Direct Synthesis of Monodisperse Hollow Molecularly Imprinted Polymers Based on Unfunctionalized SiO2for the Recognition of Bisphenol A
Shao-min LiuMeng-xing WeiXin FuXue-bin Zhang
Department of Chemistry,University of Science and Technology of China,Hefei 230026,China
I.INTRODUCTION
Molecular imprinting is a versatile and straightforward method for the preparation of polymer receptors with tailor-made recognition sites. Molecularly imprinted polymers(MIPs)possess good binding affinity,stability,and speci ficity toward target molecules and thus have been widely used in separation science,biosensing,the recognition of proteins,and drug delivery[1−4].These applications emphasize that MIPs need to be optimized to obtain high sensitivity,large binding capacity,and rapid binding kinetics[5,6].Although numerous researchers have worked toward the development of molecular imprinting technology,the heterogeneous binding sites,template leakage,low binding capacity,and slow mass transfer of molecularly imprinted polymers are still challenges[7].To solve the above mentioned problems,surface molecular imprinting technologies and hollow molecular imprinting technologies that produce a high density of effective recognition sites on the surface,thereby facilitating the template transfer and producing high adsorption capacities and fast adsorption rates for the uptake of targets in MIPs have attracted increasing attention[8,9].Hollow molecular imprinted polymers,which are obtained by removal of the core of coreCshell structured surface molecular imprinted polymers,have well-de fined morphology,uniform size,low density and large surface areas,and could exhibit improved performance in real applications[10−13].
To prepare hollow molecular imprinted polymers,core-shell structured surface molecular imprinted polymers are first prepared by imprinting shells onto cores.Previously,several carriers were used as cores to synthesize the core-shell structured surface molecular imprinted polymers[14−17].Of these,SiO2has many advantages over other carriers,including availability,low cost,easy of modification,and the ability to introduce new groups to react with imprinted precursors,and it has been studied by several researchers.Two strategies were developed to synthesize core-shell structured surface molecular imprinted polymers.One is a surface modification strategy that involves preparing molecularly imprinted polymer shells with grafted C=C covalent bonds,iniferter groups and ultrathin polymeric layers on SiO2carries[18−27].The other method is a direct surface imprinting strategy that uses tetraethoxysilane(TEOS)as a cross-linking agent to direct hydrolytic condensation with silanol groups on the surface of SiO2carriers[28].Although the abovementioned methods could effectively synthesize molecularly imprinted polymer layers on the surface of SiO2carriers,the tedious and inefficient modi fication process for the former and the lack of resistance to acids and alkalis of the latter,largely limit their large-scale preparation and application.Polymer/silica nanocomposite technologies,wherein the hydroxyl groups in the repeating units of the polymer are expected to produce weak H-bonding interactions with the residual silanol groups of SiO2,have been successfully used to prepare polymercoated silica nanocomposites[29].This strategy has several advantages,since the encapsulation of polymer silica nanoparticles is driven by the H-bonding interaction between the hydroxyl group on the surface of silica core and the hydroxyl or carbonyl group of the polymer precursors during polymerization without modi fication of the silica surface in the absence of any stabilizer or surfactant.It is intriguing to investigate whether the above strategy can be used to directly prepare surface molecular imprinted polymers.Although the H-bonding interaction between functional monomers and the silica core may affect the selectivity of synthesized molecular imprinted polymers,the easy preparation method,uniform surface coating and inexpensive carriers are highly attractive.Additionally,as it becomes easier to obtain hollow molecular imprinted polymers,their use in widespread applications will increase.

Scheme 1 Fabrication of MHMIPs.BPA=bisphenol A,MMA=methacrylic acid,EGDMA=ethylene glycol dimethacrylate,AIBN=azodiisobutyronitrile.
In this paper,we report a novel,efficient,and generally applicable method to synthesize monodisperse hollow molecularly imprinted polymer(MHMIPs).This approach uses unfunctionalized SiO2spheres as carriers and weak H-bonding interactions between functional monomer and residual silanol groups of SiO2,followed by removal of the SiO2core and molecular imprinting with an ethanol solution of HF,as shown in Scheme 1.
As a model system,we chose to imprint a methacrylic acid-based polymer shell with bisphenol A(BPA),which is a known endocrine disrupter that could easily migrate into the human body to produce adverse effects on health.To prepare MHMIPs,the imprint molecule bisphenol A(BPA)was first allowed to interact with MMA in a mixed solvent of acetonitrile and toluene,and then,SiO2spherical carriers were added;afterwards,ethylene glycol dimethacrylate(EGDMA)and azobisisobutyronitrile(AIBN)were added into the above mixture to cause direct polymerization on the surface of SiO2spheres at high temperature.Finally,the SiO2spheres and BPA molecules were etched away by an ethanol solution of HF,and MHMIPs were obtained.This strategy has three advantages:(i)molecular imprinted polymers could be directly formed on the surface of monodisperse SiO2spheres created by the Stöber method,(ii)the synthesized SiO2@MIPs and MHMIPs are highly dispersed,uniform in size and spherically structured,(iii)a high density of recognition sites located on the surface formed and helped to improve the bonding capacity and bonding rate.
II.EXPERIMENTS
A.Reagents and chemicals
Bisphenol A (BPA, purity>98%), phenol(purity>97%), tetrabromobisphenol A (TBBPA,purity>98%)and 4-tert-butyl-pyrocatechol(PTBP,purity>96%)were purchased from Sigma Reagent Co.,Ltd. (Shanghai,China). Methacrylic acid(MAA,purity>96%), acrylic amide (AM,purity>98%),4-vinyl pyridine(4-VP,purity>98%)and ethylene glycol dimethacrylate(EGDMA,purity>98%)were purchased from Alfa Aesar and puri fied by vacuum distillation before use.Azobisisobutyronitrile(AIBN,chemical grade)was purchased from Shanghai No.4 Reagent& H.V.ChemicalCompany (Shanghai,China),which was recrystallized with ethanol and then dried at room temperature in a vacuum prior to use. HPLC-grade methanol was purchased from Tedia(Fair field OH,USA).Tetraethoxysilane(TEOS),ammonium hydroxide(28 wt%NH3in water),HF solution(40%),acetonitrile and ethanol were purchased from Shanghai Chemical Reagent Co.,Ltd.and were used as received without any further purification.Doubly distilled water was used in all experiments.
