Preferential Surface Decoration of Supported Co Catalysts with Pt for Aromatic Saturation
Zheng Renyang; Zheng Peng; Dong Xiao; Zheng Aiguo; Li Huifeng; Li Mingfeng
(1. Research Institute of Petroleum Processing (RIPP), SINOPEC, Beijing 100083;2. Innovative Catalysis Program, Key Lab of Organic Optoelectronics and Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing 100084)
1 Introduction
Aromatic saturation is an important reaction both in petroleum processing and petrochemicals industry, which is used in the production of cyclohexane ― one of the key intermediates in the synthesis of Nylon-6 and Nylon-66[1-4]. Specially, the toluene/methylcyclohexane couple is a promising cyclic hydrocarbon combination for the safe storage of hydrogen[5]. The reaction of the toluene/methylcyclohexane couple is not only reversible and highly selective, but also it is free from carcinogenic products[6]. Hydrogenation of aromatics has been extensively studied and implemented in commercial processes,both on metal sul fides (Co/Ni and Mo/W) and on group VIII metals (Pt, Pd, Ru, and Ni)[7]. Despite the fact that aromatics hydrogenation over Pt is one of the most studied catalytic reactions, the Pt utilization has not been improved to a desirable level, and the effect of different Pt utilization on the hydrogenation performance is still not very clear.Combining Pt with other transiton metals to form a bimetallic structure is an effective method to improve the Pt utilization and catalytic perfromance[8-10]. Previous research has demonstrated that the supported Pt-Co bimetallic catalyst exhibits significantly higher catalytic activity for benzene hydrogenation than other Pt-based (e.g.,Pt-Ni)[1]and Co-based (e.g., Pd-Co or Ru-Co)[11]bimetallic and the corresponding monometallic catalysts, which has demonstrted a correlation with the binding energies of benzene and atomic hydrogen on the metallic surfaces based on the density functional theory calculations. The superior hydrogenation activity of Pt-Co catalyst is in line with the fact that bimetallic catalysts often show properties that are distinctly different from those of the parent metals[12]. Many investigations have been performed to correlate the electronic and catalytic properties by com-
These leading works have motivated us to explore the ideal architecture of Pt-Co bimetallic catalysts for aromatics saturation, one of which is composed of 1 atomic layer of Pt on the surface of Co nanoparticles (Figure 1).Previous TEM results revealed that 10% Co/SiO2catalysts synthesized by the impregnation method contained the relatively uniform metal particles, approximately 2 nm in size[16]. Therefore, the required mass ratio of Pt to Co is 2.3 to cover 1 atomic layer of Pt on Co nanoparticle surface composed of 10% of Co on SiO2, making the catalyst very expensive. The first objective of the present work is to explore whether the catalyst structure consisting of trace Pt (0.3%) located on the supported Co nanoparticles would enhance the catalytic performance and Pt utilization ef ficiency. However, Pt atoms would be randomly distributed in the supported Pt-Co bimetallic catalysts synthesized by the conventional co-impregnation method, which could often result in the additional formation of monometallic nanoparticles on the supports[17]. We have solved this problem by using a modified galvanic replacement method. As widely applied in sysnthesis of unsupported bimetallic catalysts,the galvanic replacement is an electrochemical process involving the oxidation of one metal by the ions of another metal having a higher reduction potential[18]. Thus,another objective of the present work is to extend the synthesis strategy via galvanic replacement from the unsupported to the supported bimetallic catalysts, and the challenge lies in how to delve into decorating the Pt atoms preferentially onto the surface of Co instead of the support.

Figure 1 The ideal architecture of Pt-Co catalyst consisting of 1 atomic layer of Pt on the surface of Co nanoparticles calculated by using a half truncated octahedron structure model
2 Experimental
2.1 Catalyst preparation
The Pt-Co bimetallic catalysts were synthesized by three methods:
(1) Co-impregnation (CI): The precursors of Co(NO3)2·6H2O and Pt(NH3)4Cl2·H2O were dissolved in deionized water and impregnated into the SiO2support.After the impregnation, the catalyst was dried at 120 °C for 4 h and then calcined in air at 290 °C for 2 h to produce 0.3%Pt-10%Co/SiO2-CI.
