Study on Co-re fining of Poplar Powder with Soybean Oil in Supercritical Methanol
Hu Jianbo; Du Zexue
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
1 Introduction
The alcoholysis of oils and fats with supercritical methanol can produce fatty acid methyl esters, which can be used as biodiesel[1-3]. The Research Institute of Petroleum Processing has been studying the technique of biodiesel production by using the sub/super critical methanol method for many years, and the technology was commercialized in 2009[4-6]. Bio-crude can be obtained from high-pressure liquefaction of lignocellulosic biomass,the typical case of which is hydrothermal liquefaction[7].However, hydrothermal liquefaction is characteristic of such disadvantages as making the content of water-soluble oil higher than that of water-insoluble oil, and resulting in low calorific value and high acidic value of the products[7-10]. To solve these problems, some researchers have explored the supercritical methanol liquefaction in place of the hydrothermal liquefaction and made some success[11-12].
Through literature analysis, we have found that the alocholysis of fats and oils and the liquefaction of lignocellulosic biomass in supercritical methanol, respectively,require almost the same reaction conditions[11-13]; in other words, the alocholysis of fats and oils and the liquefaction of lignocellulosic biomass can proceed in supercritical methanol in the same time, which we call as Biomass Co-re fining. As we know, the fatty acid methyl esters derived from oils and fats are a kind of excellent industrial solvent[14], and they may have good solubility for longchain molecular compounds that are produced by lignocellulosic biomass liquefaction and decomposition. This hypothesis was verified in the present work.
In the co-re fining process, the fatty acid methyl esters derived from oils and fats are a kind of high quality biofuel or chemical raw materials[15]; whereas the bio-crude originating from the liquefaction of lignocellulosic biomass has so poor performance that it has to be further processed before being used as biofuel or chemical raw material[7-10]. In such
2 Experimental
2.1 Materials
The sample of lignocellulosic biomass was poplar powder used in this study which was obtained from a local wood fiber powder plant. Two kinds of poplar powder were used, namely: the poplar powder 1 (P1) with its ash content at 800 °C equating to 20.5%, and the poplar powder 2 (P2) with its ash content at 800 °C being equal to 3.3%.Particles with a grain size of about 100 meshes were used in the experiments. The powder was dried in an oven at 120 °C for 4 h before use. The blend of fatty acid methyl esters (BFAME) was mainly composed of C18 methyl esters obtained from the Zhenjiang Hengshunda Bioenergy Co. Ltd. The sample of oils and fats was a first-grade soybean oil obtained from the Jinlongyu Plant. Cellulose was obtained from J&K Chemica, and hemicellulose (xylan) was otained from Sigma, while lignin (alkali) was obtained from Aldrich.
2.2 Biomass co-re fining
Based on the experimental design, certain amounts of soybean oil or BFAME, poplar powder and methanol were added into a Parr Instrument Company’s model 4575A autoclave (wth a volume of 500 mL), and the whole autoclave body was weighed as m1. After securely sealed, the autoclave was preheated under continuous stirring (at a revolution rate of 600—800 r/min) over a period of time until the specified temperature was attained. The reaction system remained in the supercritical methanol phase during the reaction period according to the experimental design.After the autoclave was cooled down to room temperature,it was opened and the reaction products were weighed as m2. So the gas yield of the liquefaction product was equal to m1-m2. The solid/liquid products were rinsed completely from the reactor with the reagent-grade acetone. The resulted suspension was filtered under atmosphere pressure through filter paper to recover the solid products (that were methanol and acetone insoluble), consisting of the un-reacted wood sample, coke/char and ash. The remaining solids were dried for 3—4 h in an oven at 105—120 °C before weighing, and the weight of solids was labeled as m3. Then the decomposition rate or conversion rate of poplar powder was (m-m3)/m, in which m was the weight of dry poplar powder added. The filtrate was evaporated under reduced pressure (~5 kPa) at 80 °C to remove the solvents (methanol and acetone). And then the extractive separation method was used to separate the remaining filtrate, which was referred to later in this article.
3 Results and Discussion
3.1 Effect of fatty acid methyl esters and soybean oil on the decomposition of lignocellulosic biomass
3.1.1 Effect of fatty acid methyl esters on the decomposition of poplar powder in supercritical methanol
The poplar powder used in the experiments was P1.The decomposition rate of direct liquefaction of poplar powder in supercritical methanol and that of poplar powder co-refined with BFAME are shown in Table 1.It can be seen from Table 1 that the decomposition rate of poplar powder in biomass co-re fining was higher than that of poplar powder achieved via direct liquefaction in methanol at three selected temperatures, i.e. 280 °C,320 °C and 360 °C, and the average increase in the decomposition rate was close to 10%, which proved that the liquefaction and decomposition of poplar powder were promoted by BFAME certainly.
