Glucose Isomerization into Fructose Catalyzed by MgO/NaY Catalyst
Bing LiLu-wei LiYing-nn DongQing ZhngWei-zheng WengHui-lin Wn
a.Anhui Laboratory of Molecule-Based Materials(State Key Laboratory Cultivation Base),College of Chemistry and Materials Science,Anhui Normal University,Wuhu 241000,China
b.College of Energy and Power,Shenyang Institute of Engineering,Shenyang 110136,China
c.State Key Laboratory of Physical Chemistry of Solid Surfaces,Xiamen University,Xiamen 361005,China
I.INTRODUCTION
Glucose can perform hydrogenation,oxidation,acetalization reaction,and is widely used in the field of food,fermentation,or fine chemicals[1–5].In addition,glucose can be isomerized to fructose under certain condition,which is a key intermediate for the production of 5-hydroxymethylfurfural(5-HMF)and levulinic acid[6–9].Fructose production on large industrial scale is mainly achieved by enzyme catalysis of glucose,and the process is carried out under very harsh reaction conditions due to the characteristic of enzyme,which causes high cost of fructose production.Meanwhile,the isomerization of glucose to fructose is a thermodynamic equilibrium controlled reaction with the enthalpy of 3 kJ/mol[10],and the reaction is reversible with the equilibrium constant of 1 at 25°C,resulting in the mixed product of 50 wt%glucose and 42−48 wt%fructose[11].Thus,a great deal of attempt has been made to develop chemical techniques for glucose conversion to fructose in the potential substitution of enzyme process by using homogeneous or heterogeneous catalysts including halides,alkaline modi fied zeolites,hydrotalcites,and other solid catalysts in water medium[9,12–15].However,glucose was found to be conversed difficultly in the reaction system with low yield of fructose.Souzaet al.[11]reported that the basic hybrid materials such as[CTA]Si-MCM-48 and[CTA]Si-MCM-50 molecular sieves were active for the isomerization of glucose into fructose and the yield of fructose was obtained to be 17.5%over the[CTA]Si-MCM-48 catalyst in water at 100°C after 2 h reaction.The basic Mg-Al hydrotalcites were found to show high catalytic activity for the reaction and the basic groups in the hydrotalcites favored the formation of fructose.Delidovichet al.[9]con firmed that the basicity of the Mg-Al hydrotalcite catalyst was one of the key factors for the isomerization of glucose into fructose in the aqueous phase and the highest yield of fructose was achieved 30%with the fructose selectivity of 89%over the catalyst with the molar ratio of Al/(Mg+Al)from 0.23 to 0.30.Notably,Sn-Beta zeolites are particularly adopted in the isomerization of glucose into fructose in water and exhibit a similar activity to the enzyme process[17].Nevertheless,Sn-Beta zeolites are restricted to be widely applied in the isomerization of glucose into fructose due to the complicated preparation procedures and long preparation period as well as the use of hydro fluoric acid(HF).
Presently,the organic solvents involving alcohols,DMSO(dimethylsulfoxide),and DMF(dimethylformamide)are employed to afford glucose for fructose formation[18–23].Sodium aluminate(NaAlO2)could catalyze glucose in the mixture of DMSO,PG(propylene glycol)and water,and the best selectivity of fructose was obtained 72%with glucose conversion of 68%at 55°C for 3 h[21].Notably,NaAlO2can be dissolved easily in the reaction solvent system and its recycle is very difficult as well as the harsh requirement of reactor.Saravanamuruganet al.[22]reported that glucose could be efficiently conversed into fructose with near 55%of the selectivity in methanol media by using H-USY zeolite at 120°C for 1 h and found that the reaction pathway was different from those in water,wherein glucose was first transformed to fructose and then subsequent formation of methyl fructoside in methanol,followed by hydrolysis to regenerate fructose with water addition.In addition,some organic amines such as triethylamine,piperazine,or ethylenediamine can catalyze the isomerization of glucose into fructose and the yield of fructose can be achieved 32%by using triethylamine at 100°C for 20 min[24].But it is not negligible that the process requires severe equipments since these organic amines are generally flammable,explosive and toxic with a strong irritant odor.Most recently,Marianouet al.[25]investigated the isomerization of glucose to fructose in aqueous media by using some heterogeneous catalysts such as Sn-or K-exchanged ZSM-5,γ-Al2O3,TiO2or MgO,and found that the formation of fructose was affected by the reaction conditions and the catalysts,wherein the highest fructose yield of 33.4%was achieved for 4%glucose solution over the oxide MgO with the glucose to MgO ratio of 8.Meanwhile,the effect of organic solvent such as DMSO orN,N-dimethylacetamide(DMA)for the glucose isomerization was also discussed,both led to the decrease in the fructose formation.
