Chemical stability,thermal behavior,and shelf life assessment of extruded modified double-base propellants
Sherif Elbasuney,Ahmed Fahd,Hosam E.Mostafa,Sherif F.Mostafa,Ramy Sadek
School of Chemical Engineering,Military Technical College,Kobry El-Kobba,Cairo,Egypt
1.Introduction
Modified double base(MDB)propellants have found wide applications in modern military and space rocketry,in view of their superior performance[1,2].It is well known that MDB propellants are evolved from double-base by integrating energetic fillers such as HMX or RDX.There is also another trend to integrate potential oxidizers such as ammonium perchlorate(AP),as well as active metal fuels such as aluminum,magnesium,and boron[3-6].This is why MDB propellants have recently been used in booster,sustainer,and dual thrust rocket motors[7-9].
MDB can exhibit a wide range of burning rate up to 40 mm/s;specific impulse can also be varied from 220 to 270 s[9-12].It has been reported that integration of stoichometric binary mixture of oxidizer-metal fuel(AP/Al),and energetic nitramine such as HMX offered a higher specific impulse(Fig.1)[9,13-15].
MDB based on binary mixture of AP/Al and HMX offered higher specific impulse by 10%and 9%respectively compared with reference formulation[9].Stoichometric binary mixture of AP/Al had a dual effect by increasing the average operating pressure and burning rate[9].This action was ascribed to the gaseous decomposition nature of AP(Equation(1)),and the exothermic oxidation of Al metal fuel which could enhance the heat of combustion,and flame temperature[1,2,7,16].
Aluminum metal fuel,with high exothermic heat of combustion(7.4 kcal/g)and excellent thermal conductivity values,tended to increase the burning rate[7,17,18].Aluminum particles are able to react not only with free oxygen resulted from oxidizer decomposition;but also it is able to react with inert decomposition gaseous products and add much more heat to the combustion process[18-20].
The great impact of HMX on ballistic performance was attributed to the positive heat of formation(+353.8 kJ/kg).HMX is a highly effective explosive material with heat of explosion 6197 kJ/kg and gaseous product of 902 L/kg[13].HMX also has a slightly negative oxygen balance which means decomposition products of low molecular weight[13,21].Much research has been directed toward the development of MDB propellants with enhanced combustion characteristics and high specific impulse[22-25].However less attention has been directed to investigate the impact of different energetic additives on chemical stability,thermal behavior,and shelf life[26].
1.1.Chemical stability of MDB propellants
The nitrate esters(nitrocellulose&nitroglycerine),the main constituents of double-base propellant,are molecules that aren't chemically stable.Their decomposition is slow in ambient conditions of temperature,pressure,and humidity.In severe environments,the chemical decomposition becomes autocatalytic[11].There are many mechanisms through which chemical decomposition can occur;these mechanisms include:
1.1.1.Chain reactions
Chain reactions start with the homolytic breaking of the weak O-NO2bond,forming nitrogen dioxide and the corresponding alkoxyl radical[27-29].These reactive free radicals immediately undergo consecutive reactions with nearby nitrate ester molecules[29].
1.1.2.Saponification(hydrolysis)
Another main decomposition pathway is the neutral to acid hydrolysis of the nitrate esters[28].This reaction is catalyzed by moisture and residual acids(which weren't fully removed after nitrate ester synthesis),or by water,or by acids formed during decomposition(Equation(3)).
A further decomposition reaction is the “enhanced hydrolysis”.This reaction was found to have low activation energy of 71 kJ/mol.Therefore it can be a dominant decomposition reaction at lower temperatures[30].
