Selective Synthesis of Different-Sized Gold Nanoclusters through HCl-Etching and-Growth Effect
Ting HuangZhi-hu SunGuo-qiang Pan
National Synchrotron Radiation Laboratory,University of Science and Technology of China,Hefei 230029,China
I.INTRODUCTION
Gold clusters in sub-nanometer size deviating from the sluggishness of bulk state exhibit fantastic properties in optics[1−3],magnetism[4,5], fluorescence and electron emissions[6,7],and catalyst[8−14],due to the discrete electronic structure of the cores.They have been intensively studied for fundamental science and practical applications in electrochemistry,imaging,labeling,sensing,and so on[7,15−19].The properties of gold clusters highly depend on their categories,and the availability of syntheses of gold clusters with various properties is the essential premise for promoting their potential applications.In this regard,numerous efforts have been made to develop effective synthetic routes for preparation of diverse gold clusters.However,the complicated reactions occurring during the synthetic process make it still hard to design synthetic routes for desirable clusters.
The synthetic protocol of gold clusters is typically controlled by the adjustment of reaction ingredients of precursor,reducing agent,and surrounding medium.For instance,the use of a weak reducing agent gives rise to the synthesis of Au19clusters[20];controlling pH values of the reaction solutions leads to the separate syntheses of Au10−12,Au15,Au18,and Au25clusters[21].Recently,numerous post-synthetic routes have been developed to transform or converge the pre-formed clusters or their mixture into relatively monodisperse clusters,through methodssuch as ligand-exchange,growth,etching,and so on. The ligand exchange reaction is illustrated by the synthesis of [Au11(dppp)5)]3+/[Au9(dpph)4]3+(dppp=1,3-bis(diphenylphosphino)propane, dpph=bis(diphenylphosphino)hexane)via the reactions of[Au11(P(4-ClC6H4)3)7(SCN)3]3+/[Au9(PPh3)8](NO3)3(PPh=triphenyl phosphine)with the dppp/dpph ligands[22,23]. Examples of post-synthetic growth include the evolution into [Au13Cl2(PMePh)10]3+,Au11(PPh3)7(4-pyS)3and Au25(PPh3)10(SEt)5Cl2starting from[Au11(PMe2Ph)10]3+,[Au9(PPh3)8]3+,and [Au11(PPh3)8Cl2]Cl, by the addition of AuCl(PMe2Ph), 4-pyridinethiol, and ethanethiol into the solutions,respectively[24−26]. Etchinginduced size conversion has been developed as an important category of synthetic routes for Au clusters.For example,aromatic thiol etching could direct the convergence of Au68-Au102clusters into Au36cluster[27];the mixed Au38to Au102clusters undergo the size focusing into Au38clusters under the etching effect of excess pheneylethylthiol[28].[Au8(PPh3)8]2+and[Au6(dppp)4]2+could also be synthesized via the etching of[Au9(PPh3)8]3+with excess PPh3and dppp respectively[29,30].HCl has also been evidenced to possess the ability to converge the nuclearity and promote the growth of polydisperse diphosphine-stabilized clusters into monodisperse Au13clusters[31]. The etching-and growth-induced size convergence is an effective strategy for cluster synthesis,and deserves further researches.
In this work,we use HCl to control the synthetic route of PPh3-capped gold clusters.Two different synthetic routes toward Au7and Au13clusters are achieved by adding HCl at different reaction time(2 and 48 h,respectively)during the traditional synthetic course of Au11.The results of time-dependent mass spectra and UV-Vis absorption spectra indicate that the HCl directs the etching of Au8-Au9intermediates into Au7clusters,while it promotes the growth of Au11to Au13clusters.The solo-H+and-Cl−sources were also used instead of HCl,and were shown to be valid for the above etching and growth processes,suggesting the HCl-directed convergence mechanism through its H+-etching and Cl–growth effects.
II.EXPERIMENTS
A.Chemicals
Chloroauricacid (HAuCl4,Alfa Aesar),triphenylphosphine(99%,Alfa Aesar),sodium borohydride(98%,Aldrich),hydrochloric acid(HCl,36%,Aldrich).All solvents were obtained from Aldrich and employed as received.AuPPh3Cl was prepared according to the method in Ref.[32].