Here,the MgO/NaY catalysts were developed and used for the isomerization of glucose to fructose in water medium without any organic solvents.The catalysts revealed excellent catalytic performance for the reaction,particularly under low concentration of glucose.NaY zeolite with the Si/Al ratio of 2.91 was activated at different calcination temperature.The effects of MgO loading,reaction temperature,glucose concentration and reaction time on the formation of fructose over the catalyst were studied.X-ray powder diffraction(XRD)and N2physical adsorption(BET)were applied to investigate the structural properties of the catalysts.Moreover,CO2temperature programmed desorption(CO2-TPD)of the catalysts was used to investigate the variety in the basicity amount and distribution of the catalyst and their effect on the selective conversion of glucose to fructose.
II.EXPERIMENTS
A.Catalyst preparation
The catalyst used in this work was prepared by the wetness impregnation method.Prior to the preparation,NaY(Si/Al=2.91)was calcined in batches at 400,500,600 and 700°C in air for 4 h,the resulted samples were noted as NaY-400,NaY-500,NaY-600 and NaY-700,respectively.After the screening of these NaY samples for the isomerization of glucose into fructose,the optimum NaY was selected for the preparation of the MgO/NaY catalyst.The loading of light MgO was in the range of 5%−30%.Brie fly,appropriate amount of NaY zeolite(particle size of 80−100 mesh)and MgO were mixed in deionized water at room temperature for 4 h under the stirring speed of 500 r/min,and then placed in air over night,followed by drying in air at 110°C for 12 h,and then the dried samples were calcined at 600°C for 4 h,the resulted catalyst was denoted as the MgO/NaY catalyst.The Mg content in the catalyst was also measured by the inductively coupled plasma(ICP)method.NaY zeolite was supplied by Nankai University catalyst Co.,Ltd.(Tianjin,China),and light MgO was bought from Shanghai Tongya Co.,LTD(Shanghai,China).
B.Catalytic activity test
The isomerization of glucose into fructose was carried out in a batch-type autoclave reactor with a capacity of 100 mL.Typically,50 mL of glucose solution and 0.1 g of the catalyst were filled in the reactor per run.The reactor was purged in advance with N2for three times so as to remove oxygen contained in the reactor,and then the reaction was performed at certain temperature under the pressure of 1.0 MPa for certain time under the stirring speed of 300 r/min.The products such as glucose,fructose and other species were measured by a Waters Alliance e2695 HPLC instrument equipped with a 410 refractive index detector and a Biorad Aminex HPX-87H column,which was maintained at 50°C with 0.005 mol/L H2SO4as mobile phase flowing at a rate of 0.55 mL/min.Quantification of the products was measured by external standard method according to the peak area of each product.All liquid products before HPLC analysis were diluted 50 times by pure water.The concentration of glucose was in the range of 1.0%−20.0%.
The glucose conversion and the fructose selectivity are calculated by the de finition formulas listed below:
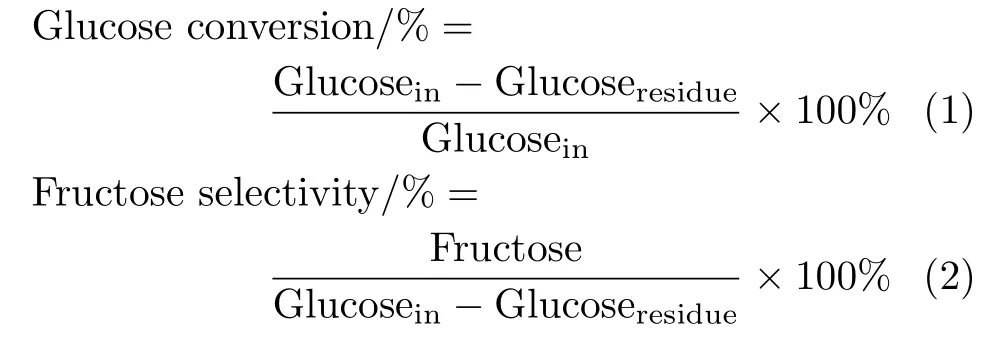
The yield of fructose was equal to the product of glucose conversion and fructose selectivity.
C.Catalyst characterization
X-ray powder diffraction(XRD)patterns were collected on a PANalytical X’pert PRO diffractometer using Cu Kα(k=0.154 nm)radiation at 40 kV and 30 mA.The 2θangles were scanned from 5°to 80°with a step of 0.016°.