1.1.3.Auto-catalytic reactions
Decomposition products of reactions(2)can further transformed in presence of moisture and oxygen as follow
Whereas the primary homolytic reaction(2)can't be suppressed,the consecutive reactions(3-6)can be slowed down nearly to zero by binding or elimination of acids,nitric oxides,and water from the system.This fact was employed for the stabilization of double-base propellants by integrating stabilizing agents[30,31].Stabilizers fulfill their purpose by reacting with the nitrogen oxides and neutralize the decomposition products[32].Conventional double-base propellants,with proper percentage of stabilizer,can offer a safe chemical life of at least 20 years[33].For modified systems containing energetic solid additives similar shelf life should be secured[34].A number of studies have been carried out on the thermal stability of MDB propellants[35-38].Complete information regarding the influence of high energy ingredients including(in organic oxidizers/high explosives)on MDB propellant stability and shelf life is vital in regards of their handling,processing,transportation,and storage.
1.2.Impact of different energetic additives on chemical stability
AP has a great impact on the degradation of propellants containing nitrate esters.Many researchers have studied the rate of stabilizer depletion and the time to ignition of such propellants[39].Asthana,Divekar et al.investigated the stability,auto ignition,and stabilizer depletion of MDB propellants containing NG and AP[40].It was noted that the inclusion of AP increased the autocatalytic behavior of MDB propellants over time[41].MDB based on AP demonstrated ease of ignition suggesting faster decomposition kinetics[42].AP-MDB propellants possess shorter shelf life than their conventional counterparts[40,43].Further research showed that MDB containing AP and NG exhibited less stability than conventional double-base[44].However,nitramine doublebase propellants exhibited relatively good thermalstability[45-49].This paper is devoted to investigate the effect of binary mixture of oxidizer/metal fuel(AP/Al)and energetic nitramine(HMX)on DB chemical stability,thermal behavior,as well as shelf life assessment.MDB formulation based on HMX demonstrated extended service life of 16 years compared to(AP/Al)-MDB which demonstrated 9 years.DSC outcomes demonstrated an increase in heat released with aging time.The released heat was increased by 31,41,and 25%for reference,(AP/Al)-MDB,and HMX-MDB formulations respectively.This thermal behavior was ascribed to the auto-catalytic thermal degradation over artificial aging.Correlation between the increase in heat released and the evolved nitrogen oxides was conducted.
2.Experimental
2.1.Manufacture of MDB formulations
Screw extrusion technique emphasizes mixing of different ingredients to ensure good homogenization,high density,and dimensional stability.This technique included many stages such as blending,followed by rolling,grinding,granulation,and finally extrusion to obtain grains of desired shape and dimensions[50].Different MDB formulations based on stoichiometric binary mixture(AP/Al),and HMX at 10 wt%solid loading level,were manufactured by screw extrusion.
2.2.Chemical stability of MDB
Evaluation of chemical stability,deals with the fact that the rate of decomposition at normal temperature is judged from decomposition at higher temperature[51,52].Quantitative stability tests were employed for fast and reliable evaluation of MDB chemical stability;they were devoted to the direct measurement of evolved gasses[53].The most commonly used quantitative stability tests are Bergmann-Junk test,and Vacuum stability test.
2.2.1.Bergmann-Junk test
Bergmann-Junk test is the main quantitative test for DB stability evaluation.In this test,5g of the tested sample was heated at 120°C for 5 h.The evolved nitrogen oxides(NOx)were entrapped in a secondary tube containing 50 ml of de-ionized water.The evolved NOx gases were quantitatively determined by titration using potassium iodide solution.The acceptable limit for Bergmann-Junk test is 10 ml of NOx/5 g sample[23,54].
Vacuum stability test is a controlling,measuring,enabling evaluation of temperature stability from measurements of evolved gases from tested sample during long term isothermal heating.This test was performed according to STANAG 4556,where 1 g of the sample was heated at 90°C for 40 h with pressure measurement reading each 1 min during the isothermal heating process.
