质子交换膜燃料电池非贵金属催化剂研究进展*
康启平,张国强,张志芸,刘艳秋,乔 佳
(1. 北京亿华通科技股份有限公司,北京 100192;2. 北京市氢燃料电池发动机工程技术研究中心,北京 100192)
0 引 言
质子交换膜燃料电池(proton exchange membrane fuel cells, PEMFCs)以其高效、低噪音、低温快速启动、零污染等优势,非常适合作为新能源环保型汽车的动力能源[1]。目前,贵金属Pt基催化剂仍然是PEMFCs使用最为普遍的催化剂,尽管PEMFCs技术在近几年取得了重大的突破,但其昂贵的价格及易中毒的缺点是造成PEMFCs难以大规模商业化的重要原因。根据美国能源部(Department of Energy,DOE)的调查研究显示(图1),在大规模生产的前提下,PEMFCs的成本中有46%是来自于所使用的Pt基催化剂。
为了降低PEMFCs的成本,研究人员通过改善催化剂结构来减少Pt负载量、提高Pt利用率和催化剂的稳定性。然而,由于Pt基催化剂的价格较高,而且抗甲醇和 CO中毒性能较差,越来越多的研究者开始对具有髙活性和髙稳定性的非贵金属催化剂进行研究(本文仅讨论金属催化剂)。非贵金属催化剂的稳定性与其阴极催化层的导电性有很大的关系,并随着阴极催化层导电性的增大而增大[2]。2017年9月,加拿大Ballard公司与日本Nisshinbo环境能源公司合作开发出全球第一款商业化的质子交换膜燃料电池非贵金属催化剂(non-precious metal catalysts, NPMC),该非贵金属催化剂是基于碳合金材料,具体成分还未见报道[3]。目前有几类非贵金属氧还原反应(oxygen reduction reaction, ORR)催化剂引起了研究人员的高度重视:金属氮碳催化剂(M/N/C)[4-6],过渡金属氧化物[7-8],过渡金属硫化物,过渡金属碳化物和氮化物等[9]。
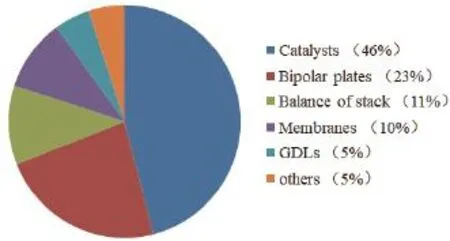
图1 PEMFCs各个组成部分的成本Fig. 1 The cost of each parts of PEMFCs
1 金属氮碳催化剂(M-N-C)
在非贵金属催化剂中,M-N-C具有比表面积大、孔径分布合理和ORR催化活性较高等优点。同时,该类催化剂能有效降低PEMFCs的成本,也具备寿命长和抗甲醇的优势,非常有希望替代价格高昂的Pt基催化剂。M-N-C首次作为ORR催化剂是从对金属大环化合物的研究开始,自从JASINSKI[10]发表了过渡金属M-N-C具有ORR催化效果之后,研究人员开始广泛关注M-N-C催化剂。M-N-C中的非贵金属M一般可以是Fe、Co、Ni、Cu、Mn等,特别是以Co和Fe为中心原子的M-N-C非贵金属催化剂研究较多[11-12]。M-N-C催化剂的催化机理和活性中心一直是该类催化剂的研究重点,但目前尚不明确。不同金属的加入对活性位点形成所起的作用不同,Co的加入可能只是单纯辅助氮原子更好地掺入碳晶格中,不直接参与形成活性中心[13],而Fe可以与周围的氮配位(Fe-Nx),直接参与形成活性中心[14]。
影响M-N-C催化剂性能的因素包括催化剂的制备方法、金属及其盐的种类、氮源种类、碳载体类型和热处理条件等[15-16]。采用模板法制备M-N-C催化剂材料,可以增加比表面积,引入足够的活性位点,从而提高M-N-C催化剂的ORR催化活性。MUN等[17]通过采用软模板法合成了有序介孔结构的Fe-N-C催化剂,这种有序介孔为催化剂提供了较高的比表面积和活性位点,采用该Fe-N-C催化剂制作的膜电极(membrane electrode assembly, MEA)其功能密度比用商业化 Pt/C催化剂的 MEA提高了40%。OSMIERI等[18]利用酞菁铁(II)为Fe、N和C源,采用介孔SiO2为模板,制备了高比表面积和孔隙率达到50%的Fe-N-C催化剂,其ORR催化按4电子过程进行,起始电位为0.83 V,略低于商业化Pt/C催化剂(如图2所示)。然而,Fe-N-C催化剂的耐久性和抗甲醇性能要明显好于商业化 Pt/C催化剂。