B.The HCl-,tetraethy lammonium chloride-,and acetic acid-directed synthetic routes of Au7and Au13clusters
NaBH4(0.0562 mmol)was added to the Au precursor(AuPPh3Cl,0.2 mmol)solution in 10 mL of ethanol and allowed to react for 2 h(route I)and 48 h(route II)at room temperature,respectively,which are followed by the addition of HCl(150µL,30 wt%)for another 24 h at 308 K.FIG.1 shows the experimental sequence of the synthesis.For route I,the reaction vessel was warped in tinfoil.The undissolved solids were discarded after the centrifugation at 15000 r/min for 5 min,and the supernatant was dried and re-dissolved in 5 mL of ethanol;this washing procedure was repeated for three times with the Au7yield of 28%on the Au atom basis.For route II,after centrifugation of the reaction solution at 15000 r/min for 5 min,the supernatant was discarded,and the solids were collected.After three times of this washing step,∼10%Au13yield was obtained on the basis of Au atoms.The non-acidic Cl−(tetraethy lammonium chloride)and non-chlorine H+(acetic acid)sources were utilized instead of HCl to repeat the above experiments in the same mole amount with HCl.

FIG.1 Schematic diagram of HCl-directed synthetic routes of Au7and Au13clusters:HCl,introduced to the Au11synthetic reaction at 2 h and 48 h directs the synthesis toward Au7and Au13clusters,respectively.
C.Mass spectrometry and UV-Vis absorption spectrum characterizations
Electrospray ionization mass spectrometry (ESIMS)measurement was performed with a Bruker Microt of mass-spectrometer operating in positive ionization mode at the temperature of 373 K nitrogen drying gas of 1µL/min.The mass spectra were collected in reflectron mode of the TOF mass spectrometer equipped with multi-step detection for obtaining maximum sensitivity;isotopic resolution was obvious throughout the entire detection mass range ofm/zof 650−2000.Calibration was conducted by reserpine(m/z=609)in FIA 10 pg(200µL/min,100:1S/N).The detection of clusters was carried out with 10µL of sample at flow rate of 0.1 mL/min in 0.1%formic acid in acetonitrile/H2O(1:1).Matrix-assisted laser desorption ionization massspectrometry(MALDI-MS)measurements were operated in positive transmission mode with a Bruker Auto flex III mass spectrometer.The UV-Vis absorption data were collected by a TU-9001 spectrometer in the wavelength range of 250−800 nm,following the correction of the background absorption by utilizing pure ethanol.
III.RESULTS AND DISCUSSION
A.Products of HCl-directed synthetic routes
Reduction of AuPPh3Cl ethanol solution by NaBH4is a traditional way toward the synthesis of PPh3-ligated Au11clusters.However,the cluster synthetic route is altered upon introduction of HCl into the Au11synthetic recipe at different time(FIG.1).Adding HCl at 2 h(route I)and 48 h(route II),respectively,directs the selective synthesis of different-sized clusters,deviating from the traditional route of Au11synthesis.Routes I and II give rise to the smaller product of Au7cluster and the larger product of Au13cluster,respectively,as evidenced by the mass spectra and UV-Vis absorption spectra below.

FIG.2(a)ESI mass spectrum of cluster product of HCl-directed route I(top)and MALDI mass spectra of cluster product of traditional route(middle)and HCl-directed route II(bottom);(b)UV-Vis spectra of cluster product of HCl-directed route I,non-controlled route,and HCl-directed route II.The inset in FIG.1(a)shows the experimental and calculated isotope patterns of[Au7(PPh3)7]2+,[Au11(PPh3)8]+and[Au11(PPh3)8]+of the[Au11(PPh3)8Cl2]+and[Au11(PPh3)8Cl4]+fragment.The experimental isotope patterns of[Au11(PPh3)8]+and[Au11(PPh3)8]+are not well-de fined due to the low resolution of MALDI-MS.