2.3.Thermal behavior of MDB
Ignition temperature is one of the main important characteristics which need to be evaluated for developed MDB formulation,in an attempt to evaluate the impact of different energetic constituents on MDB heat sensitivity.A sample of 0.1 g was introduced in a glass tube and heated at controlled rate of 5°C/min till ignition[32].Phase change with temperature,onset decomposition temperature,and heat released upon combustion are the main parameters for MDB thermal stability evaluation.Differential scanning calorimetry(DSC)measures heat flow associated with phase changes(i.e.melting),endothermic/exothermic decomposition as a function of temperature or time.DSC measurements were performed using DSC 2920 by TA instruments.2 mg of MDB propellants were heated up to 300°C at 5°C/min,under nitrogen gas flow at 5 ml/min.
2.4.Artificial aging
Artificial aging was conducted in an attempt to reduce the time scale by storing the propellant at elevated temperatures so that prediction of service life can be made in shorter times.It facilitates the planning of time-temperature profile of MDB with limited knowledge about their degradation behavior[55].Artificial aging was performed by isothermal heating at 80°C in temperature controlled oven under ambient atmospheric conditions.The developed MDB formulations were stored under isothermal heating for 4,8,14 and 28 days[56].Consequently safe storagelife of the propellant can be predicted[56].
2.5.Shelf life assessment of MDB
Van't Hoff's formula(Equation(7))enabled the estimation of inservice periods at given in-storage temperatures,from the equivalent time-temperature loads during the artificial ageing.Van't Hoff's formula has been proved by experience to be suitable to establish the time-temperature profile[57].
Where:TE,TT,F,and ΔTFare time in years at the in-service temperature(TEin0C),test time in days at the test temperature(TTin0C),reaction rate change factor per 10°C of temperature change(Fusually between 2 and 4),and temperature interval for actual valueFrespectively.Factor F was determined using Arrhenius Equation(8)[57].
Where,Eais the activation energy(kJ/mol),andRis the ideal gas constant[55].Ffactor was deduced by compiling and comparing reaction rates obtained at different temperatures[55].The range for this factor is often between 2 and 4[57].Table 1 demonstrates the accelerated ageing conditions simulating an in-use time up to 32 years at 25°C for developed MDB propellants.
The change in chemical stability of aged MDB was tracked by quantifying the evolved NOx gases with aging time.Their thermal behavior was investigated and quantified using DSC.Novel correlation between chemical stability(volume of evolved NOx gases)and thermal behavior(Heat released)was represented.
3.Results and discussions
3.1.Chemical stability of MDB
The volume of nitrogen oxides evolved from freshly manufactured MDB compositions was quantified using Bergman-Junk test.The quantified NOxare listed in Table 2.
总之,正确的水分调控对于高效、低耗地制作优质硬颗粒饲料具有重要作用。在实际生产中,由于各企业颗粒饲料产品的配方组成不同、原料质量的变异、加工环境、生产设备、蒸汽条件等的不同或客户的需要不同,都会对水分的调控技术参数提出不同要求。因此,饲料企业应重视硬颗粒饲料加工技术的研究与创新,通过加工参数的优化研究,获得实现加工优质颗粒饲料的最佳参数组合,并将这些参数组合作为生产中的控制标准,只有这样,才能使企业和用户获得最佳经济与社会效益。
The volume of NOxevolved from reference DB and MDB formulations were within the acceptable limits(10 ml of NOx/5 g sample)[54].HMX based formulation exhibited similar value of evolved NOxto reference.This indicated that HMX is compatible with double base constituents;no side chemical reactions could take place.However AP based formulation exhibited the largest volume of evolved NOxgases.This was attributed to the reactivity of AP oxidizer to react with nitroglycerine to form perchloric acid[30,58].Vacuum stability test represents a fast way of chemical stability determination.Results from vacuum stability test for freshly manufactured MDB propellants are listed in Table 3.The evolved NOx confirmed the obtained data from Berman-Junk test.
3.2.Thermal behavior of MDB
Ignition temperature test was conducted to measure the temperature of spontaneous ignition by progressive heating.Even though,MDB formulation exhibited an increase in heat released during exothermic decomposition,there was no dramatic change inignition temperature.The ignition temperature for reference,AP/Al-MDB,and HMX-MDB was found to be 171,172,170°C respectively.DSC was employed to monitor any chemical/physical changes which involve the evolution/absorption of heat.The total heat released,the maximum decomposition temperature,and the onset decomposition temperature were measured and evaluated using DSC(Fig.2).