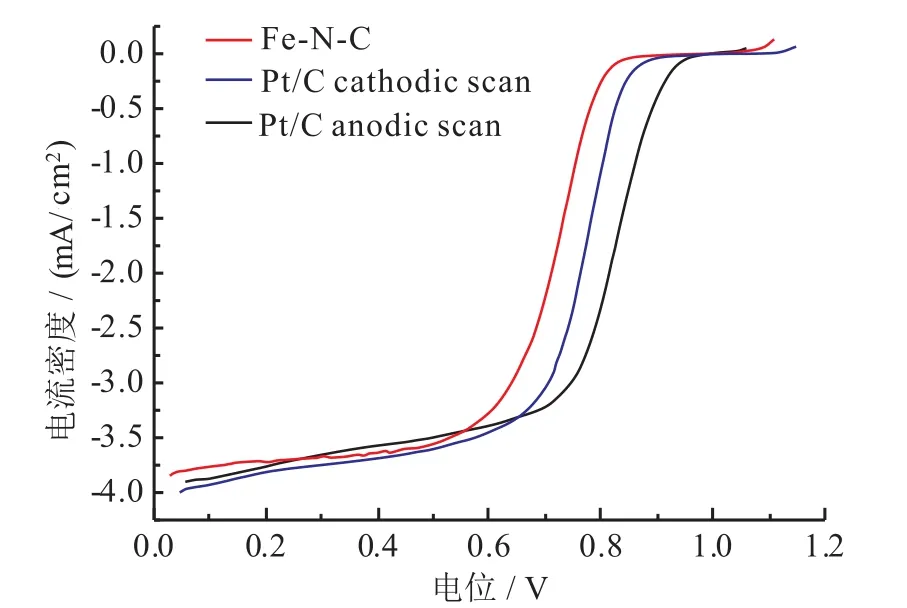
图2 Fe-N-C催化剂在0.1 M HClO4中的ORR极化曲线[18]Fig. 2 ORR activity in 0.1 M HClO4 of Fe-N-C catalyst
金属及其盐的前驱体种类对M-N-C催化剂性能的影响也非常明显。李姝玲等[19]以铁盐作为金属前驱体,BP2000为碳源,聚吡咯(PPy)为氮源,对甲基苯磺酸(TsOH)为掺杂剂,合成的Fe-PPy-TsOH/C催化剂结晶完善、颗粒大小适中且分布均匀,在0.5 mol/L H2SO4中ORR起始电位为0.72 V。采用33.3%的Co取代Fe-PPy-TsOH/C中的Fe时,由于Fe和Co的协同作用、CoFe2O4的促进作用以及催化剂中空结构导致的比表面积增大,所制得的 Fe0.666Co0.333PPy-TsOH/C催化剂氧还原催化性能得到明显提升。
氮掺杂石墨烯(N/GR)被认为是一种有效的非金属氧还原催化剂,然而N/GR的N掺杂效率非常低,仅有少数N原子掺入石墨烯骨架中,从而导致N/GR催化剂的ORR催化活性不高。LIANG等[20]通过密闭热解法制备了高N掺杂效率的Co/N/GR催化剂(如图3所示),该催化剂表现出了良好的ORR催化活性,且较商业化Pt/C催化剂具有更好的稳定性和抗甲醇性能。原因主要是由于Co纳米颗粒支撑N/GR骨架以及N掺杂原子增多,为Co/N/GR提供了较大的比表面积,尽可能地暴露Co纳米颗粒和N掺杂活性位点,且该催化剂的ORR催化按4电子过程进行。

图3 Co/N/GR催化剂合成过程示意图[20]Fig. 3 Schematic illustration of the synthesis procedure of Co/N/GR electrocatalysts
由于合成金属大环化合物难度大、成本高等原因,研究人员逐渐采用低成本的前躯物来将其替代,将不同形式的过渡金属、N、C前躯物进行热解来合成M-N-C催化剂。氮源主要有聚苯胺、聚吡咯、聚多巴胺等,这些含氮有机聚合物有序化比较高,在热解过程中容易形成有序化且稳定的N掺杂C活性层。其中,聚吡咯最早被用作合成 M-N-C催化剂的前躯物,随后研究人员发现采用聚苯胺热解合成的M-N-C催化剂ORR催化活性更好、更稳定[21]。MUTYALA等[22]通过将铁盐和聚苯胺在惰性气氛中碳化得到Fe-N-CNFs催化材料,其ORR催化按4电子过程进行,在碱性溶液中循环2 000次,波电位下降10 mV,同样测试条件下商业化20wt% Pt/C则下降了42 mV,且Fe-N-CNFs催化剂的耐久性和抗甲醇性能也要高于该商业化催化剂。
利用双金属的协同作用,同时加入Fe和Co可以显著提高M-N-C催化剂的ORR催化活性[23-24]。WU等[25]通过在氮气气氛下将含有Fe、Co、聚苯胺和C颗粒的前躯物在900℃进行热处理得到双金属氮掺杂碳(FeCo-N-C)催化剂,其 ORR起始电位高达0.93V(vs. RHE),而其半波电位也仅比商业化20wt%Pt/C低43 mV,非常有希望替代商业化Pt/C催化剂。XIA等[26]同时研究了CoFe-PANI、Fe-PANI和Co-PANI三种聚苯胺衍生的M-N-C催化剂,ORR催化活性顺序为 CoFe-PANI > Fe-PANI > Co-PANI,原因是双金属的掺入产生了协同作用,更有利于电子向吸附氧物种转移,使得其ORR催化活性要高于单一金属掺杂,而金属Co的加入使得催化剂更加稳定。