(2) Sequential Impregnation (SI): The catalyst was synthesized by a two-step process involving the impregnation and calcination of Co followed by impregnation of Pt on the support under the same conditions.
(3) Galvanic Replacement (GR): The 0.1%Pt-10%Co/SiO2was synthesized firstly by co-impregnation, and then was reduced with H2, followed by being cooled down to the room temperature and put into an aqueous solution of Pt(NH3)4Cl2·H2O with continuously bubbling of H2.Then the supernatant was removed and the resultant catalyst was dried to yield the surface-decorated bimetallic catalyst 0.3%Pt-10%Co/SiO2-GR. During the preparation process, 0.1% of Pt was applied to promote the reduction of Co, and 0.2% of Pt was decorated preferentially onto the Co surfaces.
The corresponding monometallic Pt and Co catalysts (Pt/SiO2, Co/SiO2) were also synthesized to serve as control samples.
2.2 Catalyst characterization
CO chemisorption by a volumetric static method was measured using a Micromeritics ASAP 2020C instrument.Approximately 0.20 g of the catalyst were reduced by H2at 350 °C (450 °C for monometallic Co/SiO2) for 2 h and then the system was evacuated at 350 °C for 3 h, and at 35 °C for 1 h to desorb any hydrogen from the catalysts.The CO adsorption isotherms were measured to determine the CO uptake at 35 °C.
The transmission electron microscopy (TEM) analysis was performed on the pre-reduced samples using a JEOL ARM 200F equipped with a cold- field emission gun operated at 200 kV and a Probe Cs corrector providing a point resolution of 0.8 Å at the scanning transmission electron microscopy (STEM) mode. The TEM samples were prepared by grinding and suspending the the pre-reduced samples in ethanol, and then a few droplets of this solution were placed onto a carbon-coated copper grid.
The H2-temperature-programmed reduction (H2-TPR) and the H2-temperature-programmed desorption (H2-TPD)experiments were carried out using a Micromeritics AutoChem II 2950 to determine the reduction and H2desorption behaviour of the catalysts. For each experiment, 0.10 g of calcined catalyst, 100—200 mesh in grain size, were put into an U-shaped tubular quartz reactor. Then the catalyst was exposed to a gas mixture consisting of 10.0% of H2in Ar with a temperature ramp from room temperature to 700 °C at a rate of 10 °C/min. A thermal conductivity detector (TCD) was used to detect the amount of hydrogen consumption. Furthermore, to compare the surface compositions of the catalyts, the oxidation resistance experiments via a modified TPR process were performed. The catalysts were reduced, cooled down to room temperature, and exposed to the air prior to being used for the TPR experiment.For H2-TPD experiment, about 0.20 g of the catalyst were reduced by H2at 350 °C (450 °C for monometallic Co/SiO2) for 2 h and cooled down to 50 °C. Then the catalyst was exposed to Ar with a temperature ramp from 50 °C to 400 °C at a rate of 10 °C/min. During this period, the desorption of H2was monitored with the TCD.
The X-ray fluorescence (XRF) spectrometry and the inductively coupled plasma atomic emission spectroscopy(ICP-AES) were conducted using a Rigaku ZSX Primus II spectrometer and a Perkin Elmer Optima 7300V instrument, respectively, to quantify the preferential surface decoration amount of Pt.
2.3 Catalytic evaluation
Flow reactor studies on toluene hydrogenation were carried out in a continuous flow fixed-bed micro-reactor at 110 °C and 1.0 MPa. Prior to the reaction, 0.15 g of the catalyst, diluted with quartz powder, were reduced in situ by H2at 350 °C (450 °C for monometallic Co/SiO2) for 2 h. During the reaction, the model feed consisting of 10%of toluene in heptane, with its amount being controlled by a constant flow pump at a liquid flow rate of 0.2 mL/min,was introduced by H2at a gas flow of 200 mL/min. The molar ratio of H2to toluene was calculated to be 47:1. The products were analyzed by using an online GC equipped with a flame ionization detector (FID).