N2physical adsorption of the samples was measured on a QUADRASORB SI-MP-10/Pore Master 33 instrument at−196°C using N2as adsorbent.Samples were treated at 200°C for 12 h under vacuum before N2adsorption.Surface area was calculated by BET method.The pore properties of the samples were evaluated by BJH method.
CO2temperature-programmed desorption (CO2-TPD) was performed on an automatic physical/chemical adsorption instrument(Quantachrome,USA).Typically,50 mg of the sample loaded in a quartz reactor was pretreated first with high-purity He at 500°C for 1 h. After the sample was cooled to 50°C,CO2adsorption was performed by switching He flow to a pure CO2gas and then keeping at 120°C for 30 min.Subsequently,the reactor with loaded sample was purged with high purity He to remove the gas phase or the weakly adsorbed CO2at the same temperature.The base strength distribution was measured by desorption of CO2from 50°C to 850°C at a rate of 10°C/min.The amount of desorbed CO2was detected by a thermal conductivity detector(TCD).
III.RESULTS AND DISCUSSION
A.Glucose isomerization
Solid base catalysts were proven to be effective for the isomerization of glucose.NaY zeolite was used as the carrier for the isomerization of glucose to fructose in water at 100°C,and the results showed that the conversion of glucose was affected obviously by the calcination pretreatment of NaY in the temperature range of 400−700°C(FIG.1(a)).A low conversion of glucose was observed over NaY-400 with the value of 17.3%,and the fructose selectivity and yield was 24.6%and 4.3%,respectively.A similar behavior of glucose conversion was apparent over NaY-500 and only a bit higher in the glucose conversion and the yield of fructose than those over NaY-400,indicating that the base amount in NaY calcined at low temperature was inadequate to complete the conversion of glucose.As the calcination temperature of NaY was further elevated to 600°C,higher activity for isomerization of glucose was obtained with the fructose yield of 9.3%,and the selectivity of fructose was 46.8%,which was higher than that over NaY-400 or NaY-500.This is related to the polar electrostatic field presented in the framework of NaY zeolite,and higher calcination temperature facilitates the activation of polar molecules like-CHO group in glucose molecule[26].Generally,the-CHO aldehyde group in glucose molecule can be transformed easily into-CH(OH)2under alkaline conditions and then the corresponding ketose was generated.In addition,the basicity of NaY pretreated at different temperature was calculated from CO2-TPD analysis(FIG.1(b)),and the amount and distribution of the basicity are shown in Table I.The total amount of basic sites of NaY-600 was 0.236 mmol/g,and the amount of weak and strong basic sites was 0.077 and 0.159 mmol/g,respectively.This indicates that strong basic sites are more than weak ones in NaY,and the strong basic sites can promote the isomerization of glucose.Thus,NaY-600 was suitable for acting as the support of the catalyst.For the sample NaY-700,the yield of fructose(7.4%)was less than that of NaY-600.This indicates that much higher calcination temperature to a certain extent would cause the destruction of zeolite structure inevitably and be followed by the decrease in the base amount,which can be validated by the results of CO2-TPD analysis(Table I).
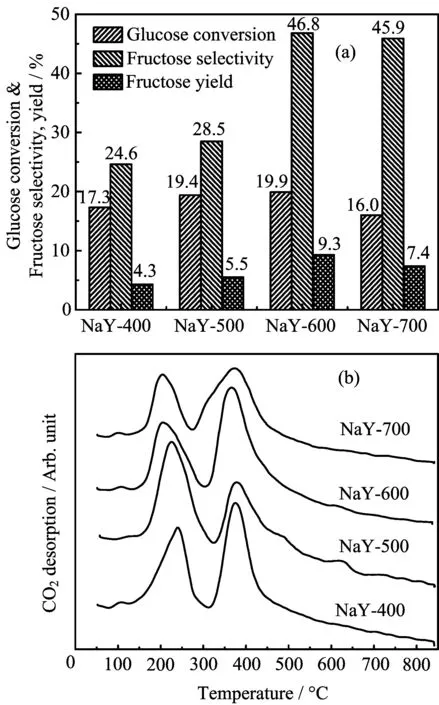
FIG.1 Glucose isomerization to fructose in water over NaY pretreated at different calcination temperature(a)and the basicity of NaY analyzed by CO2-TPD(b).Reaction conditions:5 wt%glucose solution,50 mL,0.1 g NaY,1.0 MPa,100°C,2 h.