虽然采用前躯体热解方法制备的M-N-C催化剂具有良好的ORR催化活性,但是其制备工艺复杂,热解产物的形貌和结构很难得到控制,材料性能的一致性难以得到保证。如何优化制备工艺并获得形貌和结构可控、催化性能一致的催化材料是M-N-C催化剂的主要研究方向。另外,M-N-C催化氧还原过程的活性位点和催化机理还存在争议,也有待继续深入研究。
2 过渡金属氧化物催化剂
过渡金属氧化物具有低成本、高活性和环保等特点,是一种可靠的燃料电池阴极催化剂材料。其中,Mn基和Co基氧化物催化剂的ORR催化活性最好。在Mn氧化物中,MnO、Mn2O3、Mn3O4、MnO2和MnOOH等均具有较高的ORR催化活性,且Mn氧化物催化剂的ORR催化活性和Mn价态相关[27-30]。研究结果表明,不同价态的 Mn氧化物催化剂可以通过调整烧结工艺来合成,其ORR催化活性随 Mn价态的上升而不断增强,而且高电位下得到的Mn氧化物催化剂的ORR催化活性要明显高于低电位下获得的[31]。在 Co氧化物催化剂中,CoO和Co3O4具有较高的ORR催化活性[32]。
GUO等[33]将高度分散的Co纳米颗粒与氧化石墨烯混合后在空气中加热,制得石墨烯负载的具有核壳结构的Co/CoO纳米颗粒催化剂,当CoO壳层为1 nm时,该催化剂具有最优的ORR催化活性。这种高活性一方面源于 Co核能较好地起到传导电子的作用,另一方面,Co与CoO之间的相互作用也能起到调节催化剂活性的作用。LIANG等[34-35]采用水热法在氮掺杂的碳载体上负载 Co3O4、CoO纳米颗粒,提高了金属氧化物的导电性,并利用其协同效应增强ORR催化活性。通过X射线近边吸收精细结构分析可知,该催化剂形成了金属-碳-氧和金属-碳-氮共价键,电子由氮传至金属氧化物,从而赋予了金属氧化物良好的导电性和电化学活性。将不同价态的过渡金属氧化物复合形成尖晶石结构的催化剂是过渡金属氧化物催化剂的研究重点。另外,通过利用Mn3+取代Co3O4中的部分Co3+,合成具有尖晶石结构的 MnCo2O4催化剂,该催化剂的 ORR活性得到明显提高。此外,研究人员对TiO2、NbO2和Ta2O5等过渡金属氧化物的ORR催化性能也进行了研究[36-38]。近年来,钙钛矿型氧化物因同时具有电子和离子导电性,越来越多地被用作高温燃料电池中的氧还原催化剂,其中 Ba0.5Sr0.5Co0.8Fe0.2O3-x基钙钛矿型氧化物被认为是此类材料中最具潜力的氧还原催化剂[39]。材料固有的化学性质及微观结构对过渡金属氧化物的ORR催化性能起决定性作用。其中,部分过渡金属氧化物的ORR催化活性和稳定性与Pt/C催化剂相当。
研究表明,除了单金属氧化物,含两种及以上过渡金属的氧化物也具有优异的ORR催化活性[40-44]。YANG等[45]通过前躯体热解得到晶体结构和形态可控的多孔尖晶石CoxMn3-xO4。其中,立方CoxMn3-xO4纳米棒具有优异的ORR催化活性,其ORR起始电位达到0.9 V,半波电位达到0.72 V,非常接近商业化Pt/C催化剂的催化性能,同时,还具有比商业化Pt/C催化剂更好的稳定性,10 000次循环后其催化性能基本上没有衰减。而具有四方结构的CoxMn3-xO4催化活性略低于立方 CoxMn3-xO4纳米棒。这是由于立方结构表面金属位点对O2的吸附能力要比四方结构更强,所以在同样表面积下,立方结构具有更多的活性位点。
尽管过渡金属氧化物催化剂的成本低、选择性高、催化性能好,是一类很有发展潜力的非贵金属催化剂。但是,过渡金属氧化物催化剂的制备工艺复杂且氧化物易缓慢分解及纳米粒子的氧化物容易团聚,电流密度要远低于商业化Pt/C催化剂,这些都将是过渡金属氧化物催化剂需要解决的问题。
3 过渡金属硫化物、碳化物及氮化物催化剂