B.Preparation of monodisperse SiO2spheres
Monodisperse SiO2spheres,350 nm in diameter,were prepared by using a slightly modified Stöber process[30].In a typical synthesis,18 mL of TEOS was dissolved in 182 mL of ethanol and rapidly added into a mixture of 65 mL of ethanol,100 mL of H2O,and 36 mL of ammonium.After stirring at 1000 r/min for 1 min,the stirring rate was adjusted to 450 r/min for 2 h at room temperature(25±1°C).After reaction,the resulting particles were separated from the reaction medium by repeated centrifugation(4000 r/min),ultrasonically dispersed(into ethanol)and dried at 60°C for 12 h.
C.Preparation of MHMIPs and MHNIPs
The MHMIPs were prepared as previously reported[31].In a typical synthesis,0.5 g SiO2spheres were dispersed in a mixture of 15 mL CH3CN and 55 mL toluene at room temperature with ultrasonication for 10 min.To this mixture,0.0745 g of BPA and 0.0987 g of MAA were added.After the resulting mixture was stored at 0°C for 4 h,0.7576 g of EGDMA and 40 mg AIBN were added(BPA:MAA:EGDMA=1:6:20).After purging with N2,the reaction vessel was sealed and stirred at 40°C for 2 h,at 65°C for 20 h and at 80°C for 4 h.The resulting solid was recovered by centrifugation and placed in an ethanol solution of HF(HF:ethanol=1:9,V:V)for 24 h to remove the silica matrix and imprint with molecular BPA.The MHMIPs were obtained after washing with water/ethanol and dried at 60°C for 12 h.
The synthesis strategy for MHNIPs was the same as above,except that no BPA was added.MHMIPs with different shell thicknesses were also prepared by changing the mass ratio of SiO2to the imprinted precursors.
D.Characterization of the samples
Scanning electron microscopy(SEM)measurements were taken on a Sirion 200 microscope(FEI,USA)operated at 5 kV.Transmission electron microscopy(TEM)measurements were taken on a JEOL 2010 microscope(JEOL,Japan)operated at 200 kV.Fourier transform infrared(FT-IR)experiments were performed with an EQUINOX55(Bruker,Germany)using KBr pellets containing the solid samples.UV-Vis absorption spectra of the samples were taken on a Solidspec-3700 DUV UV-Vis-NIR spectrophotometer under ambient conditions.
E.Equilibrium template binding experiments
Equilibrium template binding experiments were conducted using high-performance liquid chromatography.First,10 mg of each sample was suspended in 10 mL of a toluene solution with varying concentrations of BPA.After incubation at room temperature for 2 h,solutions were filtered using a 0.22-µm Millex-GVMilli pore filter,and the filtrate was analyzed by HPLC-DAD(Agilent 1100).Methanol was used as the mobile phase,with a flow rate of 0.8 mL/min and a detector wavelength of 276 nm.The binding amount of BPA was determined by measuring the difference between the total BPA added and the residual amount in solution.All binding analyses were performed in duplicate with mean values calculated.Meanwhile,the binding kinetics of HPMIPs with BPA were tested by monitoring the BPA concentration in the solutions as a function of time.
F.Competitive binding experiments
The binding selectivities of MHMIP and MHNIP were evaluated by measuring their competitive binding capacities towards BPA and structurally related compounds(tetrabromobisphenol A(TBBPA),phenol,andp-tert-butylphenol(PTBP))at the same concentration.A 10-mg sample of MHMIP or MHNIP was incubated with 10 mL of a mixed solution of BPA,TBBPA,phenol and PTBP in toluene at 25°C for 2 h.The amounts of BPA,TBBPA,phenol and PTBP bound to the MHMIP or MHNIP were quanti fied by HPLC-DAD.The wavelength used for this determination was 276 nm.Methanol and water with a volume ratio of 7:3 were used as the mobile phase with a flow rate of 1 mL/min.All binding analyses were performed in duplicate,and the mean values were calculated.
The relative selectivity coefficient(k′)was calculated according to the following equations:

where the distribution coefficient(kd)represents the amount of a substance adsorbed by a sorbent,the selectivity coefficient of the sorbent(k)represents the ratio of two analogues adsorbed by the same sorbent,and the relative selectivity coefficient(k′)indicates the ratio of two different sorbents.CiandCfrepresent the initial and final concentrations(µmol/L),whileV(L)andW(g)are volume of solution and mass of sorbent.
III.RESULTS AND DISCUSSION
A.Synthesis of MHMIP

FIG.1The SEM and TEM patterns of SiO2(a,b),SiO2@MIP(c,d),and MHMIP(e,f).
To obtain hollow molecularly imprinted polymers,the first step is to prepare SiO2@MIPs.Compared with other methods of SiO2@MIP synthesis,this strategy eliminates the tedious and inefficient modification process for the SiO2carriers.Additionally,the SiO2cores and imprinted molecules of synthesized SiO2@MIPs could be simultaneously removed with washing in an ethanol solution of HF,and hollow molecularly imprinted polymers could be obtained effectively. In this work,core-shell molecularly imprinted polymers were successfully synthesized through the H-bonding directed polymerization of imprinted precursors onto the surface of colloidal SiO2particles.The SiO2spheres synthesized according to the Stöber method were highly monodisperse and spherical(FIG.1(a)and(b))[30].The spheres had a highly uniform diameter of 350 nm,and their surfaces were smooth,which improved the formation of uniform core-shell structures during the imprinting polymerization. In a typical synthesis,SiO2spheres(0.5 g)were dispersed in the mixing solution of toluene and CH3CN,which contained the template(BPA),functional monomers(MMA),crosslinking agent(EGDMA)and initiator(AIBN).After polymerizing the mixture of imprint precursor with SiO2at given temperature,SiO2@MIP were obtained which were still monodisperse and spherical,and neither pure polymer aggregated nor bare SiO2spheres were observed in the reaction products,indicating that selective imprinting polymerization occurred at the surface of the SiO2spheres(FIG.1(c)and(d)).Furthermore,the shell thickness of the synthesized SiO2@MIPs is uniform.In a following process,an ethanol solution of HF(HF:ethanol=1:9,V:V),which has been used previously to obtain hollow porous molecularly imprinted polymers,was used to remove the template molecules(BPA)and SiO2carriers of SiO2@MIP[31]. From the SEM and TEM patterns of the obtained MHMIP(FIG.1(e)and(f)),it was found that the SiO2carriers were successfully removed,and uniform,monodisperse hollow molecular imprinted polymer shells were retained with no breakage. These figures indicate that this strategy is a very efficient method to obtain monodispersed hollow molecular imprinted polymers.