The mass spectrum of cluster product in HCl-directed route I is within the scope of our ESI mass spectrometer range and hence it was characterized by ESI-MS;while the mass spectra of cluster product in HCl-directed route II and the traditional route of Au11are out of the ESI-MS range and characterized by MALDI-MS with lower isotope resolution.FIG.2(a)shows the dominated mass peaks atm/z1377,2690−4260 and 4655−5964 Da,assigned to the molecular formulas of[Au7(PPh3)7]2+(calculatedm/z=1378.10 Da),Au11(PPh3)2,Au11(PPh3)8(calculatedm/z=2691.22−4263.36 Da)and Au13(PPh3)8,Au13(PPh3)12(calculatedm/z=4657.29−5697.75 Da).The inset in FIG.2(a)exhibiting the consistent experimental and calculated isotope patterns veri fies the molecular formula of[Au7(PPh3)7]2+.In addition,the UV-Vis absorption spectrum peaks of cluster products gained in HCl-directed route I and II are located at 345 and 413 nm of Au7clusters,and at 340 and 430 nm of Au13clusters,both of which are different from the Au11absorption peaks at 312,380,416,and 500 nm.Therefore,in contrast to the traditional synthesis of Au11cluster,the Au7and Au13clusters were obtained in the HCl-directed route I and II,respectively.
B.The HCl-directed cluster synthetic route I through the etching effect
To further investigate the information of HCldirected routes,we monitored these two reaction processes by time-dependent mass spectra and UV-Vis absorption spectra.The time-dependent ESI-MS and UV-Vis absorption spectra in the case of HCl-directed route I are shown in FIG.3.The mass spectra over the range ofm/z=750−2000 consist of two parts:the precursor-like Au(I)complexes(m/z≈750−1000 Da)and the formed gold clusters(m/z≈1000−2000 Da).The Au(I)complexes at various reaction time are similarly dominated atm/z≈853 and 918 Da,which are defined as[Au3PPh3]+(calculatedm/z=853.19 Da)and[Au2(PPh3)2]+(calculatedm/z=918.52 Da),respectively.The formed gold clusters vary with reaction time.Before the addition of HCl at 2 h,the mass spectrum is of a strongly dominated peak atm/z≈1936 Da as well as other weaker mass peaks atm/z≈1377,1574,and 1609.They are identi fied as[Au9(PPh3)8]2+(calculatedm/z=1936.52 Da),[Au6(PPh3)6]2+(calculatedm/z=1378.10 Da),[Au8(PPh3)6]2+(calculatedm/z=1575.75 Da),[Au7(PPh3)7]2+(calculatedm/z=1608.42 Da),as con firmed by the match between the experimental and calculated isotope patterns(FIG.3(a)and(b)). During the subsequent 22 h reaction period,the mass peak intensity of Au7progressively enhances along with the gradual weakening of Au9mass peak.Additionally,the UV-Vis absorption spectra also experience a time-dependent evolution.The initial shoulder peak at∼345 nm increasingly becomes well-defined;simultaneously,the originally distinct peak at∼413 nm is gradually damped in intensity.These results suggest the gradual etching process of Au9to Au7clusters,indicating the role of HCl as an etching reagent in route I.

FIG.3(a)Time-dependent ESI-MS of cluster evolution in the HCl-directed route I,(b)the experimental and calculated isotope patterns of mass spectra of[Au9(PPh3)8]2+,[Au8(PPh3)6]2+,and[Au6(PPh3)6]2+in FIG.3(a).(c)UV-Vis absorption spectra of cluster evolution in the HCl-directed route I.
