contentin catalyst shows much profounder effect on conversions.With the increase in Ni loading amount,the toluene conversion first increased and then decreased.The catalyst with the Ni content of 12 wt%exhibited the best catalytic performance:the conversion of toluene reached 98.1%under the experimental conditions(reaction temperature:973 K;S/C:2;toluene flow speed:2 ml·h-1).When the Ni amount was not bigger than 12 wt%,the reaction rate was elevated by increasing Ni amount.The conversion was promoted from 60.8%to 98.1%when the Ni content increased from 6 wt%to 12 wt%.As shown in Figs.2 and 5,when the Ni content increased from 6 wt%to 10 wt%,the change in mean particle size of Ni is minor,while the amount of active centers increased.This may explain the promotion in toluene conversion when the Ni content varied from 6 wt%to 9 wt%.However,further increase in the Ni amount reduced the conversion.Catalysts with the highest Ni content of 18 wt%showed even worse performance than that of the catalysts with lower Ni content.The toluene conversion was reduced from 98.1%to 65.0%when the Ni content increased from 12 wt%to 18 wt%.This may be resulted from the increased Ni particle size as shown in Fig.5.

Fig.9.Effect of nickel loading amount on conversion(reaction temperature:973 K;S/C:2;toluene flow speed:2 ml·h-1).
3.5.Stability of the Ni–AC(N)catalyst
The stability of the 12 wt%Ni-AC(N)catalyst during the steam reforming of toluene was tested and the results are shown in Fig.10.A slight decrease in the conversion of toluene(from 98.1%to 92.0%)was observed during the first 200 min.Then in the subsequent 200-700 min,the toluene conversion almost remained at 92.0%and no obvious changes of the gas composition were observed.The catalyst stability for steam reforming of toluene also has been investigated in other studies.Sekine[48]tested the stability of Ni-La1-xSrxAlO3-δ,and the conversion of toluene decreased from 60%to 40%after 180 min.Kong et al.[49]tested the stability ofNi-MgO,the toluene conversion decreased from 70%to 30%after 400 min.As reported by Zhang et al.[50],the toluene conversion decreased from 50%to 25%after 200 min over Ni-Ce-Mg-olivine catalyst.Hence,the as-prepared catalyst in our work showed a high catalytic activity and a good stability for toluene steam reforming.

Fig.10.Stability test of toluene steam reforming on Ni-AC(N)catalyst(reaction temperature:973 K;Ni content:12 wt%;S/C:2;toluene flow speed:2 ml·h-1).
Steam reforming of toluene has been conducted in other studies[8,10,15,51,52].The comparisons of catalytic performances are listed in Table 1.Hence,the as-prepared catalyst in our work showed a high catalytic activity and a good stability for toluene steam reforming.The excellent catalytic performance of Ni nanoparticles supported on AC was mainly contributed to the small particle size of Ni nanoparticles.
4.Conclusions
In this work,the mean particle size of immobilized Ni nanoparticles was adjusted by modi fications in surface properties of the support and nickel precursors.The acid treatment with the supporthelped to reduce the mean particle size of Ni nanoparticles from 22.4 nm to 5.7 nm by better wettability in aqueous solutions.The mean particle size of Ni nanoparticles was also affected by thermal decomposition products of nickel precursors.Ni nanoparticles prepared using nickel nitrate as the precursor showed the smallest mean particle size and the highest catalytic activity for toluene steam reforming.The mean diameter varied from 4.5 nm to 9.1 nm with the loading amount increased from 6 wt%to 18 wt%.The effects of residence time,S/C ratio and nickel loading amount on conversions were systematically investigated.The highest toluene conversion of 98.1%was obtained when the S/C ratio was 2,the Ni loading amount was 12 wt%and the toluene flow speed was 2 ml·h-1.Besides,Ni-AC(N)catalyst also showed high stability:the conversion of toluene only decreased from 98.1%to 92.0%after 700 min.The as-prepared catalysts are low-cost,highly catalytic active,and highly stable,and are thus providing promising alternative for treating tar-rich streams from biomass gasification.
[1]F.L.Chan,A.Tanksale,Review of recent developments in Ni-based catalysts for biomass gasification,Renew.Sust.Energ.Rev.38(2014)428-438.
[2]D.Li,M.Tamura,Y.Nakagawa,K.Tomishige,Metal catalysts for steam reforming of tar derived from the gasification of lignocellulosic biomass,Bioresour.Technol.178(2015)53-64.