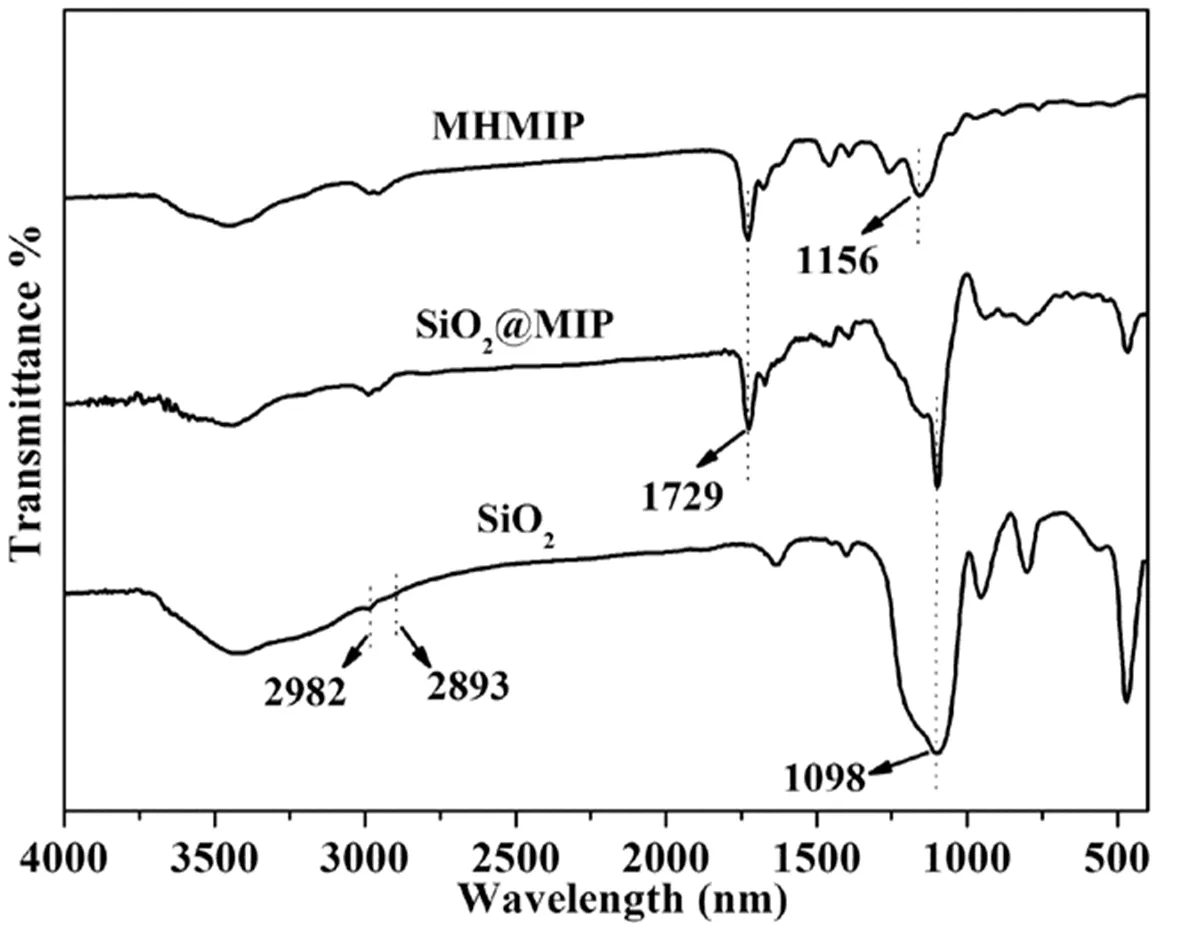
FIG.2 FT-IR spectra of the SiO2,SiO2@MIP,and MHMIP.
B.Synthetic mechanism
FT-IR spectra provide direct proof for the successful synthesis of SiO2@MIP and MHMIP nanoparticles(FIG.2).The strong peaks at 1098 and 790 cm−1in the spectrum of SiO2were due to Si−O−Si vibrations[32],in the spectrum of SiO2@MIP,the band seen at 1729 cm−1represents the C=O stretch,and the same Si−O−Si vibrations are present,indicating that the molecularly imprinted polymer has been successfully coated onto SiO2nanoparticles[33,34].After etching away the SiO2,the characteristic stretching vibration peaks of SiO2disappeared,while the characteristic stretch of molecularly imprinted polymer at 1729 cm−1was still present,which demonstrated that the SiO2matrix was removed and the MHMIP was formed[31,35].Furthermore,energy-dispersive X-rays(EDX)were used to analyze the chemical composition of MHMIP obtained after treatment with the ethanol solution of HF;this showed a major peak for carbon(shown in FIG.S1 in supplementary materials).Peaks assigned to copper and chromium came from the support grid.No other elements were observed to be present.
In a typical synthetic process,given that all other components remain constant,it was found that the formation of uniform and monodisperse MHMIPs were highly influenced by the volume ratio of toluene to CH3CN,and TEM patterns reflecting this are shown in FIG.3.As shown in FIG.3(a),no MHMIPs were obtained if only CH3CN was used as the solvent.With an increasing amount of toluene in the mixture(toluene:CH3CN=4:3 and 5:2),hollow molecularly imprinted polymers were obtained(see FIG.3(b)and(c)).Unfortunately,at those ratios,the surface of hollow molecularly imprinted polymers was somewhat uneven.Unsurprisingly,the uniform and monodisperse MHMIPs with smooth surfaces were successfully prepared if an even higher amount of toluene was used(FIG.3(d),toluene:CH3CN=5.5:1.5). However,further increasing the amount of toluene in the mixture leads to agglomeration of the synthesized MHMIPs nanospheres(FIG.3(e),toluene:CH3CN=5.5:1.5).This phenomenon is more apparent when only toluene was used as solvent in the synthesis system(FIG.3(f)).
