Solvent extraction kinetics of Sm(III),Eu(III)and Gd(III)with 2-ethylhexyl phosphoric acid-2-ethylhexyl ester☆
Zhuo Chen,Yundong Wang*
State Key Laboratory of Chemical Engineering,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
1.Introduction
Rare earth elements(REEs)play an important part in industry,and have been widely used in metallurgy,ceramics,glass,petrochemical industry,nuclear,electronics,agriculture and medicine[1-4].With the increasing demand ofhigh purity REEs,differences in extraction kinetics provide a possibility for the quantitative separation of REEs that cannot be separated in equilibrium state[5].Kovalancik et al.[6]investigated the extraction separation of Ce and Y by benzyldibutylamine(BDBA)in the presence of ethylenediaminetemlacetic acid(EDTA)as complexing agent.The concentration of Y after extraction in the organic phase was below the detection limit of the analytical method and the separation factor,αCe/Y,ranged from200 to 400.Chen etal.[7]developed a selective separation strategy for Sc(III)from yttrium and lanthanides in aqueous solution by using ionic liquids.It was shown that the extraction efficiency ofSc(III)was as high as 99.5%atpHof4.2.Sc(III)could be extracted selectively from yttrium and lanthanides by simple tuning of pH value of the aqueous phase.Sc(III)was preferentially extracted over yttrium and lanthanides with the ionic liquid extractants and the separation factor was 103.Some researchers discussed the extraction of lanthanum from the mixture solution of lanthanum nitrate and lanthanum chloride with Tri-Butyl-Phosphate(TBP),and concluded that the extraction rate increased with the increase of atomic number[8].However,there exist some basic problems such as the obscure kinetic mechanism during the mass transfer from aqueous phase to organic phase.Therefore,it is necessary to investigate the extraction kinetics of REEs for further development of system and acquisition of data.
The conventional set-up of extraction kinetics includes constant interfacial area cell[9],constant interfacial area cell with laminar flow[10],single drop technique[11-13],centrifugal partition chromatography(CPC)[14,15],stopped flow[16],growing drop cell[17]and so on.However,the following problems emerge,such as slow phase separation and difficult data acquisition.AKUFVE,integrating the reaction and separation,is used to solve the above problem effectively.No further centrifugal or settling phase separation is required,so the rapid and continuous determination and acquisition of data can be achieved.To the best of our knowledge,there has been no in-depth investigation on extraction kinetics of rare earth elements by AKUFVE until now.
In this work,the extraction mechanismwas discussed on the basis of dimeric model of P507.Then we used AKUFVE to study the extraction kinetics of medium rare earth elements Sm(III),Eu(III)and Gd(III).As for light and heavy rare earth elements,Kao[18]and Wang et al.[19]have done some researches.Here we mainly focused on the investigation ofmediumrare earth elements.The factors affecting the extraction rate such as the stirring speed,pH,concentration of REEs and P507 as wellas temperature were allanalyzed and the obtained experimental results of Sm(III),Eu(III)and Gd(III)were compared with the theoretical model.
2.Theoretical Kinetic Model
P507(HA)usually form dimers in the organic solvent,abbreviated as H2A2.And H2A2can react with REEs in two different mechanisms:one is the monomeric model and the other one is dimeric.For example,the mechanisms of VO2+reacting with P204[20]and lanthanum reacting with Cyanex 272[21]comply with the monomeric and dimeric model respectively.And the reaction order with respect to the concentration of H2A2is 0.5 and 1.0.The following experimental results indicate that the extraction kinetics of Sm,Eu and Gd are more consistent with the dimeric model.The total reaction between the REEs and extractant is shown in Eq.(1).
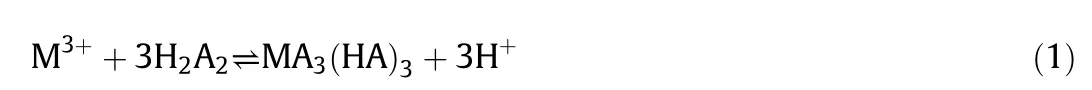
where M3+,H2A2and MA3(HA)3represent the REEs,extractant P507 and the extracted complex respectively.
According to the theory proposed by Danesi and Chiarizia[22],the following equations are considered:

where k1refers to the dissociation constant between the dimeric and monomeric form.