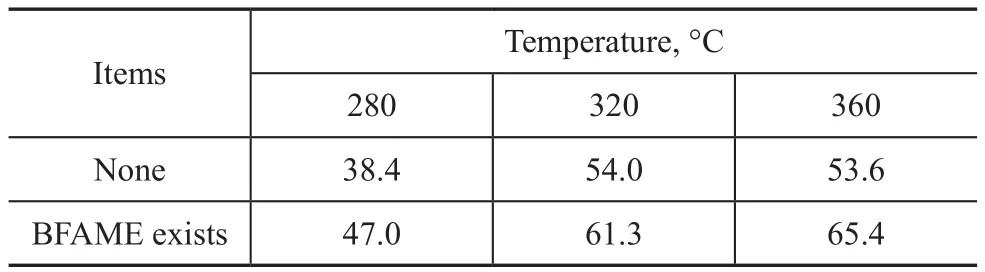
Table 1 Effect of BFAME on the decomposition of poplar powder %
3.1.2 Effect of soybean oil on the decomposition of poplar powder in supercritical methanol
The poplar powder used in the experiments was P1, with the results presented in Table 2. Similar to BFAME,soybean oil could also promote the liquefaction and decomposition of poplar powder in supercritical methanol, and the average increase in the decomposition rate was around 15%, which was higher than that achieved by BFAME, although the reaction conditions were not completely the same. The reasons are still not thoroughly studied, but there were possibly two factors that could affect the difference, namely: 1) the viscosity of soybean oil was higher than that of BFAME, which might improve the dispersion of poplar powder in supercritical methanol and promote the liquefaction and decomposition; 2) glycerol was produced when soybean oil was used[16], which might become a kind of liquefaction solvent and could promote the liquefaction and decomposition reaction.
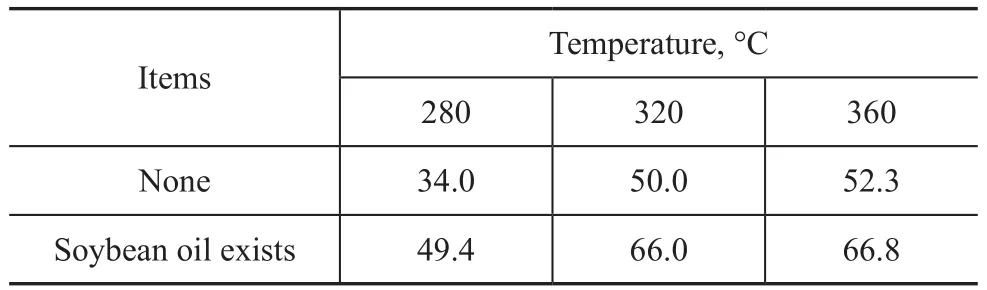
Table 2 Effect of soybean oil on the decomposition of poplar powder %
3.1.3 Effect of methyl palmitate on the decomposition of cellulose, hemicellulose and lignin in supercritical methanol
The main components of poplar powder (lignocellulosic biomass) included cellulose, hemicellulose and lignin[10,17]. The effect of methyl palmitate (reagent-grade) on the decomposition of these three kinds of compounds was studied in this work, with the results shown in Table 3. It can be seen from Table 3 that when the compounds were directly lique fied in supercritical methanol, the decomposition rate decreased in the following order: hemicellulose> cellulose >> lignin. If methyl palmitate was added to the reaction system, the decomposition rate of these three samples mentioned above all improved. The relative decomposition rate of hemicelluloses, cellulose and lignin was improved by 11.2%, 18.9% and 25.0%, respectively.That is obvious to say, for the three kinds of compounds,the more dif ficult the liquefaction, the greater the magnitude of the decomposition rate increase. It was reported[7]that the most dif ficult to be lique fied and decomposed was lignin upon being subject to high-pressure liquefaction.But it can be seen from the results of this study that the decomposition rate of lignin was significantly improved in the biomass co-re fining, which was favorable to further research on the industrial application of biomass liquefaction.
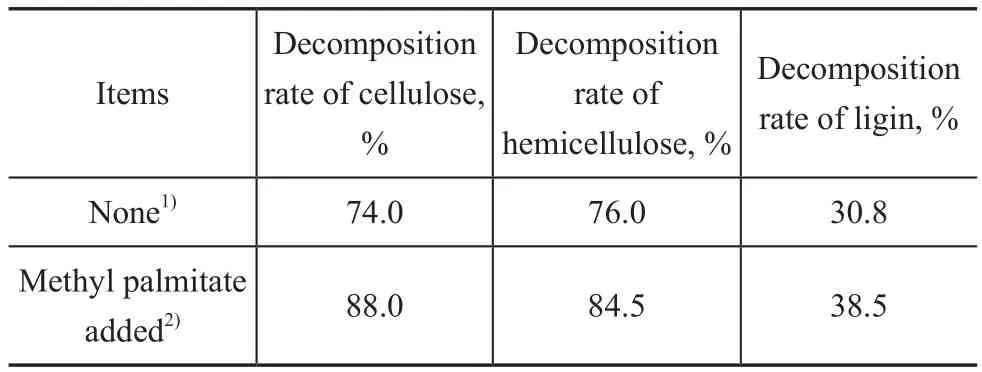
Table 3 Effect of methyl palmitate on the decomposition of cellulose, hemicellulose and lignin
3.2 Extractive separation of the products obtained from biomass co-re fining
The components of poplar powder liquefaction products are complicated, which contain highly active groups such as aldehydes[7,18]having poor thermal stability and poor storage stability[19]and many macromolecules[20], making the subsequent processing of products a gut issue. When poplar powder and soybean oil are co-refined in methanol, there are components of poplar powder liquefaction products and soybean oil derivatives in the products.Compared to the poplar powder liquefaction products,the soybean oil derivatives have good thermal stability and storage stability, so they are easier to be processed.Therefore, in order to facilitate the subsequent process-ing of co-re fining products, it is necessary to separate the poplar powder liquefaction products from the soybean oil derivatives. Because of the poor thermal stability of poplar powder liquefaction products, high-temperature vacuum distillation was not adopted in this work; however,extractive separation method under mild conditions was studied and adopted.