过渡金属硫化物是硫族化合物催化剂中 ORR催化活性最好的一类,比如 Co9S8催化剂[46]。根据密度泛函理论(DFT)研究表明,在Co9S8中,氧气是吸附在硫元素上,且氧气在(202)晶面上还原的过电势与Pt相当。另外,Co1-xS、Co4S3、CoSe2[47-51]等在碱性介质中的ORR催化按4电子过程进行,而在酸性介质中通常是按2电子过程进行。WU等[52]采用纳米 Co9S8包覆 N掺杂石墨化碳所合成的Co9S8-N-C催化剂在碱性介质中的ORR催化活性相比 Pt/C催化剂要更好。WANG等[53]采用还原氧化石墨烯负载Co1-xS纳米颗粒制备的催化剂,在酸性和碱性溶液中都具有较好的ORR催化活性。其中,在酸性溶液中的ORR起始电位达到了0.8 V,电流密度也比较高,但该催化剂的稳定性与商业化 Pt/C催化剂相比还有较大的差距。因此,提高过渡金属硫化物催化剂在酸性溶液中的稳定性将是今后的主要研究方向。
金属碳化物和氮化物由于具有较好的导电性和耐腐蚀性,被广泛应用于阴极ORR。表面碳化物或氮化物的形成可以调控催化剂的电子结构,使得 d带收缩以及电子密度增大而更接近费米能级。这样加快了电子向氧吸附物种的转移,从而使得活性金属更容易还原氧。例如钼、钨的碳化物和氮化物因具有和 Pt非常相似的 d带电子结构而具有较好的ORR催化性能。ZHONG等[54-55]研究发现Mo2N和W2N在酸性溶液中具有一定的ORR催化活性,同时还具有非常好的稳定性。QI等[56]对MoN和Mo2N的ORR催化性能和机理进行了研究,发现在酸性溶液中,MoN和Mo2N的ORR催化起始电位分别为0.75 V和0.70 V,抗甲醇性和稳定性都非常好。同时,DFT研究表明这两种氮化物的ORR催化活性提高是因其有效促进了氧分子解离。另外,研究人员还发现Fe-C、Fe-N、和CoMo-N等碳化物和氮化物也具有不错的ORR催化活性[57-60]。但是,这些碳化物和氮化物催化剂在酸性溶液中的 ORR催化稳定性比较差,还需要进一步提高。随后人们发现双金属氧氮化合物由于其协同增强效应而具有可观的ORR催化活性。CAO等[61]采用溶液浸渍法合成了CoxMo1-xOyNz/C催化剂,无论是在酸性溶液还是在碱性溶液中,该催化剂都具有较好的 ORR催化活性,其在酸性溶液中的ORR起始电位为0.645 V,稳定性也非常好。ANDO等[62]合成的碳载双金属Co-W-O-N催化剂在0.5 mol/L H2SO4中的ORR起始电位达到0.74 V,其催化活性要高于碳负载单金属Co或W氧氮化合物催化剂。
尽管过渡金属碳化物和氮化物催化剂的价格低廉、资源丰富,具有一定的催化活性和良好的抗甲醇性能,具备替代Pt基催化剂的潜力。但是,过渡金属碳化物和氮化物催化剂在酸性溶液中的催化活性和稳定性比较差,这将是过渡金属碳化物和氮化物催化剂今后的主要研究方向。
4 结束语
非贵金属催化剂具有价格低廉、资源丰富、ORR催化活性良好及抗甲醇性能等优点,有望替代Pt基催化剂在PEMFCs得到应用,从而降低成本,促进PEMFCs大规模商业化应用。虽然采用前躯热解方法制备的M-N-C催化剂具有良好的ORR催化活性,但其制备工艺复杂,材料性能的一致性难以得到保证,需要继续优化合成工艺和改善催化性能,提高催化稳定性;过渡金属氧化物的电流密度尚比商业化Pt/C催化剂低很多,还需要大幅提高;过渡金属碳化物和氮化物催化剂在酸性溶液中的 ORR催化活性和稳定性相比商业化Pt/C催化剂还有较大的差距,有待进一步研究改善。
[1]LI B, LI H, MA J X, et al. PEM fuel cells: current status and challenges for electrical vehicle applications[J].Journal of automotive safety and energy, 2010, 1(4):260-269. DOI:10.3969/j.issn.1676-8484.2010.04.002.