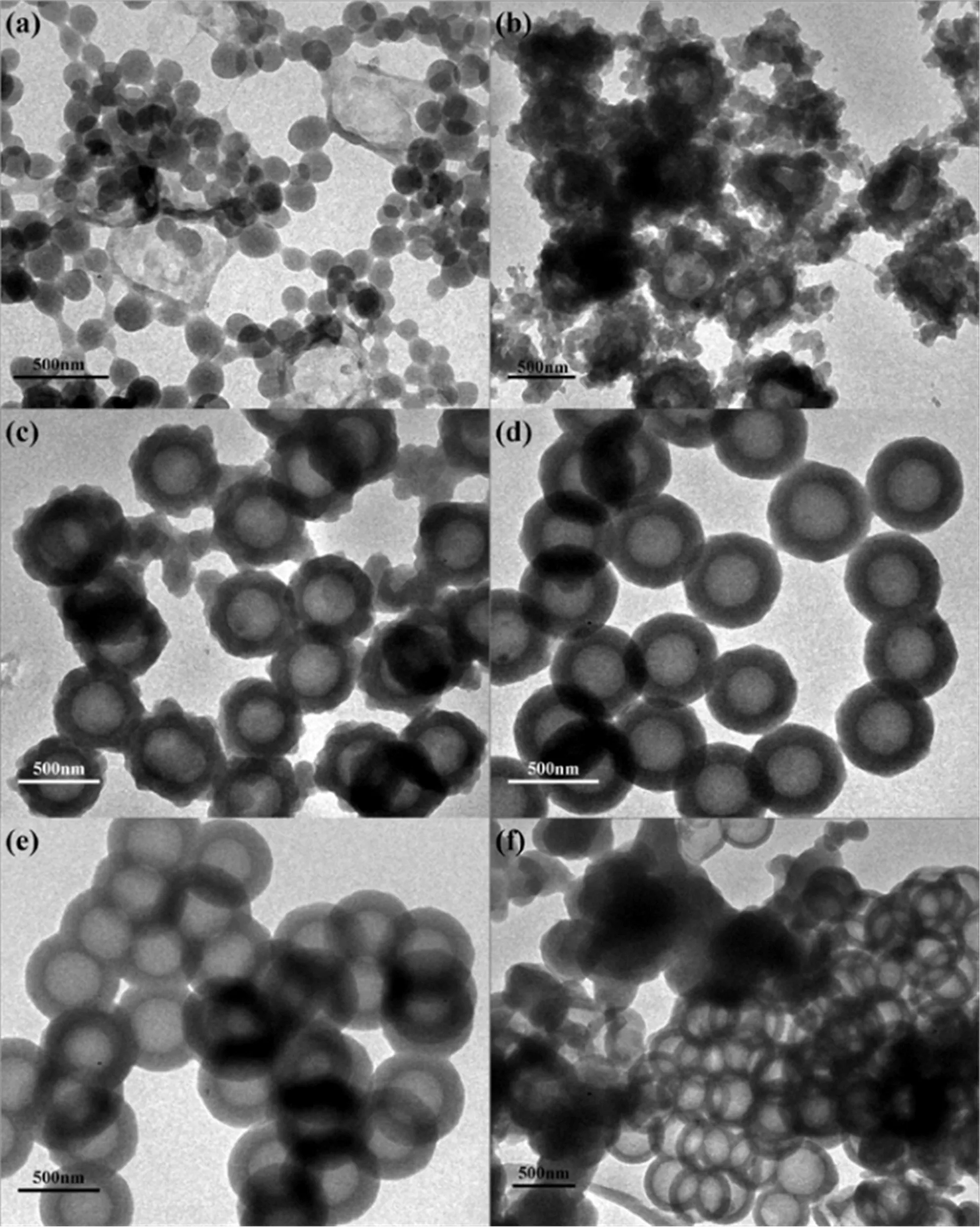
FIG.3 TEM patterns of MHMIP prepared with the volume ratio of toluene and acetonitrile equal to(a)1:0,(b)4:3,(c)5:2,(d)5.5:1.5,(e)6:1,and(f)0:1.
From the above results,we concluded that matching the solubility parameter of the developing polymer network to that of the porogen solvent is particularly important,especially when controlled formation of simultaneously uniform and monodisperse MHMIPs is desirable[36].Toluene is a nonpolar organic solvent,while CH3CN is a polar organic solvent.If toluene is used as the synthesis solvent,the H-bonding interaction of functional monomers(MMA)and template molecules(BPA)with hydroxyl groups of SiO2spheres will be beneficial for the molecularly imprinted polymers deposited onto the surface of SiO2spheres,and even the coalescence of particles[37].This phenomenon is similar to the dispersion polymerization of styrene onto a steric stabilizer in polar solvents as reported by Paineet al.[38],who found that the obtained products are changed from monodisperse to polydisperse with increasing toluene content.Furthermore,the obtained particles are more sterically stabilized in more polar solvents[39].Indeed,in our synthetic system,the final products exhibit a gradual increase of dispersity with an increased concentration of the polar solvent,CH3CN.In summary,the use of mixed toluene and CH3CN as solvent to convert SiO2@MIPs to MHMIPs is preferable,because toluene could promote the effective deposition of molecularly imprinted polymer onto the surface of SiO2spheres,while CH3CN could improve its dispersion.