Different solvents and co-re fining liquid products after removing methanol and acetone (1:1 in volume) were added into a test tube, and then the test tube was shaked prior to being subject to settling. The phenomenon was observed and recorded after 24 hours. The results are showed in Table 4. In the experiments, deionized water,heptane, pyridine, ethyl acetate and isobutyl alcohol were used individually to separate the co-re fining products. It was found that when deionized water was used,the upper oil phase and the lower water phase were observed, but there was some amount of oil in water phase which was dif ficult to be separated; when heptane was added, lots of solid appeared, which could be separated by filtration; and when other solvents were added, a homogeneous phase was observed. Obviously, the co-refining products could be separated by heptane. Upon taking into account the cost, petroleum ether in place of heptane was proposed to be used as the extractive separation solvent, which was con firmed by the experiment.The following studies adopted a 60—90 °C fraction of petroleum ether as the solvent, with the experimental procedure described as follows.
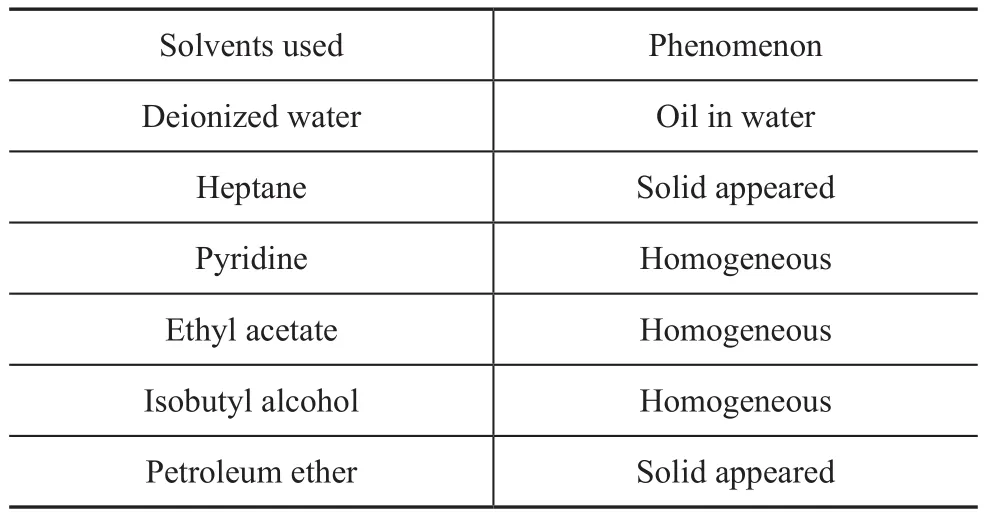
Table 4 Extractive separation of the products from biomass co-re fining by different solvents
50.0 g of P1 and 80.0 g of soybean oil were co-refined in 80.0 g methanol at 320 °C for 1.0 h, and then the liquid and solid products were separated by filtration. After removing solvents by vacuum distillation, the residual liquid was separated into two phases after adding 100 mL of petroleum ether at 20—40 °C. After cooling to room temperature (when needed) and settling for 2 hours, filtration separation was used to separate the two phases.Two products were obtained, viz.: the filtrate and the filter residue. The latter was called the bio-heavy oil, the components of which cosisted of mainly the bio-crude from poplar powder liquefaction and could be used as fuel oil in the furnace, or as the raw material for hydrocracking, hydrodeoxidation, and other applications.[18,21]The petroleum ether in the filtrate was removed by atmospheric and/or vacuum distillation at 80 °C, and the residual product was called the bio-light oil. Bio-light oil and bio-heavy oil weighed as 78.2 g and 20.3 g, respectively. The analysis of products by GC, GC-MS, IR and NMR techniques showed that the main components of the bio-light oil were fatty acid methyl esters derived from soybean oil, and the bio-light oil also contained some other soybean oil derivatives and a small amount of poplar powder liquefaction products. The boiling range of biolight oil complied with that of oils & fats, and so the biolight oil could be used as raw material for hydrodeoxidation to produce liquid fuel[22-23].