Furthermore,light MgO was used to investigate the isomerization of glucose to fructose due to its basicity and insolubility in water.The resulted MgO/NaY catalyst prepared from NaY-600 exhibited good catalytic performance for the isomerization of glucose,which was related closely to the MgO loading(FIG.2(a)).The conversion of glucose was achieved 28.8%over MgO and the yield of fructose was 13.7%,which were both higher than those over NaY.Notably,the conversion of glucose increased clearly as MgO was loaded over NaY,and revealed a monotonously increasing trend with increasing MgO loading in the range of 5%−30%,suggesting that more basic sites can facilitate the isomerization ofglucose.The maximum yield of fructose was obtained 33.8%over the 10%MgO/NaY catalyst with the selectivity of 67.3%,and then dropped as further increase in the MgO addition was up to 30%.This indicates that MgO would be well dispersed over NaY zeolite with MgO addition less than 10%and higher MgO loading would cause the aggregation of MgO and reduce the amount of effective active centers,followed by the decrease in the catalytic performance.To investigate the behavior,XRD was used for the characterization of the catalyst and the patterns are presented in FIG.2(b).The expected characteristic diffraction peaks of NaY were observed over bare NaY,and the strong diffraction peaks at 2θ=6.24°,10.14°,15.66°,and 23.61°were ascribed to(111),(220),(331)and(533),respectively,indicating the highly crystalline structure of NaY zeolite[27].The intensities of these diffraction peaks of NaY were weaken,and the decrease was visible as a function of MgO loading,implying that Mg2+ions may be embodied in the framework of NaY after calcined at 600°C for 4 h.This is also because that NaY zeolites contain the unique three-dimensional channels and a certain amount is of exchangeable cation sites[28].The weak diffraction peaks appearing at 2θ=42.8°and 62.3°were detected over all the MgO/NaY catalysts,which can also be associated with the characteristic diffraction peaks of MgO(PDF No.01-087-0651).Meanwhile,the intensities of these peaks were increased slightly as the MgO loading was enhanced to 10%,and then signi ficantly increased with the increasing MgO loading in the range of 20%−30%.These indicate that MgO particles were small and well dispersed in the NaY structure under the loading less than 10%,while larger MgO particles appeared at the higher loading of MgO.To better understand the effect of MgO loading on the basicity of the catalyst,CO2-TPD technique was also adopted and the CO2desorption curves of the catalysts are shown in FIG.2(c).Based on these curves,the amount and strength distribution of basic surface sites were calculated and the results are summarized in Table I.Two desorption peaks were observed over NaY-600,implying the occurrence of at least two types of basic sites,and the one at low temperature zone(around 240°C)could be attributed to CO2desorption on the weak basic sites,whereas the one at high temperature(round 400°C)was ascribed to CO2desorption on the strong basic sites.This is similar to that in Ref.[29].The amount of
basic sites of the catalyst increased with the addition of MgO,and the value was detected to be 0.282 mmol/g over the 5%MgO/NaY catalyst,which was higher than that over NaY.Meanwhile,the amount of the weak basic sites increased distinctly from 0.077 mmol/g to 0.155 mmol/g.MgO loaded could be embedded in the supercages of NaY zeolite,and thereby generated the corresponding basic sites.Interestingly,the amount and strength distribution of basic sites of the MgO/NaY catalyst varied with different MgO loading,especially strong basic sites.When the MgO loading increased to 10%,the basic sites of the catalyst increased clearly and the total amount of basicity was 0.352 mmol/g,which enhanced approximately 25%,wherein the amount of the strong basic sites reached 0.228 mmol/g.To further comprehend the behavior,the catalyst with the MgO loading of 7.5%or 15%was used in FIG.2(c).Another desorption peak at∼628°C was detected over the 7.5%MgO/NaY catalyst compared to other catalysts,and this peak was shifted to low temperature as the content of MgO was 10%or 15%in the catalyst.Additionally,strong basic sites were formed easily by the increased MgO,and reached the maximum in the MgO loading of 10%and then decreased.This can also infer that MgO dispersed well over the zeolite surface and a strong interaction between MgO and NaY occurred in the zeolite framework.However,further increase in the MgO loading to 20%,an apparent decline was observed in the amount of basic sites with the value of 0.241 mmol/g,wherein both weak and strong basic sites dropped,and the amount and distribution of basic sites were similar to those over NaY-600.Furthermore,there was no difference in the amount of basic sites over the catalysts with the MgO loading of 20%or 30%.This indicates that excessive MgO dispersed unevenly and aggregated over the surface of zeolite associated with the results of XRD analysis,and the super ficial phase MgO would also be reacted with CO2adsorbed into the corresponding carbonate followed by the decrease of the active basic sites.Meanwhile,a small weak desorption peak around 780°C was found over the 20%MgO/NaY or 30%MgO/NaY catalyst,and thus peak can be ascribed to the decomposition of carbonate,implying that MgO particles are aggregated easily on the surface of NaY zeolite with the MgO loading higher than 10%.