As Scheme 1 shows,the probable synthetic mechanism is due to the H-bonding interactions that exist between SiO2,MMA and BPA.To identify the interactions of SiO2with functional monomers(MMA)and template molecules(BPA),the UV-Vis absorption spectra of MMA,BPA,SiO2,SiO2+MMA,SiO2+BPA and SiO2+MMA+BPA in a mixed solution of toluene and CH3CN(toluene:CH3CN=5.5:1.5)were collected.This technique is widely used to investigate the hydrogen bond interactions in many fields[40]. As shown in FIG.4,it was found that the absorption peaks of SiO2+MMA and SiO2+BPA are moved towards longer wavelengths(Einstein shift)compared to SiO2,MMA and BPA.Meanwhile,the absorption peak of SiO2+MMA+BPA was also redshifted compared to SiO2+MMA and SiO2+BPA.These shifts were caused by the formation of hydrogen bonds and the electrostatic interactions among MMA,BPA and SiO2,which lead to MMA,BPA and SiO2self-assembling[41].Acrylamide(AM)has an amino group and a carbonyl group and is similar to MMA in that can generate double H-bonding interactions with hydroxyl groups of SiO2and BPA.If AM was used as functional monomer,it might also effectively synthesize MHMIPs.To test this hypothesis,AM was used in place of MMA to synthesize MHMIPs,with all other conditions as described above.FIG.5(a)indicates that MHMIPs were successfully prepared by replacing MMA with AM.On the other hand,4-VP could only generate single H-bonding interactions with hydroxyls of SiO2or BPA,and thus,no MHMIPs could be obtained when 4-VP was used as the functional monomer.These results further verified the synthesis mechanism of MHMIP shown in Scheme 1 and indicated that functional monomers capable of generating double H-bonding interactions with hydroxyls of SiO2and BPA are required to synthesize MHMIP nanospheres in this system.
C.Optimization of ratio of BPA:MMA:EGDMA
As mentioned above,H-bonding interactions exist in SiO2,MMA and BPA.Thus,it is necessary to determine the optimal concentrations of templates,functional monomers and cross-linking agents to obtain molecularly imprinted polymers with improved performance.

FIG.4 The UV-Vis adsorption spectra of MMA,BPA,SiO2,SiO2+MMA,SiO2+BPA and SiO2+MMA+BPA in the mixed solution of toluene and CH3CN.The amount of MMA,BPA and SiO2are 1.5 mmol,0.33 mmol,and 0.5 g,respectively.
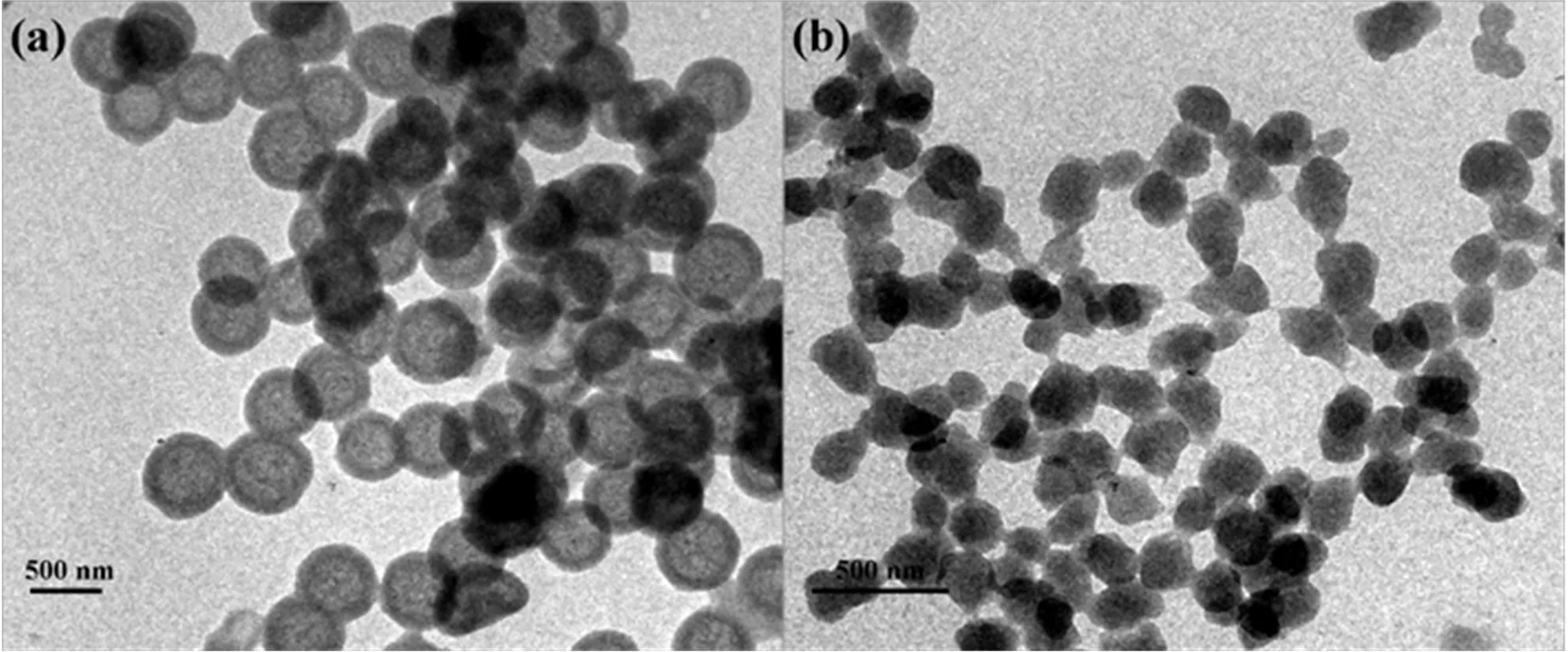
FIG.5 The TEM patterns of molecularly imprinted polymers prepared with(a)AM or(b)4-VP as functional monomers.
ThereforeMHMIPswithBPA:MMA:EGDMA=1:2:20,1:4:20,1:6:20,1:8:20,and 1:10:20 were synthesized.Meanwhile,the corresponding non-imprinted polymers were also synthesized under the same conditions used above with the exception that no template molecules were added. Their adsorption performance for BPA in a toluene solution was evaluated,and the results are given in FIG.6. It was found that when the ratio of functional monomer(MMA)increased,the adsorption capacity of the obtained MHMIPs and MHNIPs showed an increasing trend,but the adsorption selectivity was decreased.By fully comparing the adsorption performance of MHMIPs and MHNIPs prepared with different ratios of template,functional monomers,and cross-linking agents,it was found that the hollow imprinted polymer prepared with BPA:MMA:EGDMA=1:6:20 possessed a relatively large adsorption capacity and a high adsorption selectivity. As follows,the molar ratio of BPA:MMA:EGDMA=1:6:20 was used to synthesize MHMIPs and MHNIPs.