3 Results and Discussion
3.1 CO chemisorption measurements
The catalyst synthesis methods and CO chemisorption results are listed in Table 1. The bimetallic catalysts synthesized by different methods showed similar CO uptake,which was slightly higher than that of the monometallic Co catalyst. This suggests that the trace Pt has little in fluence on the number of active sites on the surfaces of the reduced catalysts, and the monometallic and bimetallic co-based catalysts would have the similar number of active sites.
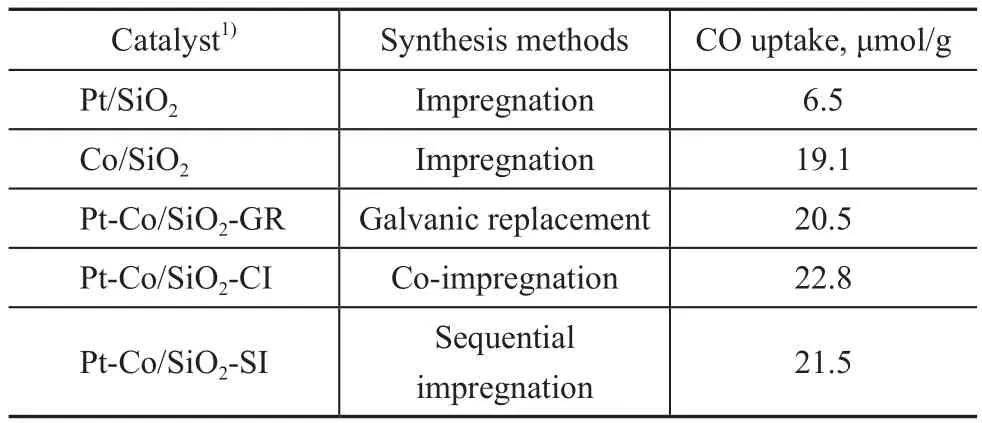
Table 1 Catalyst nomenclature, synthesis methods and CO chemisorption
3.2 TEM analysis
The representative STEM images and particle size distribution of Pt-Co/SiO2-GR are shown in Figure 2. Particle size distribution was obtained from the measurement of more than 200 particles found in several arbitrarily chosen areas of enlarged micrographs. The dominant particle size appeared to cover the diameter ranging from approximately 1 nm to 4 nm, with an average diameter of 2.6 nm.

Figure 2 Representative STEM images and particle size distribution of Pt-Co/SiO2-GR
3.3 H2-TPR
The reducibility of the calcined catalysts characterized by H2-temperature-programmed reduction (H2-TPR) is shown in Figure 3. The H2-TPR profile of Co/SiO2revealed two major peaks, α and β, which were attributed to the successive reduction of Co3+to Co2+, and further to Co0[19]. The bimetallic catalysts could be reduced at a much lower temperature than the monometallic Co catalyst. For example, the reduction temperature of the first step α decreased from approximately 274 °C for monometallic 10%Co/SiO2to 160 °C for Pt-Co/SiO2-CI and to 146 °C for Pt-Co/SiO2-SI, and the reduction temperature of the second step β showed the similar trend. Trace Pt in the bimetallic catalysts appeared to be capable of promoting the reduction of cobalt oxides through hydrogen spillover. As shown in Figure 3, Pt could be reduced at about 160 °C, which was lower than that of Co, therefore hydrogen that dissociated on the Pt surface could migrate to the surface of Co3O4and support, resulting in the reduction of Co which could be implemented at much lower temperatures in the bimetallic catalysts[20]. On the other hand, in order to compare the surface compositions of the catalyts, the oxidation resistance experiments of the reduced catalysts were carried out. The bimetallic catalysts were reduced, cooled down to room temperature and exposed to the air, and then were subject to the TPR treatment, labeled as “reduced-oxidized” in Figure 3. For the three curves of “reduced-oxidized” samples, the reduction peaks at around 260 °C could be attributed to the reduction of CoO,which represented the oxidation state of Co at room temperature. Specially, the reduction peak area for “Pt-Co/SiO2-GR (reduced-oxidized)” is only 30% of that for“Co/SiO2(reduced-oxidized)” and “Pt-Co/SiO2-CI (reduced-oxidized)”, indicating that the surface decoration of Pt can greatly suppress the oxidation of Co at room temperature.