where αHAis the distribution constant of the extractant between the interface and organic phase,HA(i)represents the extractant P507 of interface.
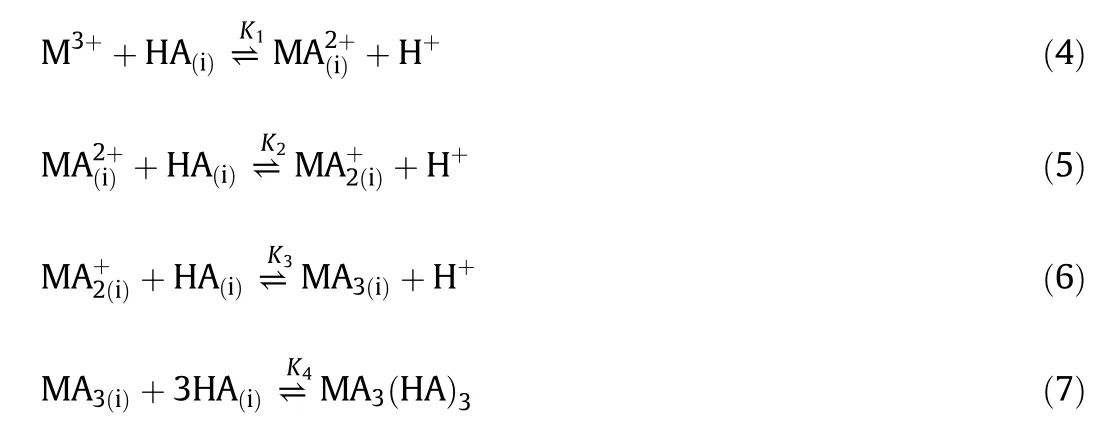
where K1,K2,K3,K4represent equilibrium constants.
The definitions of the above factors can be written in the following Eqs.(8)to(12):
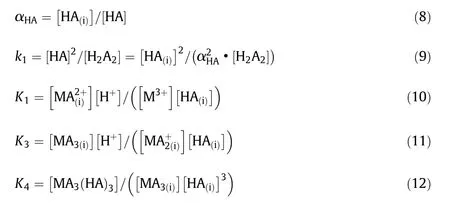
Considering Eq.(5)as the rate-controlling step[22,23],so the rate of extraction can be obtained as followed:

The definition of k is as the following Eq.(15):

Above all,Eq.(14)can be transformed into an alternative form:

3.Experimental
3.1.Reagents and materials
The extractant P507(supplied by Luoyang Aoda Chemical Co.,Ltd.)was used without further puri fication.And sulphonated kerosene(purchased from Hubei Prosperity Galaxy Chemical Co.,Ltd.)was used as the diluent.The SmCl3·6H2O,EuCl3·6H2O and GdCl3·6H2O(purity>99.9%)were purchased from the Biam Alloys Co.,Ltd.,Beijing Institute of Aeronautical Materials of AVIC.All the other reagents were analytical grade.Initial concentration of Sm(III),Eu(III),and Gd(III)was maintained at 10 g·L-1except researching the effect of REEs concentration on extraction rate.The concentration of REEs in aqueous phase before and after extraction were all determined by titration with standard disodium ethylenediaminetetraacetic acid(EDTA-2Na)using xylenol orange as indicator,and the amount of REEs extracted to organic phase was obtained by mass balance.The concentration of hydrogen ion in aqueous phase was determined using a FiveEasy pH meter with an electrode.
3.2.Apparatus and measurements
Kinetics experiments were carried out with AKUFVE[24,25],which was imported from METALLEXTRAKTION AB of Sweden.The picture of AKUFVE is shown in Fig.1.

Fig.1.Picture and structure of AKUFVE 1-injecting hole;2-mixing chamber;3-centrifugal chamber;4- flowmeter;5-condenser.
Equal volume of the organic phase and aqueous phase were added into the mixing chamber from injecting holes with funnel.At the same time,stirring,centrifuging and timing started at once.The volumes of both aqueous and organic phase were 150 ml.The stirring speed and centrifugal speed were regulated with the rotary knob which was on the front plate and the concrete values were displayed on the LED display.Compared with the other set-up,by using AKUFVE the data obtained and the operation are more efficient and reliable in reproducibility and stability.