D.The influence of the thickness of MHMIPs on adsorption performance
To investigate the thickness controllability of this method,as well as the relationship between the shell thickness and the maximum bonding capacity of MHMIPs,MHMIPs with different thicknesses were synthesized by adjusting the concentration of imprinted precursor,while the amount of SiO2,volume of the solvent and other conditions were kept constant.The TEM micrographs of MHMIPs with different thickness are shown in FIG.7.When the amount of imprinted precursor used during the polymerization was as low as 1.5(mass ratio to SiO2core),several collapsed particles indicated that the shell layer was not thick enough to support the cavities formed during the selective dissolution of the core(FIG.7(a))[42−44].Monodisperse MHMIPs were obtained when the mass ratio of imprinted precursor to silica core was in the range of 2 to 4(FIG.7(b)−(e));the sizes of the shell layer for the resultant MHMIPs were estimated from TEM characterization to be increasing from 90 nm to 160 nm,as summarized in FIG.7(f).This indicated that the obtained MHMIPs could perfectly maintain their hollow structure after the removal of the silica core.Furthermore,the method reported may be used to conveniently control the shell thickness of MHMIPs.Finally,this synthetic approach would provide a general route to the preparation of other core-shell structured silica/polymer particles,and their corresponding hollow polymers.
To understand the relationship between shell thickness and the adsorption performance of MHMIPs,the maximum binding capacities of MHMIPs with different shell thicknesses were determined and given in FIG.8.As the shell thickness increased from 90 nm to 130 nm,the binding capacity of the imprinted shell decreased gradually,and a very large decrease was observed when the shell thickness of MHMIP was increased to 160 nm.These findings are in line with previous reports[18,31].These findings also indicate that a thin shell will facilitate the complete removal of template molecules and provide the best site accessibility to target molecules,due to its high average density of effective imprinted sites on the imprinted polymer shells.

FIG.7 TEM patterns of MHMIP prepared with the mass ratio of imprint precursor:SiO2equal to(a)1.5:1,(b)2:1,(c)2.5:1,(d)3:1,and(e)4:1,and(f)the relationship between shell thickness of MHMIP and the mass ratio of imprint precursor and SiO2.

FIG.8 The interrelation between the shell thickness and the maximum binding capacities of MHMIPs.
E.Adsorptive property of MHMIPs and MHNIPs

FIG.9(a)Binding kinetics and(b)binding isotherms of MHMIP,MHNIP,SiO2@MIP and SiO2@NIP,BPA with 1 mmol/L was used for kinetics determination.
The BPA-imprinted SiO2@MIP and MHMIP,along with non-imprinted SiO2@NIP and MHNIP,obtained as described above,were tested for their ability to adsorb BPA.The materials described above were placed in a 25-mL conical flask containing 10 mL of a toluene solution of BPA.The amount of BPA adsorbed by the above materials was determined by measuring the residual BPA in solution by HPLC-DAD with a detection wavelength of 276 nm,and these results are shown in FIG.9.As indicated in FIG.9(a),the four materials exhibit fast adsorption rates,needing only 5 min to reach 80%of the adsorption capacity,which is far faster than commonly obtained BPA-MIP[33,34,41,45−47].These phenomena should be ascribed to the high monodispersion and uniform thickness of the shells.Additionally,the equilibrium adsorption performance of the four materials was determined and is shown in FIG.9(b).The adsorption capacities of MHMIP,SiO2@MIP,MHNIP and SiO2@NIP for bisphenol A are 270,210,160,and 120µmol/g,respectively.The BPA-imprinted SiO2@MIP and MHMIP exhibit higher capacities for BPA than the control samples formed without imprinted BPA.Furthermore,MHMIP and MHNIP exhibit higher adsorption capacities than SiO2@MIP and SiO2@NIP,which indicates the superiority of materials with hollow structures.The data from the binding experiments were further processed with the Scatchard equation to evaluate the binding properties of the MHMIP.Two straight lines fitting the Scatchard equation,B/F=(Bmax−B)/kd,can be drawn(shown in FIG.S2 in supplementary materials),and these give two typical dissociation constants.One dissociation constant(1105µmol/L)and a maximum number of highaffinity binding sites(429.3 µmol/g)were calculated.These further demonstrate the speci fic adsorption ability of the obtained MHMIP for BPA.