[2]BANHAM D, KISHIMOTO T, SATO T, et al. New insights into non-precious metal catalyst layer designs for proton exchange membrane fuel cells: improving performance and stability[J]. Journal of power sources,2017, 344: 39-45. DOI: 10.1016/j.jpowsour.2017.01.086.
[3]Ballard plans world’s first PEMFC product with low-cost Nisshinbo non-precious metal catalyst[J]. Fuel cells bulletin,2017, 9: 1. DOI: 10.1016/S1464-2859(17)30338-3.
[4]ZHANG R, ZHANG J, MA F, et al. Preparation of Mn-N-C catalyst and its electrocatalytic activity for the oxygen reduction reaction in alkaline medium[J]. Journal of fuel chemistry and technology, 2014, 42(4): 467-475.DOI: 10.1016/S1872-5813(14)60022-0.
[5]BAU V M, BO X J, GUO L P. Nitrogen-doped cobalt nanoparticles/nitrogen-doped plate-like ordered mesoporous carbons composites as noble-metal free electrocatalysts for oxygen reduction reaction[J]. Journal of energy chemistry, 2017, 26(1): 63-71. DOI: 10.1016/j.jechem.2016.07.005.
[6]ZHAO Q, LI Y J, LI Y T, et al. Hierarchical hybrid of Ni3N/N-doped reduced graphene oxide nanocomposite as a noble metal free catalyst for oxygen reduction reaction[J]. Applied surface science, 2017, 400: 245-253.DOI: 10.1016/j.apsusc.2016.12.203.
[7]MTUKULA A C, SHEN J, BO X J, et al. High utilization efficiency of NiCo2O4supported on porous graphene as noble metal-free catalysts for oxygen reduction reaction[J]. Journal of alloys and compounds, 2016, 655:229-237. DOI: 10.1016/j.jallcom.2015.09.185.
[8]FU G T, LIU Z Y, ZHANG J F, et al. Spinel MnCo2O4nanoparticles cross-linked with two-dimensional porous carbon nanosheets as a high-efficiency oxygen reduction electrocatalyst[J]. Nano research, 2016, 9(7): 2110-2122.DOI: 10.1007/s12274-016-1101-2.
[9]TANG Y F, CHEN T, GUO W F, et al. Reduced graphene oxide supported MnS nanotubes hybrid as a novel non-precious metal electrocatalyst for oxygen reduction reaction with high performance[J]. Journal of power sources, 2017, 362: 1-9. DOI: 10.1016/j.jpowsour.2017.07.019.
[10]JASINSKI R. A new fuel cell cathode catalyst[J]. Nature,1964, 201(4925): 1212-1213. DOI: 10.1038/2011212a0.
[11]NIU K X, YANG B P, CUI J F, et al. Graphene-based non-noble-metal Co/N/C catalyst for oxygen reduction reaction in alkaline solution[J]. Journal of power sources,2013, 243: 65-71. DOI: 10.1016/j.jpowsour.2013.06.007.
[12]CHOI I A, KWAK D H, HAN S B, et al. Doped porous carbon nanostructures as non-precious metal catalysts prepared by amino acid glycine for oxygen reduction reaction[J]. Applied catalysis b: environmental, 2017,211: 235-244. DOI: 10.1016/j.apcatb.2017.04.039.
[13]WU G, JOHNSTON C M, MACK N H, et al.Synthesis-structure-performance correlation for polyaniline-Me-C non-precious metal cathode catalysts for oxygen reduction in fuel cells[J]. Journal of materials chemistry,2011, 21(30): 11392-11405. DOI: 10.1039/c0jm03613g.
[14]LEFÈVRE M, PROIETTI E, JAOUEN F, et al.Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells[J]. Science,2009, 324(5923): 71-74. DOI: 10.1126/science.1170051.