Once the stirring started,the organic phase and the aqueous phase contacted with each other and the mass transfer process occurred.At each 30 s interval,2 ml of the aqueous phase was sampled for titrating analysis and 2 ml of the organic phase was also taken out to ensure a constant phase ratio.The kinetics experiments were carried out at ambient environment pressure and(25 ± 0.1)°C except researching the effect of temperature on extraction rate.
3.3.Data analysis
The experimentaldata were calculated as follows.Assuming that the mass transfer process could be treated as a pseudo- first-order reversible reaction with respect to the metalcation[26],the initialforward extraction rate of extraction can be expressed as:
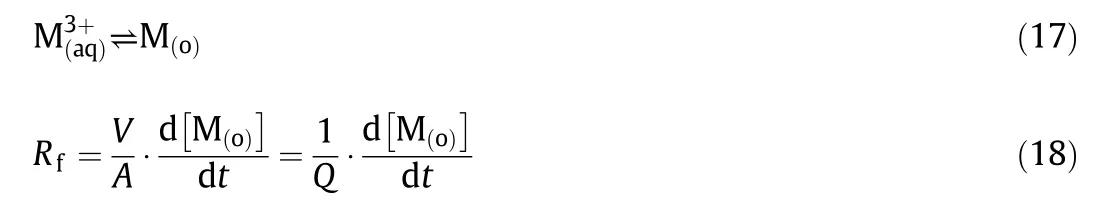
whereand M(o)represent the REEs of aqueous phase and organic phase,respectively;V is the volume of either the aqueous or organic phase and A is the phase boundary area,Q refers to the specific interfacial area;t represents the reaction time.
When data were processed,the relationship between concentration of M(o)in organic phase and t was plotted for each experiment,and the slopes of the plots were used to calculate the initial extraction rate Rf.
4.Results and Discussion
4.1.Effect of stirring speed on extraction rate
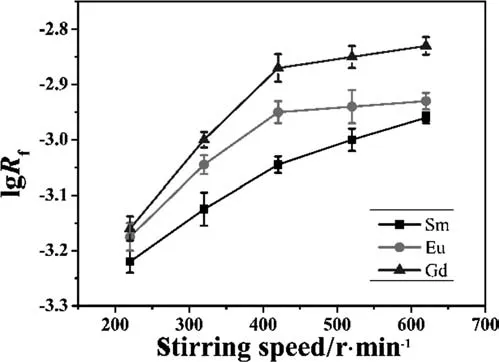
Fig.2.Dependence of lg R f on stirring speed with P507-kerosene system(experimental conditions:[M3+]=10 g·L-1,[P507]=0.35 mol·L-1,T=298 K,pH=3.00).
The effect of stirring speed on the extraction rate Rfwas investigated for P507-kerosene system and the results are shown in Fig.2.Actually diffusion and chemical reaction both have a close relationship with extraction rate.When chemical reaction is relatively slow,the resistance of the entire process is concentrated on reaction;in contrast,the extraction rate is controlled by diffusion;otherwise it is co-controlled by both chemical reaction and diffusion.With AKUFVE the relationship between the extraction rate and stirring speed is almost into a straight line with the stirring speed below 420 r·min-1.This probably results from the reduction of the boundary thickness and faster mass transfer as increasing the stirring speed,and the rate-determining step is diffusion or both of diffusion and reaction which limits the extraction rate.Above a certain stirring speed(420 r·min-1),the emulsification phenomenon occurs slightly in P507 system.Therefore in order to guarantee the same experimental condition all the other kinetics experiments were carried out at the stirring speed of 420 r·min-1.
4.2.Effect of pH on extraction rate
The relationship of extraction rate has been obtained based on the theoretical model(Eq.(16)).Logarithmic operations are performed on both sides of Eq.(16),so the following equation is obtained.