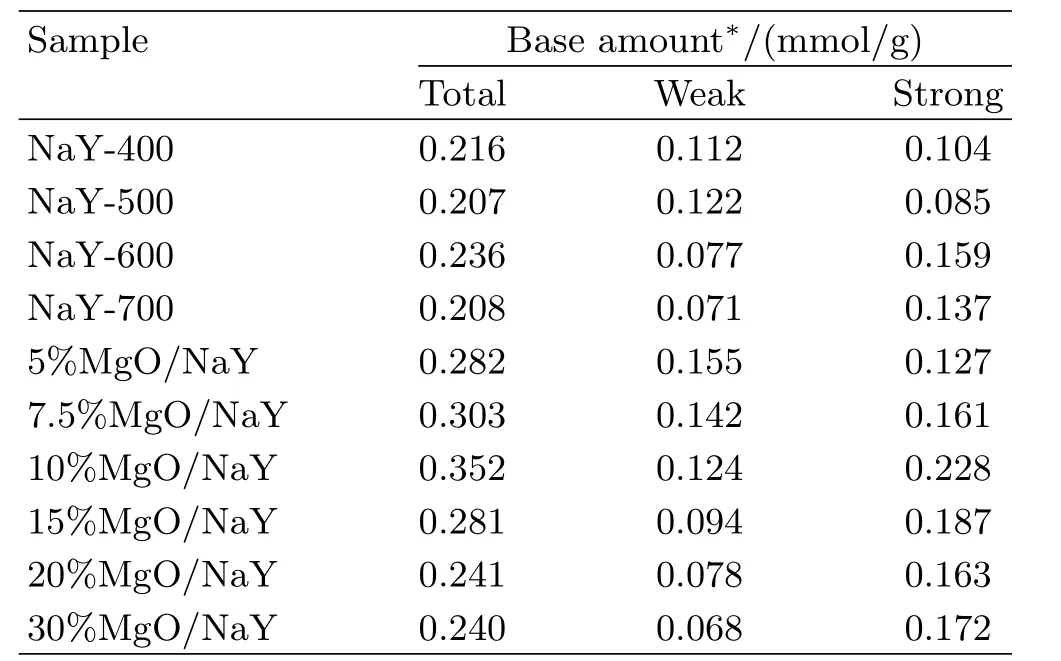
TABLE I The amount and strength distribution of basic sites over various catalysts.

FIG.2 Glucose isomerization to fructose in water over the MgO/NaY catalyst with different MgO loading(a),the structural properties of the catalyst characterized by XRD(b),and the basicity of NaY analyzed by CO2-TPD(c).Reaction conditions:5 wt%glucose solution,50 mL,0.1 g catalyst,1.0 MPa,100°C,2 h.
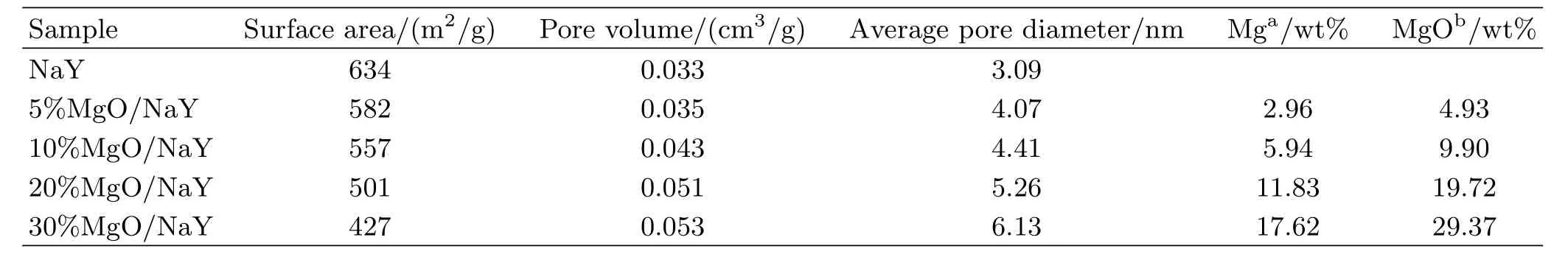
TABLE II Surface areas and pore properties of various catalysts.