[15]GOKHALE R, CHEN Y C, SEROV A, et al. Direct synthesis of platinum group metal-free Fe-N-C catalyst for oxygen reduction reaction in alkaline media[J].Electrochemistry communications, 2016, 72: 140-143.DOI: 10.1016/j.elecom.2016.09.013.
[16]OSMIERI L, ESCUDERO-CID R, ARMANDI M, et al.Fe-N/C catalysts for oxygen reduction reaction supported on different carbonaceous materials. Performance in acidic and alkaline direct alcohol fuel cells[J]. Applied catalysis b: environmental, 2017, 205: 637-653. DOI:10.1016/j.apcatb.2017.01.003.
[17]MUN Y, KIM M J, PARK S A, et al. Soft-template synthesis of mesoporous non-precious metal catalyst with Fe-Nx/C active sites for oxygen reduction reaction in fuel cells[J]. Applied catalysis b: environmental, 2018,222: 191-199. DOI: 10.1016/j.apcatb.2017.10.015.
[18]OSMIERI L, ESCUDERO-CID R, MONTEVERDE VIDELA A H A, et al. Performance of a Fe-N-C catalyst for the oxygen reduction reaction in direct methanol fuel cell: cathode formulation optimization and short-term durability[J]. Applied catalysis b: environmental, 2017,201: 253-265. DOI: 10.1016/j.apcatb.2016.08.043.
[19]李姝玲, 原鲜霞, 孔海川, 等. Fe-PPy-TsOH/C用作质子交换膜燃料电池氧电极催化剂的研究[J]. 无机材料学报, 2017, 32(4): 393-399. DOI: 10.15541/jim20160399.
[20]LIANG J W, HASSAN M, ZHU D S, et al. Cobalt nanoparticles/nitrogen-doped graphene with high nitrogen doping efficiency as noble metal-free electrocatalysts for oxygen reduction reaction[J]. Journal of colloid and interface science, 2017, 490: 576-586. DOI: 10.1016/j.jcis.2016.11.101.
[21]HU T H, YIN Z S, GUO J W, et al. Synthesis of Fe nanoparticles on polyaniline covered carbon nanotubes for oxygen reduction reaction[J]. Journal of power sources, 2014, 272: 661-671. DOI: 10.1016/j.jpowsour.2014.08.124.
[22]MUTYALA S, MATHIYARASU J. Noble metal-free Fe-N-CNFs as an efficient electrocatalyst for oxygen reduction reaction[J]. International journal of hydrogen energy, 2017. DOI: 10.1016/j.ijhydene.2017.06.133. (in Press)
[23]HAN C, BO X J, LIU J, et al. Fe, Co bimetal activated N-doped graphitic carbon layers as noble metal-free electrocatalysts for high-performance oxygen reduction reaction[J]. Journal of alloys and compounds, 2017, 710:57-65. DOI: 10.1016/j.jallcom.2017.03.241.
[24]PARK J C, CHOI C H. Graphene-derived Fe/Co-N-C catalyst in direct methanol fuel cells: Effects of the methanol concentration and ionomer content on cell performance[J]. Journal of power sources, 2017, 358:76-84. DOI: 10.1016/j.jpowsour.2017.05.018.
[25]WU G, MORE K L, JOHNSTON C M, et al.High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt[J]. Science, 2011,332(6028): 443-447. DOI: 10.1126/science.1200832.
[26]CHEN X, SUN S R, WANG X Y, et al. DFT study of polyaniline and metal composites as nonprecious metal catalysts for oxygen reduction in fuel cells[J]. Journal of physical chemistry c, 2012, 116(43): 22737-22742. DOI:10.1021/jp307055j.
[27]WANG Y G, CHENG L, LI F, et al. High electrocatalytic performance of Mn3O4/mesoporous carbon composite for oxygen reduction in alkaline solutions[J]. Chemistry of materials, 2007, 19(8): 2095-2101. DOI: 10.1021/cm062685t.
[28]CHENG F Y, SU Y, LIANG J, et al. MnO2-based nanostructures as catalysts for electrochemical oxygen reduction in alkaline media[J]. Chemistry of materials,2010, 22(3): 898-905. DOI: 10.1021/cm901698s.
[29]FENG J, LIANG Y Y, WANG H L, et al. Engineering manganese oxide/nanocarbon hybrid materials for oxygen reduction electrocatalysis[J]. Nano research,2012, 5(10): 718-725. DOI: 10.1007/s12274-012-0256-8.