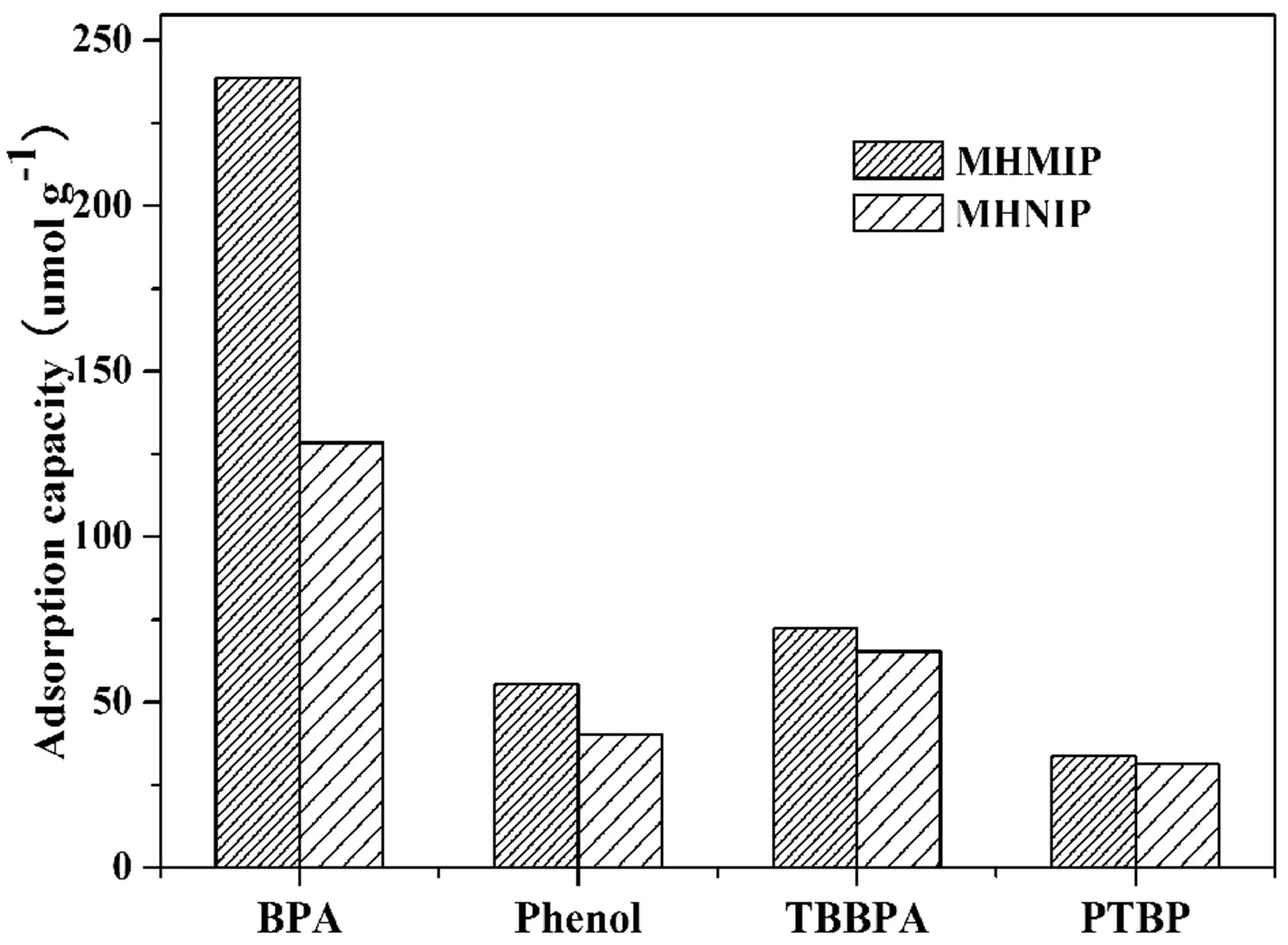
FIG.10 Binding selectivity of MHMIP and MHNIP towards BPA in the mixture solution of TBBPA,phenol,PTBP and BPA.
To determine its further applicability,the binding of the MHMIP for BPA was compared to the binding of MHMIP for three other naturally occurring,structurally related phenolic compounds:tetrabromobisphenol A(TBBPA),phenol,andp-tert-butylphenol(PTBP).The selective adsorption of BPA by MHMIP and MHNIP from a mixture of BPA,TBBPA,phenol and PTBP was determined.As seen in FIG.10,MHMIP has a much higher binding capacity for BPA than for TBBPA,phenol and PTBP.The relative selectivity coefficient(k′)values of MHMIP for TBBPA,phenol and PTBP were calculated to be 1.53,1.83 and 1.90,respectively. These results con firm the strong interactions between the template and the functional monomer,which favors the formation of high-affinity binding sites and improves the selectivity of the polymers.The obtained MHMIP also supplied an attractive sorbent for selective pre-concentration of BPA.
IV.CONCLUSION
In summary,MHMIP has been effectively synthesized by weak hydrogen bonding interactions with unfunctionalized SiO2spheres as carriers in a mixture of toluene and CH3CN,followed by the etching of the SiO2spheres and template molecules simultaneously with an ethanol solution of HF.The obtained MHMIPs possess high uniformity and monodispersion.The main factors that in fluenced the successful preparation of MHMIP were the suitable ratio of toluene to CH3CN and the use of functional monomers which can generate double H-bonding interactions.Under given conditions,the shell thickness of MHMIPs increases accordingly with increasing the mass ratio of imprint precursor to SiO2,while the uniformity and dispersion show almost no change.The obtained MHMIP has an adsorption capacity of 270µmol/g and a fast adsorption rate for BPA(approximately 5 min to reaches 80%of adsorption equilibrium).The results are promising for the enrichment and separation of BPA;these excellent properties make MHMIPs promising candidates in various applications,such as advanced separation technologies.Furthermore,this synthetic approach would provide a general route of the preparation of other core-shell structured silica/polymer particles and corresponding hollow polymers.
Supplementary materials:EDX spectrum of MHMIP and Scatchard plot of MHMIP for BPA are available.
V.ACKNOWLEDGMENTS
This work was supported by the Common Wealth Scientific Foundation for Industry of Chinese Inspection and Quarantine(No.201210071),the Ministry of National Science and Technology of China.
[1]T.Greibrokk,J.SEP.SCI.39815(2016).
[2]K.Graniczkowska,M.Putz,F.M.Hauser,S.De Saeger,and N.V.Beloglazova,Biosens.Bioelectron92,741(2017).
[3]S.A.Zaidi,Drug Deliv.23,2262(2016).
[4]D.J.Liu and M.Ulbricht,RSC Adv.7,11012(2017).
[5]C.H.Lu,W.H.Zhou,B.Han,H.H.Yang,X.Chen,and X.R.Wang,Anal.Chem.79,5457(2007).
[6]Z.D.Zhang,S.J.Zheng,J.Yang,W.M.Wang,B.Z.Liu,and X.L.Zhu,Chin.J.Chem.Phys.26,361(2013).
[7]L.X.Chen,S.F.Xu,and J.H.Li,Chem.Soc.Rev.40,2922(2011).
[8]P.P.Tang,J.B.Cai,and Q.D.Su,Chin.J.Chem.Phys.23,195(2010).
[9]X.Deng,C.Chen,J.Xie,C.Cai,and X.Chen,RSC Adv.6,43223(2016).
[10]M.A.Golse fidia,Z.Eśhaghia,and A.Sarafraz-Yazdi,J.Chromatogr.A1229,24(2012).
[11]Z.Zhang,S.F.Xu,J.H.Li,H.Xiong,H.L.Peng,and L.X.Chen,J.Agric.Food Chem.60,180(2012).