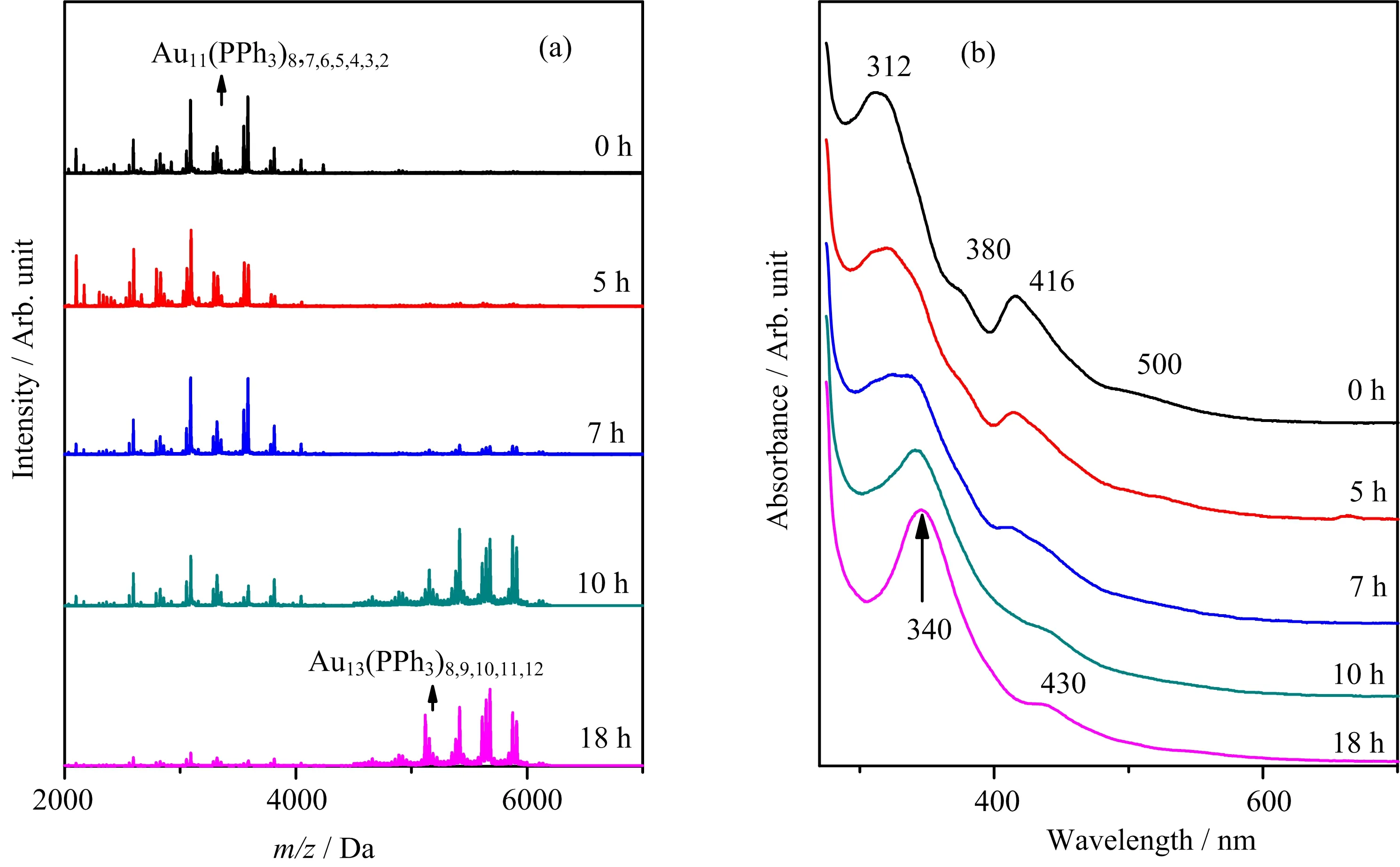
FIG.4(a)Time-dependent ESI-MS and(b)UV-Vis spectra of cluster evolution in HCl-directed route II.