Figure 3 TPR pro files of the calcined and “reducedoxidized” catalysts
3.4 H2-TPD
The H2desorption behaviour of the catalysts is exhibited in Figure 4. Both the monometallic Co/SiO2and Pt/SiO2catalysts showed very different characteristics. The H2-TPD profile of Pt/SiO2indicated an obvious peak positioned at around 76 °C, while that of Co/SiO2showed two H2desorption peaks at 88 °C and 145 °C. The different adsorption characteristics might be stemmed from the weakly adsorbed hydrogen atoms onto the Pt surface as compared to Co which could adsorb hydrogen atoms more strongly[21]. This phenomenon had also been observed on the single crystal surfaces of Co and Pt as evidenced by the experimental and theoretical studies[12,15]. On the other hand, the H2-TPD profiles of bimetallic Pt-Co/SiO2-CI and Pt-Co/SiO2-SI showed the desorption curves which were similar to those of Co/SiO2. Interestingly, H2desorption happened at 80 °C for Pt-Co/SiO2-GR, the characteristics of which were similar to those of Pt/SiO2. This result suggested that Pt-Co/SiO2-GR would provide activated hydrogen at a lower temperature than other bimetallic catalysts (Pt-Co/SiO2-CI and Pt-Co/SiO2-SI). Previous surface science studies and theoretical calculations on model bimetallic Pt-Co catalysts had shown the similar trend and the weakly bonded hydrogen had been attributed to the modification of the electronic properties of Pt by the subsurface Co atoms[22]. It is possible that such electronic effect could also play a role in the Pt-Co/SiO2-GR catalyst, which might lead to the release of the weakly bonded hydrogen to achieve a preferable catalytic performance for toluene hydrogenation.

Figure 4 H2-TPD pro files of the monometallic and bimetallic catalysts
3.5 ICP-AES and XRF Analyses
During the synthesis of Pt-Co/SiO2-GR, the driving force for the galvanic replacement reaction comes from the difference in reduction potentials (1.18 V for the Pt2+/Pt pair and -0.28 V for the Co2+/Co pair)[23]. When the reduced Co/SiO2was added to the Pt2+solution, it was assumed that a galvanic replacement reaction occurred between Pt2+and Co, leading to the preferential deposition of Pt on the surfaces of Co nanoparticles instead of SiO2support. Thereby, we have performed the ICP-AES analysis of the supernatants during the galvanic replacement reaction to verify the above assumption (Table 2). Only a trace amount of Pt2+ions(2.5%) could be removed by washing after the galvanic replacement reaction for Pt-Co/SiO2-GR, but obviously Pt2+ions (82%) could be removed by washing the control samples, which were subjected to the identical synthesis process of galvanic replacement except the process for reduction of Co. This fact indicated that the Pt located onto the catalysts existed in the state of metallic Pt, which could not be removed by washing;while that Pt in the control samples existed in the state of adsorbed Pt2+ions, most of which would be washed away after being dissolved in water. The comparing experiments of ICP-AES revealed that most of Pt was preferentially decorated onto the surface of Co instead of the support. Moreover, the XRF analysis of the solid catalysts suggested that the elemental compostion of Pt-Co/SiO2-GR was identical to that of Pt-Co/SiO2-CI(Table 3).