Surface areas and pore properties of the catalysts were also crucial for the glucose isomerization,and the results of N2physical adsorption are presented in Table II.It is clearly that surface areas of the catalysts are in fluenced distinctly by the addition of MgO in the range of 5%−30%.The surface area was 634 m2/g over NaY zeolite,and a distinct decrease was detected over the catalysts with the addition of MgO.The 10%MgO/NaY catalyst exhibited a better surface area of 557 m2/g and the pore volume of 0.043 cm3,which could promote the dispersion of MgO over NaY and the basicity of the catalyst.Higher loading of MgO would result in the sharp decrease in the surface area.The surface area was found to be only 427 m2/g when 30%of MgO was added to the catalyst with the average pore diameter of 6.13 nm.This indicates that excessive MgO concentrated on the surface of NaY,which could reduce the basicity of the catalyst associated with the results of CO2-TPD.
B.Reaction parameters optimization
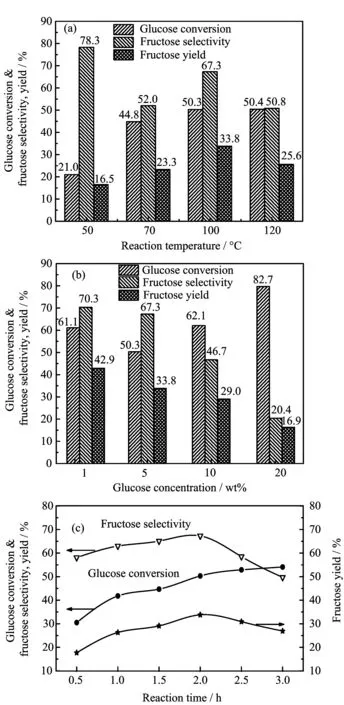
FIG.3 Glucose isomerization to fructose overthe 10%MgO/NaY catalyst under the different reaction conditions.(a)5 wt%glucose solution,50 mL,0.1 g catalyst,1.0 MPa,50−120 °C,2 h;(b)1−20 wt%glucose solution,50 mL,0.1 g catalyst,1.0 MPa,100°C,2 h;(c)5 wt%glucose solution,50 mL,0.1 g catalyst,1.0 MPa,100°C,0.5−3.0 h.
The maximum yield of fructose was achieved to be 33.8%over the heterogeneous base catalyst(10%MgO/NaY)in water,which was much higher than those reported by NaOH or some Lewis acidic catalysts such as CrCl3,AlCl3,or SnCl4[13].Recently,some organic amino acids(e.g.arginine or lysine)or organic aminine(e.g.triethylamine)were adopted as the catalyst to catalyze glucose isomerization,wherein the highest yield of fructose was about 31%[30].Notably,apart from the influence of the catalyst on the performance for glucose isomerization,the different reaction condition also affected the reaction.Effects of reaction temperature,glucose concentration and reaction time on the glucose isomerization were investigated and the results are shown in FIG.3.Seen from FIG.3(a),glucose was found to be transformed easily at high reaction temperature in the range of 50−120°C.At the reaction temperature of 50°C,the catalyst showed 21.0%of glucose conversion and 78.3%of fructose selectivity,and the yield of fructose was only 16.5%,indicating that fructose was dominated in the products under low glucose conversion.The maximum yield of fructose was obtained at the reaction temperature of 100°C with the selectivity of fructose of 67.3%.Interestingly,no obvious change in the conversion of glucose occurred as the temperature was elevated to 120°C,while a visible decrease in the fructose selectivity was observed from 67.3%to 50.8%.Meanwhile,a bit of humin was detected at the same reaction temperature,suggesting the occurrence of coking at higher temperature.As we all know,the-CHO group in glucose molecule is reactive,and the isomerization of glucose into fructose is an equilibrium controlled reaction,and this behavior is affected easily by reaction temperature.However,glucose and fructose are prone to form coke and some other side reactions occur at the temperature higher than 100°C,following by the low yield of fructose.In addition,glucose coking carried out at higher glucose concentration especially above 10%,while more fructose was formed easily at low glucose concentration(FIG.3(b)).Meanwhile,the isomerization of glucose was promoted by prolonging the reaction time(FIG.3(c)).During the initial 0.5 h,a low glucose conversion of 30.5%and the fructose selectivity of 58.1%were observed over the catalyst,and the fructose yield was achieved 17.7%.After that,a visible increase trend in the conversion of glucose was found over the catalyst with the time on stream for 3 h.However,an obvious change in the selectivity of fructose was present during the reaction.The selectivity of fructose was achieved the maximum value of 67.3%after 2 h reaction and thereafter started to drop sharply to 49.7%after 3 h reaction.Simultaneously,the formation of fructose was reduced clearly with the yield decreasing from 33.8%to 26.9%,indicating that the occurrence of fructose subsequent degradation is due to its high reactivity in the alkaline condition.Inevitably,the reversibility of fructose and glucose would lead to the decline in the yield of fructose during the extending of reaction time.