3.3 Effects of different oils & fats and fatty acid methyl esters on the composition of bio-light oil
In order to further understand the mechanism of biomass co-re fining, effects of different oils and fats and fatty acid methyl esters on the composition of bio-light oil were studied in this work. The bio-light oils obtained thereby were analyzed by using the GC-simulated distillation method[24], with the results presented in Table 5. It can be seen from Table 5 that the contents of diglycerides (or compounds in the boiling range of diglycerides, the same as methyl esters, monoglycerides and triglycerdes in Table 5 and the same compounds referred to hereinafter) varied greatly when different oils and fats and fatty acid methyl esters were used. As we all know, there are more linoleic acids containing one conjugated double bond in the soybean oil[25-26], the degree of unsaturation of which is the highest among the four chemicals[27-28], and the content of diglycerides in the bio-light oil originating from soybean oil co-re fining is the highest too. Methyl oleate contains one double bond, and the degree of unsaturation assumes the second place; meanwhile, the content of diglycerides in bio-light oil assumes the second place too. Methyl palmitate is a saturated compound, which has a lowest degree of unsaturation, and the content of diglycerides in bio-light oil is the lowest. The 24-degree palm oil mainly contains oleic acid and palmitic acid[29], and the content of diglycerides in bio-light oil is also between that of methyl oleate and methyl palmitate. It can be seen from the analyses mentioned above that the content of diglycerides in bio-light oil is seriously affected by the degree of unsaturation of oils and fats.
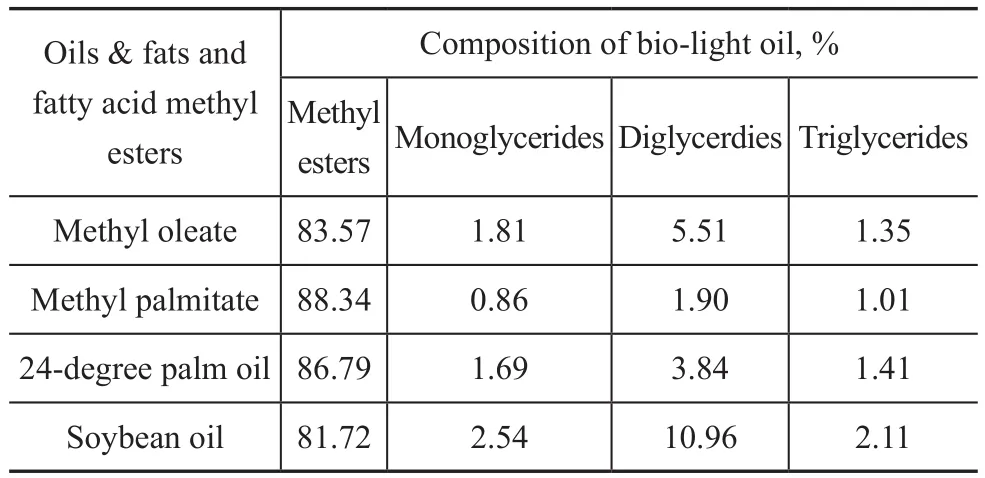
Table 5 Effects of different oils & fats and fatty acid methyl esters on the composition of bio-light oil analyzed by GC-simulated distillation method
The GC-MS analyses were also performed for the biolight oil, and the GC-MS data of bio-light oil from soybean oil or methyl palmitate co-refined with P2 are showed in Figure 1. Because no glycerol exists in methyl palmitate, no monoglyceride peaks appeared in the figure of bio-light oil derived from methyl palmitate co-refining, and also diglycerides and triglycerides did not exist.However, the monoglyceride peaks appeared obviously for the bio-light oil derived from soybean oil co-re fining because it contained associated glycerol[30]. In addition,the diglyceride peaks also appeared in the product derived from soybean oil co-refining. The peaks of diglycerides were very wide, denoting that they were overlapping peaks of several compounds and so there were some different compounds with similar boiling range of diglyceride, but we could not distinguish the different peaks in our experiment.
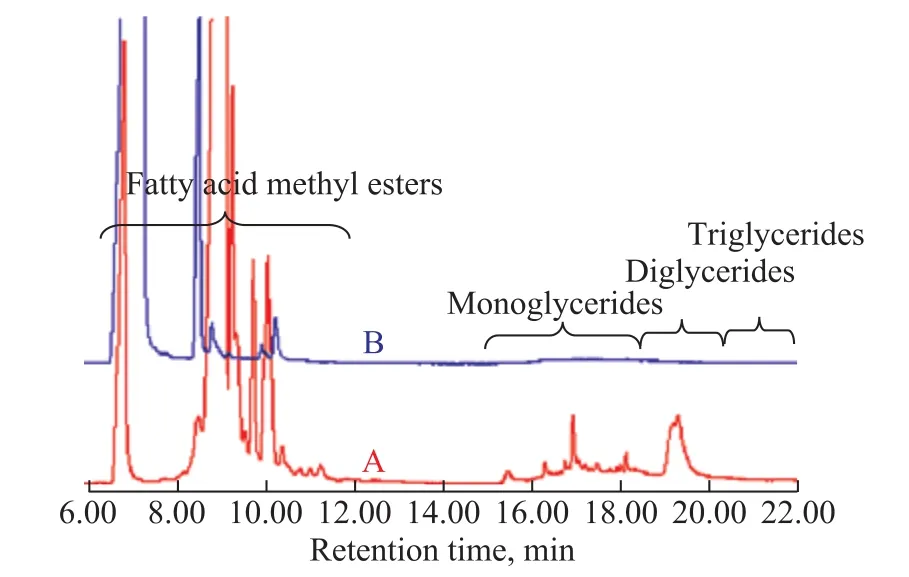
Figure 1 GC-MS figure of bio-light oil originating from soybean oil or methyl palmitate co-re fined with P2
Reaction conditions: 40.0 g of P2, 100.0 g of methanol, and 80.0 g of soybean oil (A) or methyl palmitate (B) took part in the reaction at a temperature of 320 °C for 1.0 h.