Table 1Ageing times calculated on the basis of thermal equivalent load at TE=25°C using the generalized Van't Hoff's rule with factor F=3.
All Formulations demonstrated one exothermic decomposition peak.Energetic additives did not greatly affect the maximum decomposition temperature but they positively impact the total heat released upon combustion.Summary of total heat released(J/g)and maximum peak temperature(°C)are tabulated in Table 4.
The inclusion different energetic additives including binary mixture of AP/Al,and HMX into DB propellants increased the released heat upon decomposition due to the favorable heat added by these modifiers.Formulation 2 based on HMX exhibited the highest released heat.This was ascribed to the fact that HMX decomposes with the release of large amount of heat 6197 J/g.

Table 2Quantified NOxgases evolved from freshly manufactured MDB using Bergmann-Junk test.

Table 3Vacuum stability test results of freshly developed MDB.
3.3.Shelf life assessment
The developed MDB were isothermally aged at 80°C for different periods.The increase in evolved NOxoxides was quantified with aging time and shelf life prediction using Bergman-Junk test(Table 5).
Results demonstrated that AP based formulation demonstrated the least chemical stability.This behavior was attributed to the fact that AP can degrade to form perchloric acid;which could cause rapid hydrolysis of the nitrate ester.This degradation action could accelerate the propellant decomposition(Equations(9)-(12))[30,45,58].
MDB propellants based on HMX revealed stability similar to reference formulation.This was ascribed to the high thermal stability of HMX.Furthermore,no side reactions could take place between HMX and DB constituents.Quantification of evolved NOx gases with aging time was performed using vacuum stability test(Table 6).Vacuum stability test outcomes confirmed the findings of Bergmann-Junk test.
There was an increase in volume of evolved NOxwith aging time.The volume of evolved NOx gases from HMX-MDB was higher than reference formulation but lower than AP-MDB.HMX-MDB and reference formulation exhibited similar shelf life of at least 16 years.On the other hand MDB based on binary mixture of AP/Al exhibited shelf life of 9 years.This was attributed to the induced catalytic degradation upon inclusion of AP with the formation of perchloric acid.
3.4.Thermal behavior of aged MDB
MDB demonstrated a decrease in ignition temperature with isothermal aging time(Table 7).
It is clear that sensitivity to heat of different MDB formulations increased with aging.This behavior was ascribed to the decrease in the required activation energy to start the chemical conversion[34].HMX based formulation demonstrated the highest thermalstability;this was attributed to the fact that higher energy is required for the activation of HMX compared with AP[13].The thermal behavior of aged MDB after aging period of 14 days were investigated with DSC to that of freshly manufactured formulation.DSC thermograms of aged MDB formulations ensured the findings of Bergman-Junk and Vacuum stability tests.The main findings from DSC thermograms included:shifting of maximum decomposition peak temperature to lower value,and an increase in total heat released with aging.Figs.3-5 demonstrate the DSC thermograms for fresh and aged formulations.
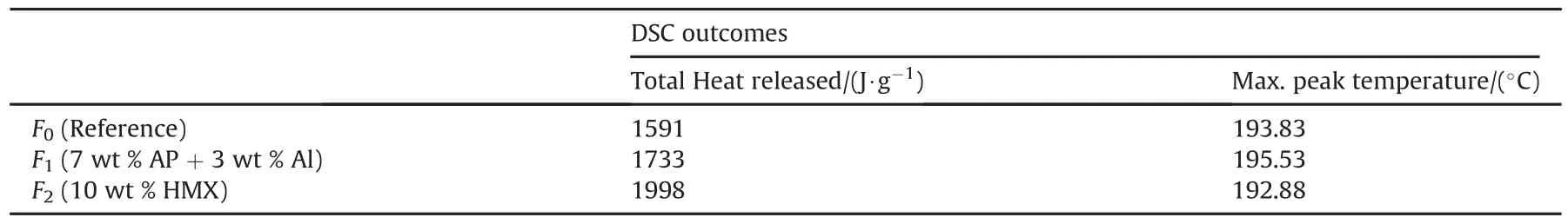
Table 4Thermal behavior characteristics of fresh manufactured MDB.