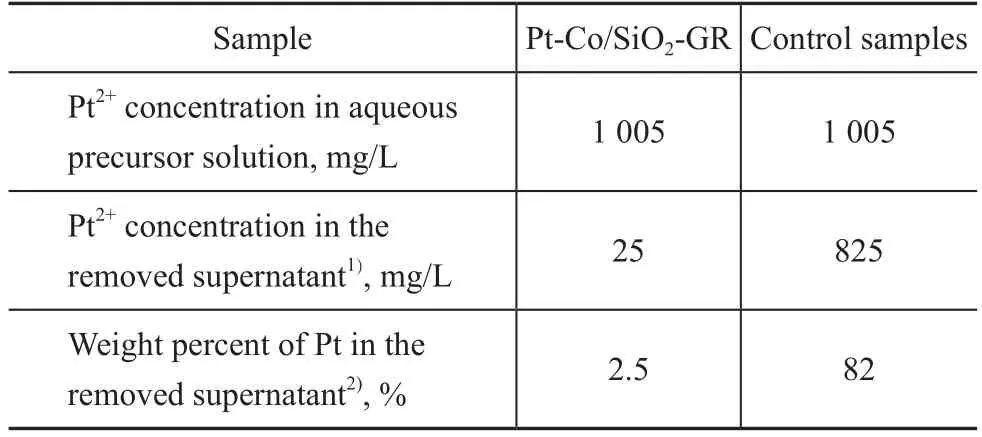
Table 2 Pt concentration during the synthesis procedure of galvanic replacement
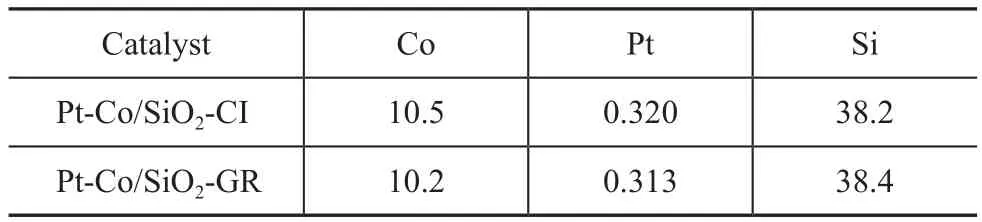
Table 3 Elemental composition of bimetallic catalysts by XRF analysis %
3.6 Catalytic performance
The flow reactor studies of toluene hydrogenation were employed to compare the catalytic performance of samples (Figure 5). Methylclohexane was the only reaction product detected by online gas chromatography. All bimetallic catalysts showed better catalytic performance than their monometallic catalysts, and the conversion rate achieved by catalyst samples decreased in the following order: Pt-Co/SiO2-GR > Pt-Co/SiO2-SI > Pt-Co/SiO2-CI > Pt/SiO2> Co/SiO2. The enhanced hydrogenation activity of the Pt-Co bimetallic catalysts was consistent with the previous catalytic evaluation in 1.2%Pt-10%Co/SiO2synthesized by the co-impreganation method[11]. More interestingly, the synthesis methods could significantly influence the catalytic performance of the bimetallic catalysts. Based on the comprehensive understanding of the performance with their synthesis methods and characterization results, we proposed three types of Pt atoms existing in the bimetallic catalysts: Pt-I located on the Co nanoparticle surfaces, Pt-II located in the Co nanoparticles, and Pt-III located on the support surfaces. Then we were able to summarize the bimetallic architecture based on different Pt atom locations: (i) For the Pt-Co bimetallic catalyst synthesized by co-impregnation, the Pt atoms should be randomly distributed in all the three types of locations; (ii) For the catalyst synthesized by sequential impregnation, the Pt atoms should be situated in the type Pt-I and Pt-III because the Pt2+ions could not diffuse into Co3O4or Co nanoparticles in the second step of impregnation; and (iii) More importantly, the catalyst synthesized via galvanic replacement should have the largest portion of Pt atoms existing in type Pt-I, which must be more similar to the active surface structure discovered by surface science study[9,12-13]and should have the maximum utilization of noble metal Pt. For the bimetallic catalyst synthesized via galvanic replacement, the most active Co atoms (highly coordinatively unsaturated) on the Co nanoparticle surfaces had been replaced by the Pt atoms, which existed as type Pt-I. Compared with Co element, the Pt element could hardly be oxidized in the air, so it would be appropriate to exhibit the oxidation resistance of Co element, as shown in the three “reduced-oxidized” curves in Figure 3. The highly dispersed Pt-I on Co nanoparticles could also lead to the release of the weakly bonded hydrogen as measured by the H2–TPD pro files in Figure 4 for Pt-Co/SiO2-GR catalyst.