3.4 Re-alcoholysis of bio-light oil
The conjugated double bond-rich soybean oil has high addition-reaction activity. In addition, there are many highly active groups in the products of poplar powder liquefaction[7,18]. These highly active groups can be condensed with double bonds at high temperature to produce new compounds. Judging from the above-mentioned research, many diglycerides were found in the bio-light oil, but the GC-MS analysis could not con firm whether they were “diglycerides” indeed . Therefore, we had designed three experiments to test them. The biolight oil was subject to re-alcoholysis in methanol under specific conditions, and the products were analyzed and normalized by GC[31]. The composition changes are compared and showed in Table 6.
It can be seen from Table 6 that the content of methyl esters decreased in all experiments and the higher the re-alcoholysis temperature, the more the methyl esters content would decrease. The content of monoglycerides decreased too, whereas that of diglycerides and triglycerides increased. These results illustrated that the side reactions happened to make methyl esters and monoglycerides become larger molecules during the bio-light oil re-alcoholysis. A small amount of relatively small molecules formed during poplar powder liquefaction existed in biolight oil as con firmed by the NMR analyses, and the molecules could react with soybean oil derivatives to form larger molecular compounds at high temperature.
Generally speaking, the contents of pure monoglycerides,diglycerides and triglycerides will all decrease in biodiesel re-alcoholysis. In other words, most so-called monoglycerides, diglycerides and triglycerides in biolight oil are not pure monoglycerides, diglycerides and triglycerides, and they are the compounds resulted from condensation of soybean oil derivatives with poplar powder liquefaction products. This fact proves that double bonds in oils and fats and their derivatives will react with highly active groups contained in the lignocellulosic biomass liquefaction products, and in order to reduce this kind of reaction, highly saturated oils and fats are needed in biomass co-re fining.
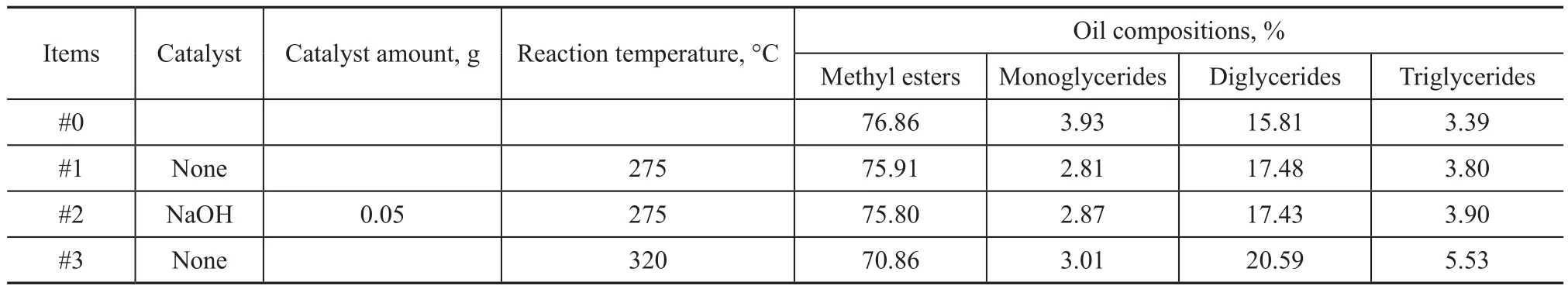
Table 6 Compositions of the products from re-alcoholysis of bio-light oil analyzed by GC normalization
3.5 Process flow and material balance of biomass core fining
In order to better understand the biomass co-re fining, the process flow and the material balance of biomass co-re fining (with poplar powder P2 being used) are introduced as follows. The process flow diagram and material balance of biomass co-re fining is showed in Chart 1. Two hypotheses are proposed in this article for biomass co-re fining:1) Gases are generated from the decomposition of poplar powder; 2) No loss in subsequent processing is detected.The decomposition rate of P2 is 82.2%, which is obtained through calculation from Chart 1, and the gasification rate is 31.7%, while the liquefaction rate is 50.5%. As the ash content of P2 is 3.3%, the actual decomposition rate,the gasification rate and the liquefaction rate are 85.0%,32.8% and 52.2%, respectively. After the removal of methanol and the light components produced by liquefaction of poplar powder, the liquid products from co-re fining are separated by extraction with petroleum. 14.2 g of bio-heavy oil and 87.0 g of bio-light oil were produced eventually. According to SH/T 0246—1992 (a standard method of SINOPEC for testing trace water content in oils), the mass fraction of water in bio-light oil was about 2.9%, which was equivalent to about 2.5 g of water in bio-light oil. Therefore, the actual weight of oil was 84.5 g. According to the result of GC-simulated distillation method, the mass fraction of light components, the boiling point of which was below the boiling point of fatty acid methyl esters, was about 4.0%, and the mass fraction of methyl esters was 75.1%, while the mass fraction of monoglycerides, diglycerides and triglycerides reached 16.8%. The total mass fraction was equal to 95.9%.