C.Catalyst reusability and regeneration
Heterogeneous catalysts used for glucose isomerization in water inevitably deactivated during the catalytic process. Some polystyrene supported base catalysts such as PS-TBD,PS-DBU or PS-BEMP were found to lose the activity of glucose isomerization after four cycles,and the deactivation of the catalysts could be restrained in the presence of sodium chloride[17].Herein,to discuss the reusability of the 10%MgO/NaY catalyst for glucose isomerization to fructose in water,the catalyst was employed repeatedly for three times and the results are presented in FIG.4(a).The catalytic performance of the catalyst was found to decrease after the reusability test.Glucose conversion and fructose selectivity were 47.4%and 61.5%after the catalyst was used for 2 times,respectively,which were slightly lower than those of the catalyst applied in the first entry.However,glucose conversion was dropped to 38.0%in the third entry,and the yield of fructose was only 17.5%,indicating that the catalyst was not stable in the process.The XRD patterns of the catalysts after reaction are shown in FIG.4(b).There was some difference in the structure of the catalyst after different reaction times.The intensities of the MgO diffraction peaks reduced obvi-ously as the increasing reaction time of the catalyst.After the catalyst was used three times,the diffraction peaks of MgO were very weak,and these peaks occurred again as the spent catalyst was regenerated at 600°C for 4 h(FIG.4(b)),implying that MgO in the catalyst was dissolved inevitably in water to magnesium hydroxide species even though no distinct diffraction was observed under the reaction condition.Meanwhile,the catalytic activity of the regenerated catalyst for glucose isomerization was achieved similar to that from the fresh catalyst(FIG.4(a)).In addition,no obvious change was found in the characteristic diffraction peaks of NaY during the reusability test.These indicate that NaY is stable in the reaction and MgO is one of the key factors for the formation of fructose associated with the test results.Therefore,the deactivated catalyst could be regenerated by simple calcination,and some other measurements would be allowed to improve the stability of the catalyst in the further investigation.

FIG.4(a)Reusability of the 10%MgO/NaY catalyst and(b)the XRD patterns for the catalyst after reaction or regeneration.
IV.CONCLUSION
The MgO/NaY catalyst exhibited high catalytic performance for the isomerization of glucose into fructose in water.There have been obvious effects of MgO loading,reaction temperature and reaction time on the formation of fructose.The conversion of glucose was found to be increased monotonically among the experiments,while the selectivity of fructose was restricted and a maximal value of 67.3%was achieved with the yield of 33.8%over the 10%MgO/NaY catalyst at 100°C for 2 h.Higher MgO loading and reaction temperature would cause the decrease in the yield of fructose as well as extending reaction time due to the high continuous reactivity of fructose.The XRD results showed that the 10%MgO/NaY catalyst would have a good interaction between MgO species and NaY in the zeolite framework,thus promoting well dispersion of MgO.Meanwhile,CO2-TPD results revealed that the catalyst contained the optimum amount and distribution of base for the reaction.These facilitate the generation of fructose from glucose by adjusting the tautomerization between aldehyde and ketone group in the alkaline environment.
V.ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of Anhui Province(No.1708085MB39)and Natural Science Foundation of Liaoning Province(No.20141097)and the National Natural Science Foundation of China(No.21206162)and Open Project of State Key Laboratory of Solid Surface Physical Chemistry,Xiamen University(No.201412)and Research Fund for Doctoral Program of Anhui Normal University(No.2014bsqdjj41).
[1]X.G.Zhang,L.J.Durndell,M.A.Isaacs,C.M.A.Parlett,A.F.Lee,and K.Wilson,ACS Catal.11,7409(2016).
[2]R.R.Ramsubhag and M.W.Peczuh,Arkivoc5,92(2011).
[3]Y.Shu,B.Li,J.Y.Chen,Q.Xu,H.Pang,and X.Y.Hu,ACS Appl.Mater.Inter.3,2360(2018).
[4]S.K.Chaudhuri and D.R.Lovley,Nat.Biotechnol.10,1229(2003).
[5]S.Schimpf,C.Louis,and P.Claus,Appl.Catal.A318,45(2007).
[6]A.Osatiashtiani,A.F.Lee,D.R.Brown,J.A.Melero,G.Morales,and K.Wilson,Catal.Sci.Technol.4,333(2014).