[30]TAN Y M, XU C F, CHEN G X, et al. Facile synthesis of manganese-oxide-containing mesoporous nitrogen-doped carbon for efficient oxygen reduction[J]. Advanced functional materials, 2012, 22(21): 4584-4591. DOI:10.1002/adfm.201201244.
[31]王俊, 魏子栋. 非贵金属氧还原催化剂的研究进展[J].物理化学学报, 2017, 33(5): 886-902. DOI: 10.3866/PKU.WHXB201702092.
[32]LIANG Y, LI Y, WANG H, et al. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts[J]. Journal of the American chemical society, 2012, 134(7): 3517-3523.DOI: 10.1021/ja210924t.
[33]GUO S J, ZHANG S, WU L H, et al. Co/CoO nanoparticles assembled on graphene for electrochemical reduction of oxygen[J]. Angewandte chemie international edition, 2012, 51(47): 11770-11773. DOI: 10.1002/anie.201206152.
[34]LIANG Y Y, WANG H L, DIAO P, et al. Oxygen reduction electrocatalyst based on strongly coupled cobalt oxide nanocrystals and carbon nanotubes[J].Journal of the American chemical society, 2012, 134(38):15849-15857. DOI: 10.1021/ja305623m.
[35]LIANG Y Y, LI Y G, WANG H L, et al. Co3O4nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction[J]. Nature materials, 2011,10(10): 780-786. DOI: 10.1038/nmat3087.
[36]WU G, NELSON M A, MACK N H, et al. Titanium dioxide-supported non-precious metal oxygen reduction electrocatalyst[J]. Chemical communications, 2010,46(40): 7489-7491. DOI: 10.1039/C0CC03088K.
[37]SASAKI K, ZHANG L, ADZIC R R. Niobium oxide-supported platinum ultra-low amount electrocatalysts for oxygen reduction[J]. Physical chemistry chemical physics, 2008, 10(1): 159-167. DOI: 10.1039/B709893F.
[38]IMAI H, MATSUMOTO M, MIYAZAKI T, et al.Structural defects working as active oxygen-reduction sites in partially oxidized Ta-carbonitride core-shell particles probed by using surface-sensitive conversionelectron-yield X-ray absorption spectroscopy[J]. Applied physics letters, 2010, 96(19): 191905. DOI: 10.1063/1.3430543.
[39]SUNTIVICH J, MAY K J, GASTEIGE H A, et al. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles[J]. Science, 2011,334(6061): 1383-1385. DOI: 10.1126/science.1212858.
[40]INDRA A, MENEZES P W, SAHRAIE N R, et al.Unification of catalytic water oxidation and oxygen reduction reactions: amorphous beat crystalline cobalt iron oxides[J]. Journal of the American chemical society,2014, 136(50): 17530-17536. DOI: 10.1021/ja509348t.
[41]ZHAO A Q, MASA J, XIA W, et al. Spinel Mn-Co oxide in N-doped carbon nanotubes as a bifunctional electrocatalyst synthesized by oxidative cutting[J].Journal of the American chemical society, 2014, 136(21):7551-7554. DOI: 10.1021/ja502532y.
[42]WANG L X, GENG J, WANG W H, et al. Facile synthesis of Fe/Ni bimetallic oxide solid-solution nanoparticles with superior electrocatalytic activity for oxygen evolution reaction[J]. Nano research, 2015,8(12): 3815-3822. DOI: 10.1007/s12274-015-0881-0.
[43]HASSAN D, EL-SAFTY S, KHALIL K A, et al. Carbon supported engineering NiCo2O4hybrid nanofibers with enhanced electrocatalytic activity for oxygen reduction reaction[J]. Materials, 2016, 9(9): 759. DOI: 10.3390/ma9090759.
[44]CHENG F Y, SHEN J, PENG B, et al. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts[J]. Nature chemistry, 2011, 3(1): 79-84. DOI: 10.1038/nchem.931.
[45]YANG H C, HU F, ZHANG Y J, et al. Controlled synthesis of porous spinel cobalt manganese oxides as efficient oxygen reduction reaction electrocatalysts[J].Nano research, 2016, 9(1): 207-213. DOI: 10.1007/s12274-016-0982-4.
[46]SIDIK R A, ANDERSON A B. Co9S8as a catalyst for electroreduction of O2: quantum chemistry predictions[J].The journal of physical chemistry b, 2006, 110(2):936-941. DOI: 10.1021/jp054487f.
[47]Feng Y J, Alonso-Vante N. Nonprecious metal catalysts for the molecular oxygen-reduction reaction[J]. Physica status solidi (b), 2008, 245(9): 1792-1806. DOI:10.1002/pssb.200879537.