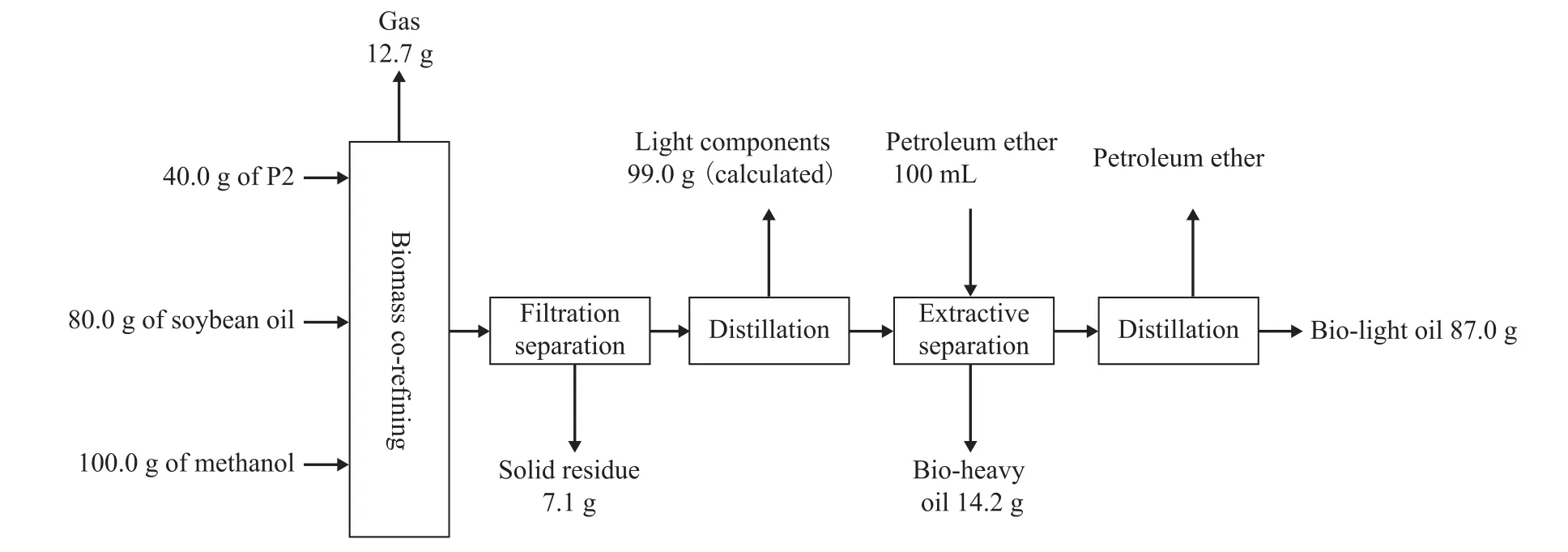
Chart 1 Process flow diagram and material balance of biomass co-re fining
The process flow diagram and material balance of poplar powder (P2) directly lique fied in supercritical methanol is showed in Chart 2. In this process, 20.0 g of P2 and 90.0 g of methanol were subject to reaction at a temperature of 320 °C over a reaction duration of 1.0 h. After cooling down to room temperature, the solid/liquid products were rinsed completely from the reactor with the reagent-grade acetone. The resulted suspension was filtered under atmospheric pressure through filter paper to recover the filtrate, and then 40.0 g of BFAME was added into the filtrate. Subsequent treatment was just the same as biomass co-refining. Through calculation based on the data in Chart 2, the decomposition rate,the gasification rate and the liquefaction rate of P2 were 70.0%, 31.0% and 39.0, respectively. But as the ash content of P2 was equal to 3.3%, the actual decomposition rate, gasification rate and liquefaction rate were 72.4%,32.0% and 40.4%, respectively.
It can be calculated from Chart 2 and Chart 3 that the decomposition rate of biomass co-re fining increased by 12.6% as compared with that of direct liquefaction of P2 in supercritical methanol, and the gasification rate showed the similar trend, denoting that the liquefaction rate increased by 11.8%. Judging from the reaction conditions adopted in the two experiments, the reaction temperature, reaction time and solid-liquid ratio were the same. Therefore, it can be seen that the liquefaction rate was significantly improved in biomass co-refining as compared with the case of direct liquefaction, and the amount of methanol used was obviously cut down for biomass co-re fining.
In Chart 2, the yield of bio-light oil was 42.6 g. According to the test method SH/T 0246—1992, the mass fraction of water in bio-light oil was about 4.1%, denoting that about 1.7 g of water were contained in bio-light oil, so the actual weight of oil was 40.9 g. Since it was assumed that the purity of BFAME was 100%, so the yield of oil from P2 direct liquefaction was 0.9 g, and the yield rate based on dry P2 was 4.5%.