[7]V.Choudhary,S.H.Mushrif,C.Ho,A.Anderko,V.Nikolakis,N.S.Marinkovic,A.I.Frenkel,S.I.Sandler,and D.G.Vlachos,J.Am.Chem.Soc.135,3997(2013).
[8]X.C.Li,K.H.Peng,X.H.Liu,Q.N.Xia,and Y.Q.Wang,ChemCatChem9,2739(2017).
[9]I.Delidovich and R.Palkovits,J.Catal.7,1(2015).
[10]M.Moliner,Y.Román-Leshkov,and M.S.Davis,Proc.Natl.Acad.Sci.USA107,6164(2010).
[11]R.O.L.Souza,D.P.Fabiano,C.Feche,F.Rataboul,D.Cardoso,and N.Essaye,Catal.Today195,114(2012).
[12]V.Choudhary,A.B.Pinar,R.F.Lobo,D.G.Vlachos,and S.I.Sandler,Chem.Sus.Chem.6,2369(2013).
[13]J.Q.Tang,X.W.Guo,L.F.Zhu,and C.W.Hu,ACS Catal.5,5097(2015).
[14]Q.Yang,W.Lan,and T.Runge,ACS Sustainable Chem.Eng.4,4850(2016).
[15]B.Li,L.W.Li,Q.Zhang,W.Z.Weng,and H.L.Wan,Catal.Commun.99,20(2017).
[16]J.Ohyama,Y.T.Zhang,J.Ito,and A.Satsuma,Chem-CatChem9,2864(2017).
[17]J.M.Carraher,C.N.Chelsea,and J.P.Tessonnier,ACS Catal.5,3162(2015).
[18]S.Despax,B.Estrine,N.Hoffmann,and J.Le Bras,Catal.Commun.39,35(2013).
[19]S.Saravanamurugan,M.Paniagua,J.A.Melero,and A.Riisager,J.Am.Chem.Soc.135,5246(2013).
[20]L.Ren,Q.Guo,P.Kumar,M.Orazov,D.Xu,S.M.Alhassan,K.A.Mkhoyan,M.E.Davis,and M.Tsapatsis,Angew.Chem.Int.Ed.54,10848(2015).
[21]G.Q.Zhang,P.Feng,W.P.Zhang,H.Liu,C.X.Wang,H.J.Ma,D.G.Wang,and Z.J.Tian,Microporous Mesoporous Mater.247,158(2017).
[22]X.L.Meng,P.Li,M.M.Du,and P.J.Ji,Ind.Eng.Chem.Res.56,8428(2017).
[23]N.Deshpande,L.Pattanaik,M.R.Whitaker,C.T.Yang,L.C.Lin,and N.A.Brunelli,J.Catal.353,205(2017).
[24]C.Liu,J.M.Carraher,J.L.Swedberg,C.R.Herndon,C.N.Fleitman,and J.P.Tessonnier,ACS Catal.4,4295(2014).
[25]A.A.Marianou,C.M.Michailof,A.Pineda,E.F.Iliopoulou,K.S.Triantafyllidis,and A.A.Lappas,ChemCatChem8,1100(2016).
[26]K.S.Walton,M.B.Abney,and M.D.LeVan,Microporous Mesoporous Mater.91,78(2006).
[27]Y.Huang,K.Wang,D.Dong,D.Li,M.R.Hill,A.J.Hill,and H.Wang,Microporous Mesoporous Mater.127,167(2010).
[28]Y.C.Shi,W.Zhang,H.Zhang,F.Tian,C.Jia,and Y.Chen,Fuel Process.Technol.110,24(2013).
[29]P.Sun,D.H.Yu,K.M.Fu,M.Y.Gu,Y.Wang,H.Huang,and H.J.Ying,Catal.Commun.10,1345(2009).
[30]Q.Yang,M.Sherbahn,and T.Runge,ACS Sustainable Chem.Eng.4,3526(2016).
 CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
CHINESE JOURNAL OF CHEMICAL PHYSICS2018年2期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Investigation on Preparation and Anti-icing Performance of Superhydrophobic Surface on Aluminum Conductor
- Effects of Praseodymium Doping on Conductivity and Oxygen Permeability of Cobalt-Free Perovskite-Type Oxide BaFeO3−δ
- Study of Cadmium-Doped Zinc Oxide Nanocrystals with Composition and Size Dependent Band Gaps
- Analysis of Solvent Effect on Mechanical Properties of Poly(ether ether ketone)Using Nano-indentation
- Direct Synthesis of Monodisperse Hollow Molecularly Imprinted Polymers Based on Unfunctionalized SiO2for the Recognition of Bisphenol A
- Agent-Based Network Modeling Study of Immune Responses in Progression of Ulcerative Colitis