C.The HCl-directed cluster synthetic route II through the growth effect
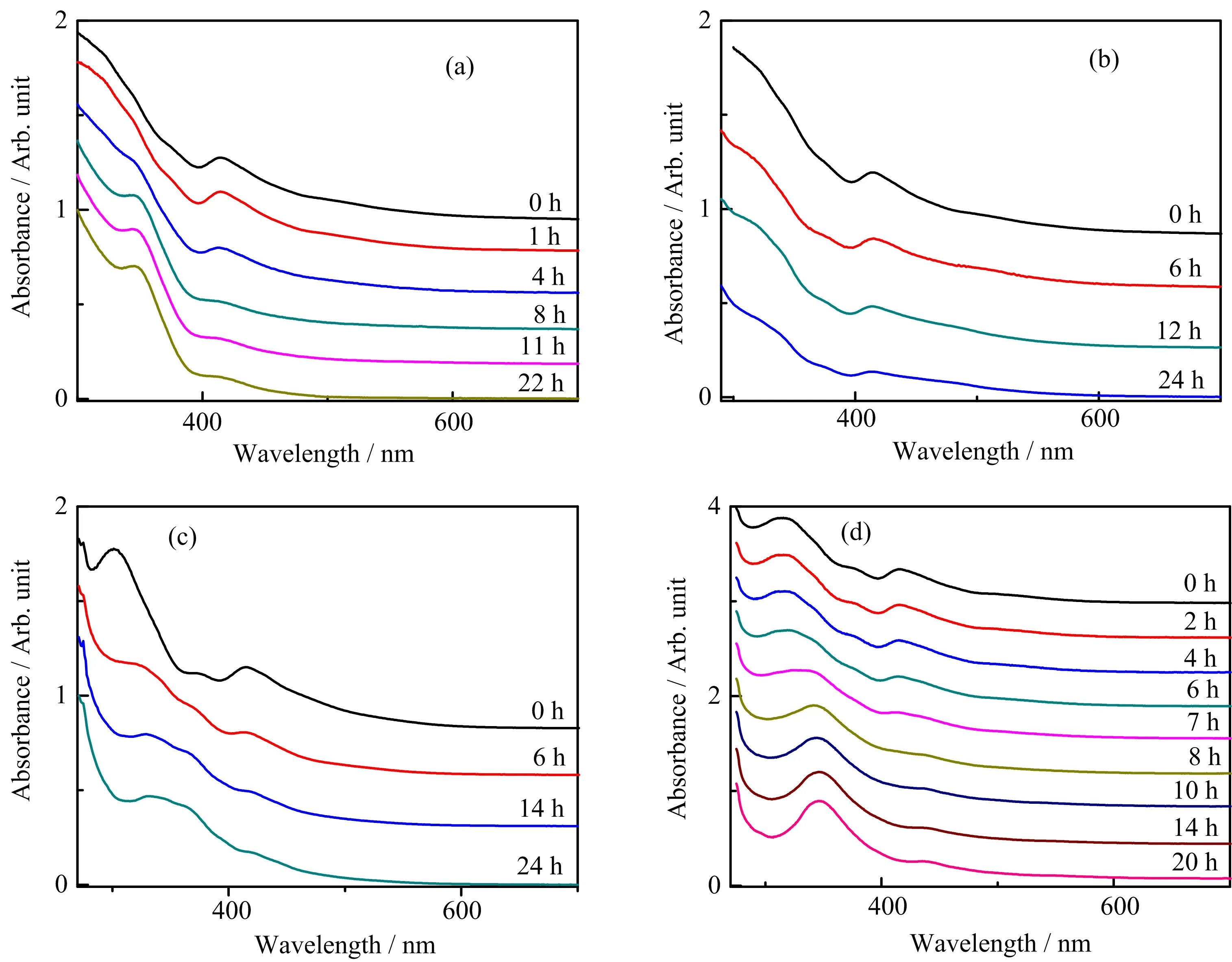
FIG.5 Time-dependent UV-Vis absorption spectra of cluster evolution after the addition of acetic acid(a,c)and tetraethylammonium chloride(b,d)at 2 h/48 h,respectively.
FIG.4 displays the cluster evolution process during the HCl-directed route II.After 48 h reduction of AuPPh3Cl,the main product of Au11clusters is obvious,as shown by the mass spectrum peaks of Au11and its fragments(FIG.4(a))and the well-defined Au11characteristic UV-Vis absorption peaks(FIG.4(b)).The initially prepared Au11is labelled as 0 h in FIG.4.Within addition of HCl for 18 h,the Au11peaks gradually fade away while the Au13mass peaks emerge and become more and more intense;besides,the originally well-de fined Au11characteristic UV-Vis absorption peaks at∼312,380,416,and 500 nm progressively shift toward the Au13characteristic absorption peaks at∼340 and 430 nm.The consistent analyses of mass spectra and absorption spectra both evidence the growth from Au11to Au13clusters,implying the role of HCl in promoting the cluster growth in the HCldirected route II.