[3]C.P.B.Quitete,R.C.P.Bittencourt,M.M.V.M.Souza,Steam reforming of tar using toluene as a model compound with nickel catalysts supported on hexaaluminates,Appl.Catal.A Gen.478(20)(2014)234-240.
[4]H.de Lasa,E.Salaices,J.Mazumder,R.Lucky,Catalytic steam gasification of biomass:Catalysts,thermodynamics and kinetics,Chem.Rev.111(9)(2011)5404-5433.
[5]P.McKendry,Energy production from biomass(part 3):Gasification technologies,Bioresour.Technol.83(1)(2002)55-63.
[6]M.Koike,Y.Hisada,L.Wang,D.Li,H.Watanabe,Y.Nakagawa,K.Tomishige,High catalytic activity of Co-Fe/α-Al2O3in the steam reforming of toluene in the presence of hydrogen,Appl.Catal.B Environ.140(2013)652-662.
[7]K.Polychronopoulou,K.Giannakopoulos,A.M.Efstathiou,Tailoring MgO-based supported Rh catalysts for puri fication ofgas streams from phenol,Appl.Catal.B Environ.111(2012)360-375.
[8]J.Ashok,Y.Kathiraser,M.L.Ang,S.Kawi,Bi-functional hydrotalcite-derived NiOCaO-Al2O3catalysts for steam reforming of biomass and/or tar model compound at low steam-to-carbon conditions,Appl.Catal.B Environ.172(2015)116-128.
[9]Y.Shen,Chars as carbonaceous adsorbents/catalysts for tar elimination during biomass pyrolysis or gasification,Renew.Sust.Energ.Rev.43(2015)281-295.
[10]S.Y.Park,G.Oh,K.Kim,M.W.Seo,H.W.Ra,T.Y.Mun,J.G.Lee,S.J.Yoon,Deactivation characteristics of Ni and Ru catalysts in tar steam reforming,Renew.Energ.105(2017)76-83.
[11]S.Anis,Z.A.Zainal,Tar reduction in biomass producer gas via mechanical,catalytic and thermal methods:A review,Renew.Sust.Energ.Rev.15(5)(2011)2355-2377.
[12]D.Wang,W.Yuan,W.Ji,Use of biomass hydrothermal conversion char as the Ni catalyst support in benzene and gasification tar removal,Trans.ASABE 53(3)(2010)795-800.
[13]Y.Shen,K.Yoshikawa,Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis—A review,Renew.Sust.Energ.Rev.21(2013)371-392.
[14]G.W.Huber,S.Iborra,A.Corma,Synthesis of transportation fuels from biomass:Chemistry,catalysts,and engineering,Chem.Rev.106(9)(2006)4044-4098.
[15]J.Ashok,S.Kawi,Steam reforming of toluene as a biomass tar model compound over CeO2promoted Ni/CaO-Al2O3catalytic systems,Int.J.Hydrog.Energy 38(32)(2013)13938-13949.
[16]L.M.Zhou,T.R.Zhang,Z.L.Tao,J.Chen,Ni nanoparticles supported on carbon as efficient catalysts for the hydrolysis of ammonia borane,Nano Res.7(5)(2014)774-781.
[17]A.Shoaib,M.W.Ji,H.M.Qian,J.J.Liu,M.Xu,J.T.Zhang,Noble metal nanoclusters and their in situ calcination to nanocrystals:Precise control of their size and interface with TiO2nanosheets and their versatile catalysis applications,Nano Res.9(6)(2016)1763-1774.
[18]M.Chareonpanich,Z.G.Zhang,A.Nishijima,A.Tomita,Effect of catalysts on yields of monocyclic aromatic hydrocarbons in hydrocracking of coal volatile matter,Fuel 74(10)(1995)1636-1640.
[19]S.Meesuk,J.P.Cao,K.Sato,Y.Ogawa,T.Takarada,The effects of temperature on product yields and composition of bio-oils in hydropyrolysis of rice husk using nickel-loaded brown coal char catalyst,J.Anal.Appl.Pyrolysis 94(2012)238-245.
[20]Z.Min,P.Yimsiri,M.Asadullah,S.Zhang,C.Z.Li,Catalytic reforming of tar during gasification.Part II.Char as a catalyst or as a catalyst support for tar reforming,Fuel 90(7)(2011)2545-2552.
[21]S.R.Li,J.L.Gong,Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions,Chem.Soc.Rev.43(21)(2014)7245-7256.
[22]D.Li,L.Zeng,X.Y.Li,X.Wang,H.Y.Ma,S.Assabumrungrat,J.L.Gong,Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation,Appl.C at al.B Environ.176(2015)532-541.