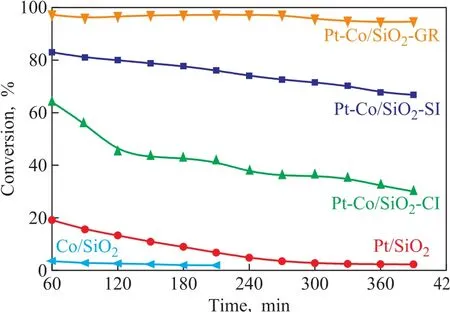
Figure 5 Flow reactor study of toluene hydrogenation reaction conditions: H2: toluene = 47:1, catalyst amount:0.15 g, pressure: 1.0 MPa, temperature: 110 °C
4 Conclusions
We explored three methods, including galvanic replacement, co-impregnation and sequential impregnation, to synthesize the supported Pt-Co bimetallic catalyst for toluene hydrogenation. Results from CO chemisorption showed that the surface decoration of Pt had little in fluence on the number of active sites on the surfaces of the reduced Co-based catalysts. The TEM measurements showed the dominant nanoparticles having an average diameter of 2.6 nm. Pt surface decoration could greatly suppress the oxidation of Co at room temperature as verified by H2-TPR tests and could provide activated hydrogen at a lower temperature by H2-TPD. The ICP-AES characterization of catalyst synthesis process revealed that most of Pt was preferentially decorated onto the surface of Co instead of the support. These characterization results had demonstrated that their properties and catalytic performance were dependent on the Pt atom locations in the bimetallic catalysts.The galvanic replacement applied in the synthesis of the surface decorated Pt-Co bimetallic catalyst was superior to the co-impregnation and sequential impregnation methods for the manufacture of aromatics saturation catalysts. These results could offer a promising and ef ficient way by using the galvanic replacement method to synthesize efficient bimetallic hydrogenation catalysts that could be provided with surface decoration of trace noble metals.
Acknowledgements:This work was supported by the National Science Foundation of China (Grant No. U1663224) and the SINOPEC Research Project (Grant No. 115059 and R16092).
[1] Lu S L, Lonergan W W, Bosco J P, et al. Low temperature hydrogenation of benzene and cyclohexene: A comparative study between γ-Al2O3supported PtCo and PtNi bimetallic catalysts[J]. Journal of Catalysis, 2008, 259(2): 260–268
[2] Pushkarev V V, An K, Alayoglu S, et al. Hydrogenation of benzene and toluene over size controlled Pt/SBA-15 catalysts: Elucidation of the Pt particle size effect on reaction kinetics[J]. Journal of Catalysis, 2012, 292(4): 64–72
[3] Zheng R Y, Xin J, Zhang R Q, et al. In fluence of hydrotreating depth on the properties of LCO[J]. Petroleum Processing and Petrochemicals, 2014, 45(10): 1–7 (in Chinese)
[4] Zheng R Y, Lu S L, Zhu Y X. Effect of metal loadings on Pt-Co/AC bimetallic catalysts for low temperature hydrogenation of benzene[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2014, 30(2): 211–217 (in Chinese)
[5] Alhumaidan F, Cresswell D, Garforth A. Hydrogen storage in liquid organic hydride: Producing hydrogen catalytically from methylcyclohexane[J]. Energy & Fuels, 2011, 25(10):4217–4234
[6] Stanley J N G, Heinroth F, Weber C C, et al. Robust bimetallic Pt–Ru catalysts for the rapid hydrogenation of toluene and tetralin at ambient temperature and pressure[J].Applied Catalysis A: General, 2013, 454: 46–52
[7] Santi D, Rabl S, Calemma, V, et al. Effect of noble metals on the strength of Brönsted acid sites in bifunctional zeolites[J]. ChemCatChem, 2013, 5(6): 1524–1530
[8] Liu L, Lou H, Chen M. Selective hydrogenation of furfural over Pt based and Pd based bimetallic catalysts supported on modified multiwalled carbon nanotubes (MWNT)[J].Appl Catal A, 2018, 550: 1–10