D.The HCl-directed etching and growth mechanism
Summarizing the above results,HCl plays distinct roles of etch ant and growth pro motor in directing the Au7and Au13cluster syntheses,respectively.For further exploration of HCl etching and growth-control mechanism,parallel experiments involving only H+or Cl−by using acidic acetic acid or chlorine tetraethylammonium chloride were conducted,respectively,instead of HCl to repeat the above reactions of route I and II.These processes were monitored by time-dependent UV-Vis absorption spectra(FIG.5).
Inspection of FIG.5 shows that acetic acid could result in the similar changes of absorption spectra to that occurring in the HCl-directed etching process from Au9to Au7clusters(FIG.5(a)),while it could not cause Au11grow into Au13clusters as the Au13characteristic absorption peaks are absent(FIG.5(c)).On the contrary,during the 20−24 h period of adding tetraethylammonium chloride,Au9could not be converted into Au7clusters since the absorption spectra at different reaction time show few distinction(FIG.5(b)),but Au11could grow into Au13clusters as the characteristic peaks of Au13emerge eventually(FIG.5(d)).These data indicate that H+and Cl−are,respectively,the effective part for the etching-controlled synthetic route I for Au7clusters and the growth-controlled synthetic route II for Au13clusters.
Based on all the above experiments,we propose a picture depicting the HCl-etching and-growth control for the cluster synthetic routes,as shown in FIG.6.In the case of etching reaction,the interaction of H+with a certain surface Au(I)-PPh3oligomer weakens the connection between Au(I)-PPh3and the residue gold clusters(abbreviated as Aum),and the release of Au-P part is thus facilitated with the production of smaller gold clusters(abbreviated as Aun).In the case of HCldirected growth,due to the excess Cl−in the solution the coordination of Au-Cl is favorably formed,which is easy to attach to the preformed gold clusters(abbrevi-ated as Aux),leading to the formation of larger gold clusters(abbreviated as Auy)[33].

FIG.6 Schematic illustration of HCl-directed etching and growth mechanism:the etching is mainly achieved via the H+action to break the Au(I)-PPh3oligomer of clusters;the growth is mainly achieved via the attachment of Au-Cl species to the clusters.
IV.CONCLUSION
In summary,two synthetic routes of different-sized Au clusters are achieved via the HCl etching and growth effects.Through introducing HCl to the reaction solution of traditional Au11synthesis at different time of 2 and 48 h after the addition of reducing agent(NaBH4),respectively,Au7and Au13clusters could be selectively obtained in a controllable way.Time-dependent mass spectra and UV-Vis absorption spectra reveal the distinct roles of HCl as etchant and growth promotor in directing the Au7and Au13cluster synthesis,respectively.Parallel experiments on independent synthetic routes involving only H+or Cl−illustrate the main role of H+-etching and Cl-assisted growth in HCl-directed cluster synthetic routes.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.11475176,No.U1632263,and No.21533007),and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China(No.11621063).
[1]R.C.Jin,C.J.Zeng,M.Zhou,and Y.X.Chen,Chem.Rev.116,10346(2016).
[2]R.C.Jin,Nanoscale7,1549(2015).
[3]K.Konishi,Gold Clusters,Colloids andNanoparticles I,D.Mingos Ed.,Cham:Springer,49(2014).
[4]C.Wang,Y.G.Yao,and Q.J.Song,J.Mater.Chem.C3,5910(2015).
[5]Y.Negishi,W.Kurashige,Y.Niihori,T.Iwasa,and K.Nobusada,Phys.Chem.Chem.Phys.12,6219(2010).
[6]H.H.Deng,F.F.Wang,X.Q.Shi,H.P.Peng,A.L.Liu,X.H.Xia,and W.Chen,Bios.Bioel.83,1(2016).
[7]J.Sun and Y.D.Jin,J.Mater.Chem.C2,8000(2014).
[8]Y.Zhu,H.F.Qian,B.A.Drake,and R.C.Jin,Angew.Chem.Int.Ed.Engl.122,1317(2010).
[9]Y.Zhu,H.F.Qian,M.Z.Zhu,and R.C.Jin,Adv.Mater.22,1915(2010).
[10]Y.Zhu,Z.K.Wu,C.Gayathri,H.F.Qian,R.R.Gil,and R.C.Jin,J.Catal.271,155(2010).