[23]D.Li,X.Y.Li,J.L.Gong,Catalytic reforming of oxygenates:State of the art and future prospects,Chem.Rev.116(19)(2016)11529-11653.
[24]H.Y.Ma,L.Zeng,H.Tian,D.Li,X.Wang,X.Y.Li,J.L.Gong,Efficient hydrogen production from ethanol steam reforming over La-modi fied ordered mesoporous Ni-based catalysts,Appl.Catal.B Environ.181(2016)321-331.
[25]X.Y.Li,D.Li,H.Tian,L.Zeng,Z.J.Zhao,J.L.Gong,Dry reforming of methane over Ni/La2O3nanorod catalysts with stabilized Ni nanoparticles,Appl.Catal.B Environ.202(2017)683-694.
[26]J.Tao,C.Dong,Q.Lu,H.Liao,X.Du,Y.Yang,E.Dahlquist,Catalytic cracking of biomass high-temperature pyrolysis tar using NiO/AC catalysts,Int.J.Green Energy 12(8)(2015)773-779.
[27]A.E.Aksoylu,M.Madalena,A.Freitas,M.F.R.Pereira,J.L.Figueiredo,The effects of different activated carbon supports and support modifications on the properties of Pt/AC catalysts,Carbon 39(2)(2001)175-185.
[28]S.X.Liu,X.Chen,X.Y.Chen,Z.F.Liu,H.L.Wang,Activated carbon with excellent chromium(VI)adsorption performance prepared by acid-base surface modi fication,J.Hazard.Mater.141(1)(2007)315-319.
[29]G.G.Stavropoulos,P.Samaras,G.P.Sakellaropoulos,Effect of activated carbons modi fication on porosity,surface structure and phenol adsorption,J.Hazard.Mater.151(2-3)(2008)414-421.
[30]T.Hou,L.Yuan,T.Ye,L.Gong,J.Tu,M.Yamamoto,Y.Torimoto,Q.Li,Hydrogen production by low-temperature reforming of organic compounds in bio-oil over a CNT-promoting Ni catalyst,Int.J.Hydrog.Energy 34(22)(2009)9095-9107.
[31]M.Rashidi,A.Tavasoli,Hydrogen rich gas production via supercritical water gasification of sugarcane bagasse using unpromoted and copper promoted Ni/CNT nanocatalysts,J.Supercrit.Fluids 98(2015)111-118.
[32]M.Ruan,J.Guan,D.He,T.Meng,Q.Zhang,The hydrogenation of aromaticnaphthalene with Ni2P/CNTs,RSC Adv.5(71)(2015)57700-57703.
[33]D.X.Zheng,J.L.Gong,C.Jin,P.Li,H.L.Bai,Exchange bias effect modulated anisotropic magnetoresistance in Fe/YMnO3multiferroic bilayers,Mater.Lett.156(2015)125-128.
[34]G.J.Yu,J.L.Gong,D.Z.Zhu,S.X.He,J.Q.Cao,Z.Y.Zhu,Efficient synthesis of carbon nanotubes over rare earth zeolites by thermal chemical vapor deposition at low temperature,Diam.Relat.Mater.15(9)(2006)1261-1265.
[35]G.Wu,C.Zhang,S.Li,Z.Han,T.Wang,X.Ma,J.L.Gong,Hydrogen production via glycerol steam reforming over Ni/Al2O3:In fluence of nickel precursors,ACS Sustain.Chem.Eng.1(8)(2013)1052-1062.
[36]M.Zhou,H.Zhu,L.Niu,G.Xiao,R.Xiao,Catalytic hydroprocessing of furfural to cyclopentanol over Ni/CNTs catalysts:Model reaction for upgrading of bio-oil,Catal.Lett.144(2)(2014)235-241.
[37]K.Esumi,M.Ishigami,A.Nakajima,K.Sawada,H.Honda,Chemical treatment of carbon nanotubes,Carbon 34(1)(1996)279-281.
[38]C.H.Li,K.F.Yao,J.Liang,In fluence of acid treatments on the activity of carbon nanotube-supported catalysts,Carbon 41(4)(2003)858-860.
[39]Y.Tian,S.Zhong,X.Zhu,A.Huang,Y.Chen,X.Wang,Mesoporous carbon spheres:Synthesis,surface modi fication and neutral red adsorption,Mater.Lett.161(2015)656-660.
[40]Y.Zhai,Y.Dou,X.Liu,B.Tu,D.Zhao,One-pot synthesis of magnetically separable ordered mesoporous carbon,J.Mater.Chem.19(2009)3292-3300.