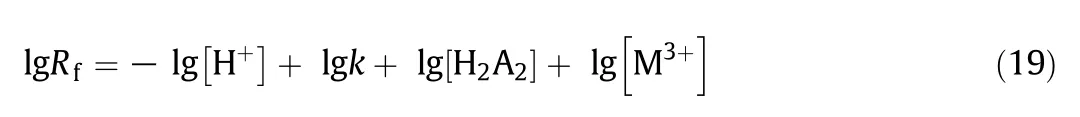
The effect of pH on the experiments was investigated from the range of 1.00 to 4.00 at fixed concentration of rare earth elements and extract ant as shown in Fig.3.The slope of lg-lg relationship was the reaction order of hydrogen ion concentration.The experimental results showed that the reaction order of hydrogen ion concentration for Sm(III),Eu(III),and Gd(III)is approximate to-1,which validated the validity of theoretical model.Besides the rate constant k can be calculated according to the intercept.It was also concluded that the extraction rate raised as the pH increased from 1.00 to 3.00,for example,the logarithm of Sm(III)ranges from-4.70 to-3.05 in P507 system,after that the extraction rate decreases.Therefore,the highest extraction rate can be reached at the pH of 3.00.Actually,the extract ant cannot be fully dissociated in strong acidic solution,so the extraction rate is not the maximum.However,in weak acidic solution hydrolysis of REEs usually occurs and REEs become M(OH)3precipitation.The hydrolysis product cannot be extracted,hence hydrolysis reduces the rate of extraction sharply.

Fig.3.Dependence of lg R f on lg[H+]with P507-kerosene system(experimental conditions:[M3+]=10 g·L-1,[P507]=0.35 mol·L-1,T=298 K,stirring speed=420 r·min-1).
4.3.Effect of P507 concentration in organic phase on extraction rate
The effect of the organic phase concentration was investigated in P507-kerosene system at fixed concentration of rare earth elements and hydrogen ion as shown in Fig.4.The reaction order of the extract ant for Sm(III),Eu(III),and Gd(III)was validated and the rate constant k was also obtained according to the similar method as described in Section 4.2.
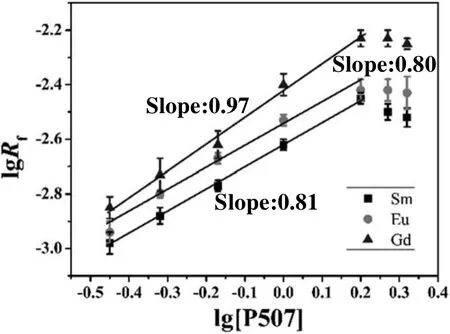
Fig.4.Dependence of lg R f on lg[P507](experimental conditions:[M3+]=10 g·L-1,stirring speed=420 r·min-1,T=298 K,pH=3.00).
In Fig.4,it can be seen that with increasing extractant concentration,the extraction rate also linearly increase and the slopes of lg Rfversus lg[P507]plot are approximately equal to 1.0 in the lower extract ant concentration ranges.By contrast,in the higher extract ant concentration ranges,the rate does not increase correspondingly and the slopes trend to zero.The reasonable explanation may be this fact:the extraction rate depends on the extractant interfacial concentration rather bulk concentration[27].The extract ant in the interface increases with the increase of bulk concentration in lower P507 concentration ranges,and the extraction rate also increases.Otherwise P507 concentration in the interface is greatly excessive after dissociation and distribution in higher extractant concentration ranges.Therefore,the reaction order does not change with increasing extractant concentrations.
4.4.Effect of REEs concentration in aqueous phase on extraction rate
The effect of REEs concentration was also investigated.As shown in Fig.5,the slopes of the plots for these three REEs are close to 1.The results indicate that the mass transfer process can be treated as a pseudofirst-order reversible reaction with respect to the metal cation.

Fig.5.Dependence of lg R f on lg[M3+]with P507-kerosene system(experimental conditions:pH=3.00,[P507]=0.35 mol·L-1,T=298 K,stirring speed=420 r·min-1).
4.5.Effect of temperature on extraction rate
The impact of temperature on extraction rate was investigated from 20 °C to 50 °C.The results are shown in Fig.6.Arrhenius equation describes the relationship between temperature and rate constant.


Fig.6.Dependence of lg k on temperature with P507-kerosene system(experimental conditions:[M3+]=10 g·L-1,[P507]=0.35 mol·L-1,pH=3.00,stirring speed=420 r·min-1).
where k is rate constant,A is pre-exponential factor,R refers to gas constant,Eaand T represent activation energy and temperature respectively.We perform logarithmic operations on both sides of Eq.(20),so the following equation is obtained.Then Eacan be calculated by the slope of the fitting line.