All investigated MDB formulations exhibited similar thermal behavior with aging.This behavior encompasses an increase in heatreleased as well as a decrease in the temperature at maximum heat released.This thermal behavior was ascribed to the degradation of MDB over aging.MDB propellants could degrade by thermal decomposition of NC and NG,which might start with the homolytic breakdown of the O-NO2bond[55].This reaction might be catalyzed by moisture and residual acids formed as products during the decomposition process[55].Table 8 summarized the increase in total heat released of aged formulations,to fresh manufactured formulations.

Table 5Bergmann-Junk test results after aging at 80°C.

Table 6Quantification of NOxwith aging using vacuum stability test.

Table 7Ignition temperature for aged MDBP.
DSC out comes ensured the findings of Bergmann-Junk and Vacuum stability tests.The total heat released was increased by 31,41 and 25%for reference formulation,binary mixture of(AP&Al),and HMX respectively.HMX based formulation demonstrated superior thermal stability.This behavior was attributed to the great consumption of heat energy for the activation of HMX compared to AP,as well as the reactivity of AP toward NG.

Table 8The increase in heat released with isothermal aging time.
4.Conclusion
MDB based on HMX exhibited good chemical and thermal stabilities using quantitative chemical stability tests and DSC respectively.MDB based on HMX exhibited service life of 16 years,similar to reference formulation.MDB based on AP demonstrated service life of 9 years.Low service life of MDB based on AP was ascribed to the reactivity of AP towards NG with the formation of perchloric acid.All MDB formulations exhibited an increase in evolved NOx,and total heat released with aging time.The increase in heat released by 31%was found to be equivalent to evolved NOxgases of 6.2 cm3/5 g and 2.5 cm3/1 g for Bergman-Junk,and Vacuum stability test respectively.These values should not be exceeded for safe storage.This manuscript shaded the light on HMX which offered MDB with balanced ballistic performance,thermal and chemical stability,as well as extended service life.
[1]Sadek R,Kassem M,Abdo M,Elbasuney S.Spectrally adapted red flare tracers with superior spectral performance.Def Technol 2017:1-7.
[2]Sadek R,Kassem M,Abdo M,Elbasuney S.Novel yellow colored flame compositions with superior spectral performance.Def Technol 2017;13(1):33-9.
[3]Meda L,G.L.M.,Braglia R,Abis L,Gallo R,Severini F,et al.A wide characterization of aluminum powders for propellants.In:Proceedings of the 9-IWCP,novel energetic materials and applications,grafiche g.s.s,Bergamo;November 2004.
[4]Yetter Richard A,G.A.R.,Son Steven F.Metal particle combustion and nanotechnology.In:Proceedings of the combustion institute,32;2009.
[5]Han X,W.T.,Lin ZK,Han DL,Li SF,Zhao FQ,et al.RDX/AP-CMDB propellants containing fullerenes and carbon black additives.Def Sci J 2009;59:284-9.
[6]Elbasuney S,Fahd A,Mostafa HE.Combustion characteristics of extruded double base propellant based on ammonium perchlorate/aluminum binary mixture.Fuel 2017;208:296-304.
[7]Mocella JACCJ.Chemistry of pyrotechnics,basic principles and theory.USA:Taylor&Francis Group,an informa business;2010.p.60-96.
[8]Davenas A.Solid rocket propulsion technology.Elsevier Science;2012.
[9]Fahd A,Mostafa HE,Elbasuney S.Certain ballistic performance and thermal properties evaluation for extruded modified double-base propellants.Central Eur J Energ Mater 2017;14(3).