[12]H.Xiang,M.Peng,H.Li,S.Peng,and S.Shi,J.Pharm.Biomed.Anal.133,75(2017).
[13]D.Fan,H.Li,S.Shi,and X.Chen,J.Chromatogr.A1470,27(2016).
[14]S.R.Carter and S.Rismmer,Adv.Funct.Mater.14,553(2014).
[15]Y.Y.Zhao,Y.X.Ma,H.Li,and L.Y.Wang,Anal.Chem.84,386(2012).
[16]C.H.Lu,Y.Wang,Y.Li,H.H.Yang,X.Chen,and X.R.Wang,J.Mater.Chem.19,1077(2009).
[17]K.Qian,G.Z.Fang,and S.Wang,Chem.Commun.47,10118(2011).
[18]D.M.Gao,Z.P.Zhang,M.H.Wu,C.G.Xie,G.J.Guan,and D.P.Wang,J.Am.Chem.Soc.129,7859(2007).
[19]J.X.Liu,H.Chen,Z.Lin,and J.M.Lin,Anal.Chem.82,7380(2010).
[20]M.Q.He,M.J.Meng,J.C.Wan,J.He,and Y.S.Yan,Polym.Bull.68,1039(2012).
[21]W.Luo,L.Zhu,C.Yu,H.Tang,H.Yu,X.Li,and X.Zhang,Anal.Chim.Acta.618,147(2008).
[22]W.H.Zhao,N.Sheng,R.Zhu,F.D.Wei,Z.Cai,M.J.Zhai,S.H.Du,and Q.Hu,J.Hazard.Mater.179,223(2010).
[23]C.Gonzato,M.Courty,P.Pasetto,and K.Haupt,Adv.Funct.Mater.21,3947(2011).
[24]M.M.Titirici and B.Sellergren,Chem.Mater.18,1773(2006).
[25]C.Sulitzky,B.Rückert,A.J.Hall,F.Lanza,K.Unger,and B.Sellergren,Macromolecules35,79(2002).
[26]C.H.Lu,W.H.Zhou,B.Han,H.H.Yang,X.Chen,and X.R.Wang,Anal.Chem.79,5457(2007).
[27]K.Wybrańska,W.Niemiec,K.Szczubiazka,M.Nowakowska,and Y.Morishima,Chem.Mater.22,5392(2010).
[28]X.M.Jiang,W.Tian,C.D.Zhao,H.X.Zhang,and M.C.Liu,Talanta72,119(2007).
[29]H.Zou,S.S.Wu,and J.Shen,Chem.Rev.108,3893(2008).
[30]W.Stober,A.Fink,and E.Bohn,J.Colloid Interf.Sci.26,62(1968).
[31]X.B.Zhang,J.Li,B.You,H.W.Tong,G.P.Yong,and S.M.Liu,RSC Adv.2,9778(2012).
[32]Z.Lin,Z.W.Xia,J.N.Zheng,D.Zheng,L.Zhang,H.H.Yang,and G.N.Chen,J.Mater.Chem.22,17914(2012).
[33]N.Griffete,H.Li,A.Lamouri,C.Redeuilh,K.Chen,C.Z.Dong,S.Nowak,S.Ammar,and C.Mangeney,J.Mater.Chem.22,1807(2012).
[34]H.Zhou,Y.P.Xu,H.W.Tong,Y.X.Liu,F.Han,X.Y.Yan,and S.M.Liu,J.Appl.Polym.Sci.128,3846(2013).
[35]T.K.Mandal,M.S.Fleming,and D.R.Walt,Chem.Mater.12,3481(2000).
[36]J.F.Wang,P.A.G.Cormack,D.C.Sherrington,and E.Khoshdel,Angew.Chem.Int.Ed.42,5336(2003).
[37]J.F.Zhou,C.Ma,S.Zhou,P.L.Ma,F.R.Chen,Y.Qi,and H.X.Chen,J.Chromatogr.A1217,7478(2010).
[38]A.J.Paine,W.Luymes,and J.McNulty,Macromolecules23,3104(1990).
[39]B.Peng,E.Wee,A.Imhof,and A.Blaaderen,Langmuir28,6776(2012).
[40]T.R.Zhang,Q.Zhang,J.P.Ge,J.Goebl,M.W.Sun,Y.S.Yan,Y.S.Liu,C.L.Chang,J.H.Guo,and Y.D.Yin,J.Phys.Chem.C113,3168(2009).
[41]Y.F.Shi,H.L.Lv,X.F.Lu,Y.Y.Huang,Y.Zhang,and W.Xue,J.Mater.Chem.22,3889(2012).
[42]G.Y.Liu,X.L.Yang,and Y.M.Wang,Polymer48,4385(2007).
[43]T.S.Deng and F.Marlow,Chem.Mater.24,536(2012).
[44]G.Y.Liu,L.Y.Li,X.L.Yang,and Z.Dai,Polym.Adv.Technol.19,1922(2008).
[45]M. C. Cela-Pérez, M. M. Castro-López, A.Lasagabáster-Latorre, J. M.López-Vilaríno, M.V.González-Rodríguez,and L.F.Barral-Losada,Anal.Chim.Acta706,275(2011).
[46]W.Rao,R.Cai,Y.Yin,F.Long,and Z.Zhang,Talanta128,170(2014).
[47]Y.M.Ren,W.Q.Ma,J.Ma,Q.Wen,J.Wang,and F.B.Zhao,J.Colloid.Interf.Sci.367,355(2012).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Analysis of Solvent Effect on Mechanical Properties of Poly(ether ether ketone)Using Nano-indentation
- Effects of Praseodymium Doping on Conductivity and Oxygen Permeability of Cobalt-Free Perovskite-Type Oxide BaFeO3−δ
- Study of Cadmium-Doped Zinc Oxide Nanocrystals with Composition and Size Dependent Band Gaps
- Glucose Isomerization into Fructose Catalyzed by MgO/NaY Catalyst
- Selective Synthesis of Different-Sized Gold Nanoclusters through HCl-Etching and-Growth Effect
- Agent-Based Network Modeling Study of Immune Responses in Progression of Ulcerative Colitis