The activation energy for Sm(III),Eu(III),and Gd(III)are 26.80 kJ·mol-1,13.40 kJ·mol-1and 11.10 kJ·mol-1,respectively.It is generally believed that when Eais greater than 42 kJ·mol-1,extraction process is usually determined by chemical reaction[28].When Eais lower than 20 kJ·mol-1,the main resistance is diffusion.Otherwise the extraction rate is controlled by both diffusion and chemical reaction.The activation energy results indicate that the extraction is determined by diffusion of Eu and Gd in P507-kerosene system and Sm is controlled by chemical reaction and diffusion.However,Danesi and Vandegrift[26]reported that Eawas not sufficient for the determination of the kinetics process in the multi-component chemical reaction system.Therefore,several comprehensive analysis methods are required for the determination of the control procedure in further research.
Above all,the correlation equations are determined as:

As shown in Fig.7,the correlation as Eqs.(22)to(24)show good coincidence with the experimental values,most relative error is within±30%.Wu et al.[29]proposed that the monomeric and dimeric models would be suitable when the reaction order with respect to[H2A2]is 0.5 and 1.0 respectively,so the experimental results obtained in the present study for Sm(III),Eu(III)and Gd(III)are expected to be best fitted by the dimeric model of P507.
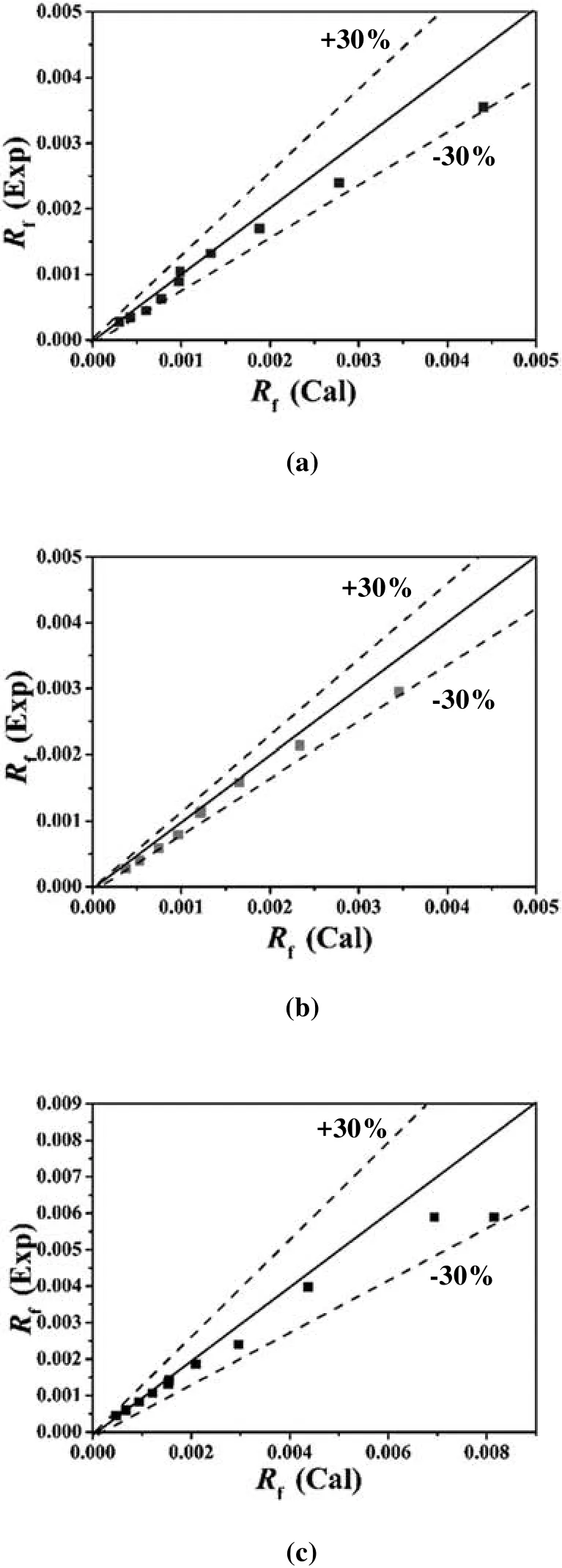
Fig.7.(a)-(c)Comparison of calculated and experiment values of Sm(III),Eu(III)and Gd(III).