[10]CS,D.,Ultra-ultrahigh burning rate composite modified double-base propellants containing porous ammonium perchlorate.1990.
[11]Davenas A.Solid rocket Motor Design.Progress in Astronautics and Aeronautics,AIAA.;1996.
[12]Sutton GPB,O.Solid propellants.In:Rocket propulsion elements.Wiley;2011.p.475-512.
[13]Gautarn GK,S.M.P.,Joshi AD,Mulage KS,Singh SN.Study of energetic nitramine extruded double-base propellants.Def Sci J 1998;48(2).
[14]A,Z.,HMX and RDX:combustion mechanism and influence on modern double-base propellant combustion.J Propuls Power,1995.
[15]Elbasuney S,Fahd A,Mostafa HE.Combustion characteristics of extruded double base propellant based on ammonium perchlorate/aluminum binary mixture.Fuel 2017;208(Supplement C):296-304.
[16]Mohamed AK,Mostafa HE,Elbasuney S.Nanoscopic fuel-rich thermobaric formulations:chemical composition optimization and sustained secondary combustion shock wave modulation.J Hazard Mater 2016;301:492-503.
[17]Mohamed AK,Mostafa HE,Elbasuney S.Nanoscopic fuel-rich thermobaric formulations:chemical composition optimization and sustained secondary combustion shock wave modulation.J Hazard Mater 2016;301:492-503.
[18]Elbasuney S,Elsaidy A,Kassem M,Tantawy H.Stabilized super-thermite colloids:a new generation of advanced highly energetic materials.Appl Surf Sci 2017;419:328-36.
[19]Meyer R,J.K.,Homburg A.Explosives.Sixth Edition ed.sixth ed.Weinheim:Wiley-VCH&Co.KGaA;2007.
[20]Yaman Hayri,Ercan Degˇirmenci VÇ.Experimental investigation of the factors affecting the burning rate of solid rocket propellants.Fuel 2014;115:794-803.
[21]Mocella JACCJ.Chemistry of pyrotechnics,basic principles and theory.USA:Taylor&Francis Group,an informa business;2010.
[22]CS.,D.,Ultra-ultrahigh burning rate composite modified double-base propellants containing porous ammonium perchlorate.1990.
[23]Meyer R,Kohler J,Homburg A,editors.Explosives.sixth ed.Weinheim:Wiley;2007.
[24]Cohen-NIr.Combustion characteristics of advanced nitramine-based propellants.Int Symp Combust 1991;18:195-205.
[25]Kubota N.Survey of rocket propellants and the combustion characteristics.Fundam Solid Propellant Combust 1984.
[26]Sutton GP,Biblarz O.Solid propellant rocket fundamentals(p 426-430).In:Rocket propulsion elements.Wiley;2011.p.426-30.
[27]G.B.Manelis,G.M.N.,Y.I.Rubtsov,V.A.Strunin,Thermal decomposition and combustion of explosives and propellants.
[28]Albrecht,G.,Milit¨artechnik,1987.5,267.
[29]Bohn MA.The use of kinetic equations to evaluate the ageing behaviour of energetic materials-possible problems.In:11th symp.on chemical problems connected with the stability of explosives,Bastad;1998[Sweden].
[30]Manelis GB.In:Francis T,editor.Thermal decomposition and combustion of explosives and propellants;2003.p.210-5.
[31]Vogelsanger B,B.O.,Schadeli U,Antenen D,Ryf K.Ballistic shelf life of propellants for medium and small calibre ammunition-influence of deterrent diffusion and nitrocellulose degradation.In:19th internafional symposium of ballisfics;2001.
[32]Nobelkrut B.Analytical methods for powders and explosives.1974[Sweden].
[33]Davenas A.Solid rocket propulsion technology.New York:Pergamon Press;1993.
[34]Vogelsanger B.Chemical stability,compatibility and shelf life of explosives.2004.Chimia.
[35]Hartman K-0,Musso RC.The thermal decomposition of nitroglycerine and its relation to the stability of CMDB propellants.CA:The Combustion Institute;1972.p.29.WSCI 72-30.