It can be also seen from Chart 2 that the yield of bioheavy oil originating from the direct liquefaction of 20.0 g of P2 in methanol was 2.1 g, and the yield rate based on dry P2 was 10.5%. Then according to Chart 1, the yield rate of bio-heavy oil originating from biomass co-re fining was 35.5%, which had far exceeded the former. The reasons were not clearly known, but might be supposed as follows:
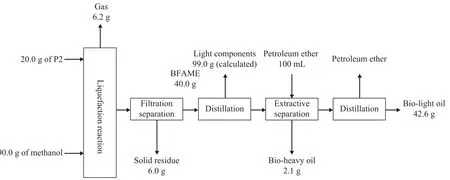
Chart 2 Process flow diagram and material balance of P2 directly lique fied in methanol
In the co-re fining process, macromolecules obtained from the liquefaction of poplar powder might be dissolved by oils and fats and their derivatives such as fatty acid methyl esters, which were con firmed previously to be capable of improving the liquefaction rate of poplar powder, and the yield of bio-heavy oil was improved in that way. (2)Fatty acid methyl esters and glycerol were produced by complete methanolysis of soybean oil, but the mass fraction of free glycerol in the bio-light oil in Chart 1 was equal to only 0.13% as analyzed by GC technique. The most probable reason could be that free glycerol was condensed with the liquefaction products of poplar powder to produce larger molecules, which were separated into the bio-heavy oil when they were extracted by petroleum ether and filtered, so that the final yield of bio-heavy oil was improved. In order to reduce the condensation reaction, the high acid value fats and oils such as acidic oil should be used in the co-re fining reaction. (3) Linoleic acid and oleic acid were rich in the soybean oil, and they had a relatively strong addition-reaction activity. These acids might be condensed with macromolecules originating from the liquefaction of poplar powder to produce new larger molecular compounds which were separated into the bioheavy oil. If the amount of oils and fats derivatives in bioheavy oil was oversupplied in the future, low temperature hydrogenation of feedstock should be considered to convert linoleic acid and/or oleic acid to less unsaturated fatty acids to reduce this condensation reaction.
4 Conclusions
Biomass co-refining was studied in this work to verify the promotion of poplar powder liquefaction and decomposition by soybean oil and fatty acid methyl esters, the separation method of the liquid products was studied,and the components of bio-light oil separated from the liquid products were analyzed. The decomposition rate of poplar powder was improved by fatty acid methyl esters as well as soy bean oil. Bio-light oil and bio-heavy oil were obtained when the extractive separation method was used after biomass co-re fining. Bio-light oil was mainly composed of derivatives of oils and fats, which could be refined to biodiesel or could be used as raw material of chemicals. Bio-heavy oil was mainly composed of poplar powder liquefaction products, which could be used as fuel oil in the furnace, or raw materials of hydrocracking, and hydrodeoxidation to produce biofuel. Some products in the boiling range of diglycerides were produced if unsaturated oils and fats were used in biomass co-re fining. The yield of the bio-heavy oil from biomass co-re fining was far more than that from the direct liquefaction of poplar powder in supercritical methanol.
Acknowledgement:This research was supported by the SINOPEC Corporation (No. S113077).
Reference
[1] Sawangkeaw R, Bunyakiat K, Ngamprasertsith S. Effect of co-solvents on production of biodiesel via transesterification in supercritical methanol [J]. Green Chem, 2007, 9: 679–685
[2] Hajjari M, Tabatabaei M, Aghbashlo M, et al. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization [J]. Renew Sust Energ Rev, 2017, 72: 445–464
[3] Daud N M, Abdullah S R S, Hasan H A, Yaakob Z. Production of biodiesel and its wastewater treatment technologies:A review [J]. Process Saf Environ Prot, 2015, 94: 487–508
[4] Du Z X,Liu X X,Jiang Y S, et al. Pilot-plant test of alcoholysis of vegetable oils with super/sub-critical methanol to biodiesel [J]. Petrochem Techno, 2014, 43(11):1296–1304(in Chinese)
[5] Du Z X, Tang Z, Wang H J, et al. Research and development of a sub-critical methanol alcoholysis process for producing biodiesel using waste oils and fats [J]. Chin J Catal, 2013, 34(1): 101–115
[6] Du Z X, Wang H J, Jiang Y S, et al. Industrial Application and life cycle analysis for biodiesel produced by SRCA process from waste oils and fats [J]. Acta Petrolei Sinica(Pet Process Sect), 2012, 28(3): 353–361 (in Chinese)
[7] Peterson A A, Vogel F, Lachance R P, et al. Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies[J]. Energy &Environ Sci, 2008, 1: 32–65
[8] Yu Y, Lou X, Wu H. Some recent advances in hydrolysis of biomass in hot compressed water and its comparisons with other hydrolysis methods [J]. Energy Fuels, 2008, 22: 46-60