5.Conclusions
The extraction kinetics of Sm(III),Eu(III)and Gd(III)with P507 by AKUFVE were studied.Firstly,the extraction mechanism was obtained according to the dimeric model of P507.Then 420 r·min-1was considered as the optimal stirring speed of the following experiments.Next the factors that affect the extraction rate such as pH,concentration of REEs and P507 were also analyzed.The results showed that the extraction mechanism changed with the increasing concentration of P507.The experiments with different temperature were also carried out,it turned out that the values of Eafor Sm(III),Eu(III),and Gd(III)extracted by P507 were 26.80 kJ·mol-1,13.40 kJ·mol-1and 11.10 kJ·mol-1respectively,the resistance of the entire process was concentrated on diffusion or both of diffusion and reaction.Finally,the correlation equations were obtained on the basis of the above experiments,and the theoretical results were consistent with the experimental data,most relative error was within±30%.
[1]T.Dutta,K.H.Kim,M.Uchimiya,E.E.Kwon,Global demand for rare earth resources and strategies for green mining,Environ.Res.150(1)(2016)182-190.
[2]M.Feng,C.Xu,J.Kynicky,L.Zeng,W.L.Song,Rare earth element enrichment in Palaeoproterozoic Fengzhen carbonatite from the North China block,Int.Geol.Rev.58(15)(2016)1940-1950.
[3]M.Panigrahi,M.Grabda,D.Kozak,A.Dorai,E.Shibata,Liquid-liquid extraction of neodymium ions from aqueous solutions of NdCl3by phosphonium-based ionic liquids,Sep.Purif.Technol.171(1)(2016)263-269.
[4]A.Cuvier,L.Pourcelot,A.Probst,J.Prunier,R.G.Le,Trace elements and Pb isotopes in soils and sediments impacted by uranium mining,Sci.Total Environ.566(1)(2016)238-249.
[5]J.A.Dound,H.F.Aly,Kinetic approach for Er(III)and Am(III)separation using selective thenoyltrifluoro acetone-triphenylarsine oxide systems,Proceedings of the International Solvent Extraction Conference,Japan 1996,pp.475-480.
[6]J.Kovalancik,M.Galova,Extraction separation of rare-earth elements by amines in the presence of complexing agents,J.Radioanal.Nucl.Chem.162(1)(1992)35-46.
[7]Y.Chen,H.Wang,Y.Pei,Selective separation ofscandium(III)from rare earth metals by carboxyl-functionalized ionic liquids,Sep.Purif.Technol.178(5)(2017)261-268.
[8]D.F.Peppard,G.W.Mason,Separation of rare earths by solvent extraction,US Pat.2955913(1960).
[9]J.B.Lewis,The mechanism of mass transfer of solutes across liquid-liquid interfaces,Chem.Eng.Sci.3(6)(1954)248-259.
[10]Z.Zheng,J.Lu,D.Q.Li,G.X.Ma,The kinetics study in liquid-liquid systems with constant interfacial area cell with laminar flow,Chem.Eng.Sci.53(13)(1998)2327-2333.
[11]N.S.Awward,H.A.Ibrahium,Kinetic extraction of titanium(IV)from chloride solution containing Fe(III),Cr(III)and V(V)using the single drop technique,Environ.Chem.Eng.1(5)(2013)65-72.
[12]R.K.Biswas,M.A.Hayat,Kinetics of solvent extraction of zirconium from chloride medium by D2EHPA in kerosene using the single drop technique,Hydrometallurgy 65(2)(2002)205-216.
[13]K.Durrani,C.Hanson,M.A.Hughes,Droplet phenomena in the Ni/Na/D2EHPAIH20 system,Metall.Trans.B 8(4)(1976)169-174.
[14]S.Muralidharan,H.Fresier,Cpc tool for practical separation of metals and fundamental investigations of chemical mechanisms,ISEC 1(2)(1996)427-432.
[15]G.X.Ma,H.Freiser,S.Muralidharan,Interfacial catalysis of formation and dissociation of tervalent lanthanide complexes in two-phase systems,Anal.Chem.69(4)(1997)2827-2834.