[36]Elrick,D.E.,US Patent 3.1975.
[37]S.W.Beckwith and H.B.Carroll,J.,Spacecraft Rockets,in Spacecraft Rockets,.1985.p.156-161.
[38]Machida H,A.Y.,Sumikawa K,Suzuki N,Fukuda T,Sumi K,et al.In:Seventeenth int.Jahrestag Fraunhofer inst.Treib explosivst.,Karlsruhe;1986.
[39]United States,O.T.A.C.,Disposal of chemical weapons:alternative technologies:DIANE Publishing.
[40]Asthana SN,C.N.D,Singh H.J Hazard Mater 1989;21:35-46.
[41]Guidelines for safe storage and handling of reactive materials.Wiley;2010.
[42]Zukas JA,Walters W,Walters WP.Explosive effects and applications.New York:Springer;2002.
[43]Bromberger CG,H.R.B.,Conduit CP,Howard AJ.The stability of colloidal propellants:Part 3:high impulse compositions.London,UK:Explosives Research Development Establishment;1960.
[44]Conduit CP.The stability of colloidal propellants:Part 5:the rates of heat generation and critical charge sizes for a composite modified cast double-base propellants.London,UK:Explosives Research Development Establishment;1962.
[45]Bunyan P.In:12th symposium on the chemical problems connected with the stability of explosives;2001[Sweden].
[46]Teipel U.Energetic materials:particle processing and characterization.Wiley;2006.
[47]Asthana SN,R.B.G.,Singh H.J Hazard Mater 1990;23:235-44.
[48]Ruth Tunnell MA,Dale Roz,Tod Dave,Proud William G.Ammonium perchlorate,friend or Foe?Part 1:the influence of this antioxidizer on the aging behavior of propellant compositions.Propellants Explos Pyrotech 2010;35:1-7.
[49]Bhalerao MM,G.K.G.,Subramanian GV,Singh SN.Nitramine double base propellants.Def Sci J 1996;46:207-14.
[50]Lewis TJ.The effect of processing variations on the ballistics of fast burning extruded double base propellants.In:AIAA 14th joint propulsion conference;1978.
[51]Yan Q-L,Li X-J,Wang Y.Combustion mechanism of double-base propellant containing nitrogen heterocyclic nitroamines(I):the effect of heat and mass transfer to the burning characteristics.Combust Flame 2009;156(3):633-41.
[52]Huggins RA.Energy storage.US:Springer;2010.
[53]Zihlman FA.Method of testing propellant stability.Google Patents;1960.
[54]Frys O,P.B.,Eisner A,Skladal J,Ventura K.Utilization of new non-toxic substances as stabilizers for nitrocellulose-based propellants.Propell Explos Pyrotech 2011;23:22-9.
[55]Djalal Trache aKK.Study on the influence of ageing on thermal decomposition of double-base propellants and prediction of their in-use time.Fire Mater 2013;37:328-36.
[56]Jelisavac L.Life-time prediction of double-base propellants in accordance with Serbian and NATO standards.Sci Tech Rev 2010;60(1):12-8.
[57]MA B.Prediction of equivalent time-temperature loads for accelerated ageing to simulate preset in-storage ageing and time-temperature profile loads.In:Proceeding of the 40th international annual conference of ICT;2009[Germany,Karlsruhe].
[58]Lurie B,V.K.,Svetlov B.In:11th symposium on the chemical problems connected with the stability of explosives;1998.p.267-87.Sweden.
- Defence Technology的其它文章
- Approximate ballistics formulas for spherical pellets in free flight
- An experimental study on shock wave mitigation capability of polyurea and shear thickening fluid based suspension pads
- Contemplation on some cyclic N8isomers-A DFT treatment
- Cold metal transfer(CMT)technology-An overview
- Numerical and experimental study of wave shaper effects on detonation wave front
- Microstructure,properties and hot workability of M300 grade maraging steel