[9] Yu W, Porosoff M D, Chen J G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts[J]. Chemical Reviews, 2012, 112(11): 5780–5817
[10] Zheng R Y, Humbert M P, Zhu Y X, et al. Low-temperature hydrogenation of the C=O bond of propanal over Ni-Pt bimetallic catalysts: From model surfaces to supported catalysts[J]. Catalysis Science & Technology, 2011, 1(4):638–643
[11] Lu S L, Menning C A, Zhu Y X, et al. Correlating benzene hydrogenation activity with binding energies of hydrogen and benzene on Co-based bimetallic catalysts[J]. Chem Phys Chem, 2009, 10(11): 1763–1765
[12] Chen J G, Menning C A, Zellner M B. Monolayer bimetallic surfaces: Experimental and theoretical studies of trends in electronic and chemical properties[J]. Surface Science Reports, 2008, 63(5): 201–254
[13] Chen J G, Qi S T, Humbert M P, et al. Rational design of low-temperature hydrogenation catalysts: Theoretical predictions and experimental verification[J]. Acta Physico-Chimica Sinica, 2010, 26(4): 869–876
[14] Humbert M P, Chen J G. Correlating hydrogenation activity with binding energies of hydrogen and cyclohexene on M/Pt(111) (M = Fe, Co, Ni, Cu) bimetallic surfaces[J].Journal of Catalysis, 2008, 257(2): 297–306
[15] Khan N A, Murillo L E, Chen J G. Observation of novel low-temperature hydrogenation activity on Co/Pt(III) surfaces[J]. Journal of Physical Chemistry B, 2004, 108(40):15748–15754
[16] Zheng R Y, Porosoff M D, Weiner J L, et al. Controlling hydrogenation of C=O and C=C bonds in cinnamaldehyde using silica supported Co-Pt and Cu-Pt bimetallic catalysts[J]. Applied Catalysis A-General, 2012, 419: 126–132[17] Li L, Zhou L, Ould-Chikh S, et al. Controlled surface segregation leads to efficient coke-resistant nickel/platinum bimetallic catalysts for the dry reforming of methane[J].ChemCatChem, 2015, 7(5): 819–829
[18] Wang Z, Zheng A G, Zheng R Y, et al. Selective ring opening of methylcyclopentane over surface-decorated Ir-Co bimetallic catalysts synthesized by galvanic replacement reaction[J]. RSC Adv, 2016, 6(107): 105063–105069
[19] Steen E V, Sewell G S, Makhothe R A, et al. TPR study on the preparation of impregnated Co/SiO2catalysts[J]. Journal of Catalysis, 1996, 162(2): 220–229
[20] Prins R. Hydrogen spillover: Facts and fiction[J]. Chemical Reviews, 2012, 112(5): 2714–2738
[21] Zheng R Y, Zhu Y X, Chen J G G. Promoting low-temperature hydrogenation of C = O bonds of acetone and acetaldehyde by using Co-Pt bimetallic catalysts[J]. Chem-CatChem, 2011, 3(3): 578–581
[22] Khan N A, Murillo L E, Chen J G. Observation of novel low-temperature hydrogenation activity on Co/Pt(111) surfaces[J]. Journal of Physical Chemistry B, 2004, 108(40):15748–15754
[23] Wang S R, He J, Xie J L, et al. Synthesis of bimetallic systems using replacement reactions[J]. Applied Surface Science, 2008, 254(7): 2102–2109
- 中国炼油与石油化工的其它文章
- Fabrication of the Core-Shell Structured ZSM-5@Mg(Al)O and Its Catalytic Application in Propane Dehydrogenation
- Study on Co-re fining of Poplar Powder with Soybean Oil in Supercritical Methanol
- Controllable Synthesis of Mixed-Phase TiO2 with Small Anatase and Rutile Particle and Its Enhanced Photocatalytic Activity
- Synthesis of Core-Shell HZSM-5@SBA-15 Composite and Its Performance in the Conversion of Methanol to Aromatics
- Oxidation of Dibenzothiophene in Model Diesel Using Hydroperoxide Generated via In-Situ Reaction of Octane with Oxygen
- Effects of Gasoline with Ester Additives on the Swelling Behavior of Rubbers