[41]Y.Bang,S.Park,S.J.Han,J.Yoo,J.H.Song,J.H.Choi,K.H.Kang,I.K.Song,Hydrogen production by steam reforming of lique fied natural gas(LNG)over mesoporous Ni/Al2O3catalyst prepared by an EDTA-assisted impregnation method,Appl.Catal.B Environ.180(2016)179-188.
[42]S.Helveg,C.López-Cartes,J.Sehested,P.L.Hansen,B.S.Clausen,J.R.Rostrup-Nielsen,F.Abild-Pedersen,J.K.Nørskov,Atomic-scale imaging of carbon nano fiber growth,Nature 427(2004)426-429.
[43]J.R.A.Sietsma,J.D.Meeldijk,M.Versluijs-Helder,A.Broersma,A.Jos Van Dillen,P.E.De Jongh,K.P.De Jong,Ordered mesoporous silica to study the preparation of Ni/SiO2ex nitrate catalysts:Impregnation,drying,and thermal treatments,Chem.Mater.20(9)(2008)2921-2931.
[44]J.R.A.Sietsma,J.D.Meeldijk,J.P.Breejen,M.Versluijs-Helder,A.Jos Van Dillen,P.E.De Jongh,K.P.De Jong,The preparation of supported NiO and Co3O4nanoparticles by the nitric oxide controlled thermal decomposition of nitrates,Angew.Chem.Int.Ed.46(24)(2007)4547-4549.
[45]S.B.Ren,S.Zhou,P.Zhang,Z.C.Wang,Z.P.Lei,C.X.Pan,H.F.Shui,Highly dispersed Ni/SBA-15 catalysts prepared with different nickel salts as nickel precursors:Effects of activation atmospheres,J.Fuel Chem.Technol.42(5)(2014)591-596.
[46]C.Hoang-Van,Y.Kachaya,S.J.Teichner,Y.Arnaud,J.A.Dalmon,Characterization of nickel catalysts by chemisorption techniques,X-ray diffraction and magnetic measurements:Effects of support,precursor and hydrogen pretreatment,Appl.Catal.46(1989)281-296.
[47]W.Xu,Z.Liu,A.C.Johnston-Peck,S.D.Senanayake,G.Zhou,D.J.Stacchiola,E.A.Stach,J.A.Rodriguez,Steam reforming of ethanol on Ni/CeO2:Reaction pathway and interaction between Ni and the CeO2support,ACS Catal.3(5)(2013)975-984.
[48]Y.Sekine,D.Mukai,Y.Murai,S.Tochiya,Y.Izutsu,K.Sekiguchi,N.Hosomura,H.Arai,E.Kikuchi,Y.Sugiura,Steam reforming of toluene over perovskite-supported Ni catalysts,Appl.Catal.A Gen.451(2013)160-167.
[49]M.Kong,Q.Yang,J.Fei,X.Zheng,Experimental study of Ni/MgO catalyst in carbon dioxide reforming of toluene,a model compound of tar from biomass gasification,Int.J.Hydrog.Energy 37(18)(2012)13355-13364.
[50]R.Zhang,H.Wang,X.Hou,Catalytic reforming of toluene as tar model compound:EffectofCe and Ce-Mg promoterusing Ni/olivine catalyst,Chemosphere 97(2014)40-46.
[51]U.Oemar,M.L.Ang,W.F.Hee,K.Hidajat,S.Kawi,Perovskite LaxM1-xNi0.8Fe0.2O3catalyst for steam reforming of toluene:Crucial role of alkaline earth metal at low steam condition,Appl.Catal.B Environ.148(2014)231-242.
[52]U.Oemar,M.L.Ang,Y.C.Chin,K.Hidajat,S.Kawi,Role of lattice oxygen in oxidative steam reforming of toluene as a tar model compound over Ni/La0.8Sr0.2AlO3catalyst,Catal.Sci.Technol.5(7)(2015)3585-3597.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Transport hindrances with electrodialytic recovery of citric acid from solution of strong electrolytes
- Experimental investigation on CO2-light crude oil interfacial and swelling behavior
- Biosynthesis of 4-hydroxyphenylpyruvic acid from L-tyrosine using recombinant Escherichia coli cells expressing membrane bound L-amino acid deaminase☆
- Process development for producing a food-grade glucose solution from rice straws
- Carbon dioxide induced degradation of diethanolamine during absorption and desorption processes
- Biodegradation of natural and synthetic estrogens in moving bed bioreactor