[16]H.Freiser,New tools for old questions in solvent extraction chemistry,ISEC 1(6)(1996)11-16.
[17]M.A.Hughes,Rate of extraction of cobalt from an aqueous solution to D2EHPA in a growing drop cell,Hydrometallurgy 13(3)(1985)249-264.
[18]H.C.Kao,P.S.Yen,R.S.Juang,Solvent extraction of La(III)and Nd(III)from nitrate solutions with 2-ethylhexylphos phonic acid mono-2-ethylhexyl ester,Chem.Eng.J.119(2-3)(2006)167-174.
[19]X.Wang,S.Meng,D.Li,Extraction kinetics of ytterbium(III)by 2-ethylhexylphosphonic acid mono-(2-ethylhexyl)ester in the presence of isooctanol using a constant inter facial cell with laminar flow,Sep.Purif.Technol.71(1)(2010)50-55.
[20]R.K.Biswas,M.G.K.Mondal,Kinetics of VO2+extraction by D2EHPA,Hydrometallurgy 69(1)(2003)117-133.
[21]M.I.Saleh,M.F.Bari,M.S.Jab,B.Saad,Kinetics of lanthanum extraction from nitrateacetato medium by Cyanex 272 in toluene using the single drop technique,Hydro metallurgy 67(1)(2002)45-52.
[22]P.R.Danesi,R.Chiarizia,The kinetics of metal solvent extraction,CRC Crit.Rev.Anal.Chem.10(1)(1980)1-126.
[23]H.Hou,J.Xu,Y.Wang,Solvent extraction performance of Pr(III)from chloride acidic solution with 2-ethylhexyl phosphoric acid-2-ethyl hexyl ester(EHEHPA)by using membrane dispersion micro-extractor,Hydrometallurgy 156(10)(2015)116-123.
[24]D.S.Flett,J.A.Hartlage,D.R.Spink,D.N.Okuhara,The extraction of copper by an alkylated 8-hydroxy quinolone,J.Inorg.Nucl.Chem.37(9)(1975)1967-1971.
[25]J.D.Miller,R.L.Atwood,Discussion of the kinetics of copper solvent extraction with hydroxy oximes,J.Inorg.Nucl.Chem.37(12)(1975)2539-2542.
[26]P.R.Danesi,G.F.Vandegrift,Kinetics and mechanism of the interfacial mass transfer of europium(3+)and americium(3+)in the system bis(2-ethylhexyl)phosphaten-dodecane-sodium chloride-hydrochloric acid-water,J.Phys.Chem.85(24)(1981)3646-3651.
[27]G.F.Vandegrift,E.P.Horwitz,The mechanism of interfacial mass transfer of calcium in the system:Di(2-ethylhexyl)phosphoric acid in dodecane-dilute nitric acid,J.Inorg.Nucl.Chem.39(8)(1997)1425-1432.
[28]J.F.Yu,C.Ji,Interfacial chemistry and kinetics-controlled reaction mechanism of organo phosphoric acid mixed extraction systems,Chem.J.Chin.Univ.13(2)(1992)224-226.
[29]D.B.Wu,X.L.Wang,D.Q.Li,Extraction kinetics of Sc(III),Y(III),La(III)and Gd(III)from chloride medium by Cyanex 302 in heptane using the constant interfacial cell with laminar flow,Chem.Eng.Process.46(1)(2007)17-24.
 Chinese Journal of Chemical Engineering2018年2期
Chinese Journal of Chemical Engineering2018年2期
- Chinese Journal of Chemical Engineering的其它文章
- Surface chemical characterization of deactivated low-level mercury catalysts for acetylene hydrochlorination☆
- Insight into fouling behavior of poly(vinylidene fluoride)(PVDF)hollow fiber membranes caused by dextran with different pore
- Protein adsorption onto diethylaminoethyl dextran modi fied anion exchanger:Effect of ionic strength and column behavior☆
- Gas emission source term estimation with 1-step nonlinear partial swarm optimization-Tikhonov regularization hybrid method☆
- Dissolution of antibiotics mycelium in ionic liquids:Performance and mechanism☆
- Kinetic studies on extra heavy crude oilupgrading using nanocatalysts by applying CFD techniques☆