[9] Toor S S, Rosendahl L, Rudolf A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies[J]. Energy, 2011, 36(5): 2328–2342
[10] Kabir G, Hameed B H. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals [J]. Renew Sust Energ Rev, 2017, 70: 945–967
[11] Yang Y. Production of Bio-crude from Forestry Waste by Hydro-liquefaction in Sub-/Super-critical Methanol and Upgrading of Bio-crude by Hydrotreating[D]. Ontario Canada: Lakehead University, 2009
[12] Mazaheri H, Lee K T, Bhatia S, et al. Sub/supercritical liquefaction of oil palm fruit press fiber for the production of bio-oil: Effect of solvents [J]. Bioresour Technol, 2010,101: 7641–7647
[13] Farobie O, Matsumura Y. Biodiesel production in supercritical methanol using a novel spiral reactor [J]. Procedia Environ Sci, 2015, 28: 204–213
[14] Hu J B, Du Z X, Tang Z, et al. Study on the solvent power of a new green solvent: Biodiesel [J]. Ind Eng Chem Res,2004, 43: 7928–7931
[15] Min E Z, Du Z X, Hu J B. An exploratory study on developing biore finery based on vegetable oils [J]. Sci Technol Rev, 2005, 23(5): 15–17
[16] Melero J A, Iglesias J, Morales G. Heterogeneous acid catalysts for biodiesel production: current status and future challenges [J]. Green Chem, 2009, 11: 1285–1308
[17] Carpenter D, Westover T L, Czernik S, et al. Biomass feedstocks for renewable fuel production: a review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors [J].Green Chem, 2014, 16: 384–406
[18] Yang T H, Jie Y F, Li B S, et al. Catalytic hydrodeoxygenation of crude bio-oil over an unsupported bimetallic dispersed catalyst in supercritical ethanol [J]. Fuel Process Technol, 2016, 148: 19–27
[19] Chen D Y, Zhou J B, Zhang Q S, et al. Evaluation methods and research progresses in bio-oil storage stability [J]. Renew Sust Energ Rev, 2014, 40: 69–79
[20] Oh S, Hwang H, Choi H S, et al. Investigation of chemical modifications of micro- and macromolecules in bio-oil during hydrodeoxygenation with Pd/C catalyst in supercritical ethanol [J]. Chemosphere, 2014, 117: 806–814
[21] Guo S J, Lu J X, Chang J, et al. Research progress of the upgrading of bio-oil [J]. Mod Chem Ind, 2016, 7: 37–41
[22] Pattanaik B P, Misra R D. Effect of reaction pathway and operating parameters on the deoxygenation of vegetable oils to produce diesel range hydrocarbon fuels: A review [J].Renew Sust Energ Rev, 2017, 73: 545–557
[23] Galadima A, Muraza O. Catalytic upgrading of vegetable oils into jet fuels range hydrocarbons using heterogeneous catalysts: A review [J]. J Ind Eng Chem, 2015, 29(25): 12–23
[24] Mäder A, Zimon A, Fleischmann A, et al. In fluences of entrainers to engine oil to improve the drag-out of biodiesel – Experiments and simulations [J]. Fuel, 2014, 117, Part A: 488–498
[25] Jaselskis B, Stemm N L, Johnston W D. Determination of the fatty-acid composition of soybean oil by high-pressure liquid chromatograph [J]. Talanta, 1982, 29(1): 54–56
[26] Sawada M M, Venâncio L L, Toda T A, et al. Effects of different alcoholic extraction conditions on soybean oil yield,fatty acid composition and protein solubility of defatted meal [J]. Food Res Int, 2014, 6: 662–670
[27] Gao J, Wang M Z, Jing Y J, et al. Impacts of the unsaturation degree of long-chain fatty acids on the volatile fatty acid pro files of rumen microbial fermentation in goats in vitro [J]. J Integr Agric, 2016, 15(12): 2827–2833
[28] Liu P, Sun S, Hou H, et al. Effects of fatty acids with different degree of unsaturation on properties of sweet potato starch-based films[J]. Food Hydrocolloids, 2016, 61:351–357
[29] Yang Q P, Liang S H, Yang R N, et al. Composition analysis of palm oils with different melting points [J]. J Henan Univ Technol (Nat Sci Ed), 2015, 36(1): 27–31
[30] Bashiri H, Pourbeiram N. Biodiesel production through transesterification of soybean oil: A kinetic Monte Carlo study [J]. J Mol Liq, 2016, 223: 10–15
[31] Li C X, Tang Z, Yang H Y. Determination of fatty acid methyl esters and glycerides in biodiesel samples by GC [J].J Instrum Anal, 2005, 24(5): 66–68
- 中国炼油与石油化工的其它文章
- Synthesis of Core-Shell HZSM-5@SBA-15 Composite and Its Performance in the Conversion of Methanol to Aromatics
- Effects of Gasoline with Ester Additives on the Swelling Behavior of Rubbers
- Fabrication of the Core-Shell Structured ZSM-5@Mg(Al)O and Its Catalytic Application in Propane Dehydrogenation
- ZSM-5/MAPO Composite Catalyst for Converting Methanol to Ole fins in a Two-Stage Unit with a Dimethyl Ether Pre-Reactor
- Oxidation of Dibenzothiophene in Model Diesel Using Hydroperoxide Generated via In-Situ Reaction of Octane with Oxygen
- Controllable Synthesis of Mixed-Phase TiO2 with Small Anatase and Rutile Particle and Its Enhanced Photocatalytic Activity