[48]FENG Y J, HE T, ALONSO-VANTE N. In situ free-surfactant synthesis and ORR-electrochemistry of carbon-supported Co3S4and CoSe2nanoparticles[J].Chemistry of materials, 2008, 20(1): 26-28. DOI:10.1021/cm7024763.
[49]GANESAN P, PRABU M, SANETUNTIKUL J, et al.Cobalt sulfide nanoparticles grown on nitrogen and sulfur codoped graphene oxide: an efficient electrocatalyst for oxygen reduction and evolution reactions[J]. ACS catalysis,2015, 5(6): 3625-3637. DOI: 10.1021/acscatal.5b00154.
[50]FENG Y J, HE T, ALONSO-VANTE N. Carbonsupported CoSe2nanoparticles for oxygen reduction reaction in acid medium[J]. Fuel cells, 2010, 10(1):77-83. DOI: 10.1002/fuce.200900038.
[51]ZHOU Y X, YAO H B, WANG Y, et al. Hierarchical hollow Co9S8microspheres: solvothermal synthesis,magnetic, electrochemical, and electrocatalytic properties[J].Chemistry-a European journal, 2010, 16(39): 12000-12007.DOI: 10.1002/chem.200903263.
[52]WU G, CHUNG H T, NELSON M, et al. Grapheneriched Co9S8-N-C non-precious metal catalyst for oxygen reduction in alkaline media[J]. ECS transactions, 2011,41(1): 1709-1717. DOI: 10.1149/1.3635702.
[53]WANG H L, LIANG Y Y, LI Y G, et al. Co1-xS-graphene hybrid: a high-performance metal chalcogenide electrocatalyst for oxygen reduction[J]. Angewandte chemie international edition, 2011, 50(46): 10969-10972. DOI: 10.1002/anie.201104004.
[54]ZHONG H X, ZHANG H M, LIU G, et al. A novel non-noble electrocatalyst for PEM fuel cell based on molybdenum nitride[J]. Electrochemistry communications,2006, 8(5): 707-712. DOI: 10.1016/j.elecom.2006.02.020.
[55]ZHONG H X, ZHANG H M, LIANG Y M, et al. A novel non-noble electrocatalyst for oxygen reduction in proton exchange membrane fuel cells[J]. Journal of power sources, 2007, 164(2): 572-577. DOI: 10.1016/j.jpowsour.2006.11.080.
[56]QI J, JIANG L H, JIANG Q, et al. Theoretical and experimental studies on the relationship between the structures of molybdenum nitrides and their catalytic activities toward the oxygen reduction reaction[J]. Journal of physical chemistry, 2010, 114(42): 18159-18166.DOI: 10.1021/jp102284s.
[57]XIAO M L, ZHU J B, FENG L G, et al. Meso/macroporous nitrogen-doped carbon architectures with iron carbide encapsulated in graphitic layers as an efficient and robust catalyst for the oxygen reduction reaction in both acidic and alkaline solutions[J].Advanced materials, 2015, 27(15): 2521-2527. DOI:10.1002/adma.201500262.
[58]YIN H, ZHANG C Z, LIU F, et al. Hybrid of iron nitride and nitrogen-doped graphene aerogel as synergistic catalyst for oxygen reduction reaction[J]. Advanced functional materials, 2014, 24(20): 2930-2937. DOI:10.1002/adfm.201303902.
[59]ZHANG S M, ZHANG H Y, LIU Q, et al. Fe-N doped carbon nanotube/graphene composite: facile synthesis and superior electrocatalytic activity[J]. Journal of materials chemistry a, 2013, 10(1): 3302-3308. DOI:10.1039/C2TA01351G.
[60]SUN T, WU Q, CHE R C, et al. Alloyed Co-Mo nitride as high-performance electrocatalyst for oxygen reduction in acidic medium[J]. ACS catalysis, 2015, 5(3):1857-1862. DOI: 10.1021/cs502029h.
[61]CAO B F, VEITH G M, DIAZ R E, et al. Cobalt molybdenum oxynitrides:synthesis,structural characterization, and catalytic activity for the oxygen reduction reaction[J]. Angewandte chemie international edition, 2013, 52(41): 10753-10757. DOI: 10.1002/anie.201303197.
[62]ANDO T, IZHAR S, TOMINAGA H, et al.Ammonia-treated carbon-supported cobalt tungsten as fuel cell cathode catalyst[J]. Electrochimica acta, 2010,55(8): 2614-2621. DOI: 10.1016/j.electacta.2009.12.039.
——庆祝中国共产党成立一百周年贵金属纪念币展