[11]Y.M.Liu,H.Tsunoyama,T.Akita,and T.Tsukuda,Chem.Commun.46,550(2010).
[12]Y.M.Liu,H.Tsunoyama,T.Akita,S.H.Xie,and T.Tsukuda,ACS Catal.1,2(2011).
[13]G.Ramakrishna,O.Varnavski,J.Kim,D.Lee,and T.Goodson,J.Am.Chem.Soc.130,5032(2008).
[14]M.A.H.Muhammed,A.K.Shaw,S.K.Pal,and T.Pradeep,J.Phys.Chem.C112,14324(2008).
[15]R.C.Jin,Nanoscale2,343(2010).
[16]L.Y.Chen,C.W.Wang,Z.Q.Yuan,and H.T.Chang,Anal.Chem.87,216(2015).
[17]N.Y.Hsu and Y.W.Lin,New J.Chem.40,1155(2016).
[18]W.Chen and S.W.Chen,Angew.Chem.Int.Ed.Engl.48,4386(2009).
[19]D.Lee,R.L.Donkers,G.L.Wang,A.S.Harper,and R.W.Murray,J.Am.Chem.Soc.126,6193(2004).
[20]Z.K.Wu,M.A.MacDonald,J.Chen,P.Zhang,and R.C.Jin,J.Am.Chem.Soc.133,9670(2011).
[21]Y.Yu,X.Chen,Q.F.Yao,Y.Yu,N.Yan,and J.P.Xie,Chem.Mater.25,946(2013).
[22]F.Vollenbroek,J.W.A.van der Velden,J.J.Bour,and J.M.Trooster,Rec.Trav.Chim.Pays Bas100,375(1981).
[23]M.Schulzand M.Jansen,Z.Anorg.Allg.Chem.633,2326(2007).
[24]F.A.Vollenbroek,J.J.Bour,and J.W.A.van der Veden,Recl.Trav.Chim.Pays-Bas.99,137(1980).
[25]C.E.Briant,B.R.Theobald,J.W.White,L.K.Bell,D.M.P.Mingos,and A.J.Welch,J.Chem.Soc.Chem.Commun.201(1981).
[26]F.A.Vollenbroek,J.J.Bour,J.M.Trooster,and J.W.A.van der Velden,J.Chem.Soc.Chem.Commun.907(1978).
[27]P.R.Nimmala and A.Dass,J.Am.Chem.Soc.133,9175(2011).
[28]H.F.Qian,Y.Zhu,and R.C.Jin,ACS Nano3,3795(2009).
[29]J.W.A.van der Velden,J.J.Bour,J.J.Steggerda,P.T.Beurskens,M.Roseboom,and J.H.Noordik,Inorg.Chem.21,4321(1982).
[30]Y.Kamei,Y.Shichibu,and K.Konishi,Angew.Chem.Int.Ed.Engl.50,7442(2011).
[31]Y.Shichibu and K.Konishi,Small6,1216(2010).
[32]L.Malatesta,L.Naldini,G.Simonetta,and F.Cariati,Coord.Chem.Rev.1,255(1966).
[33]L.N.Yang,H.Cheng,Y.Jiang,T.Huang,J.Bao,Z.H.Sun,Z.Jiang,J.Y.Ma,F.F.Sun,Q.H.Liu,T.Yao,H.J.Deng,S.X.Wang,M.Z.Zhu,and S.Q.Wei,Nanoscale7,14452(2015).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Analysis of Solvent Effect on Mechanical Properties of Poly(ether ether ketone)Using Nano-indentation
- Effects of Praseodymium Doping on Conductivity and Oxygen Permeability of Cobalt-Free Perovskite-Type Oxide BaFeO3−δ
- Study of Cadmium-Doped Zinc Oxide Nanocrystals with Composition and Size Dependent Band Gaps
- Glucose Isomerization into Fructose Catalyzed by MgO/NaY Catalyst
- Direct Synthesis of Monodisperse Hollow Molecularly Imprinted Polymers Based on Unfunctionalized SiO2for the Recognition of Bisphenol A
- Agent-Based Network Modeling Study of Immune Responses in Progression of Ulcerative Colitis
