泛素-蛋白酶体途径在生殖细胞减数分裂过程中的作用及机制
彭馥芝 董莲花 冉茂良 翁 波 陈 斌*
(1.湖南农业大学动物科学技术学院,长沙 410128;2.畜禽遗传改良湖南省重点实验室,长沙 410128)
泛素-蛋白酶体途径(ubiquitin-proteasome pathway,UPP)通过三酶级联将泛素连接于靶蛋白以使其泛素化,为介导真核生物蛋白质降解调节的主要途径。此外,泛素化在蛋白质稳定性[1]、蛋白质运输[2]、细胞分化[3]、细胞周期进程[4]等过程中发挥重要作用,与脂质代谢[5]、肌肉发育[6]、神经元形态发生[7]等生理过程相关,UPP异常则导致肌肉萎缩[8]、炎症反应[9]、睾丸肿瘤[10]等病变发生。研究证实,泛素相关组件及去泛素酶在动物配子生成中普遍表达,在减数分裂过程中的减数分裂同源重组[11]、减数分裂性染色体失活[12]、卵母细胞减数分裂恢复[13]、第一极体(the first polar body,PBI)排出[14]及精卵融合[15]等过程中发挥着重要作用,UPP的紊乱或UPP组件的突变将导致这些生物过程发生损伤及配子发育缺陷。例如,初级精母细胞粗线期染色体联会重组,组蛋白多聚泛素化,人工敲除组蛋白泛素化出现减数分裂中断并最终导致不育[16];圆形精子细胞中,组蛋白泛素化有助于鱼精蛋白替换[17];卵母细胞缺失Cullin4(CUL4)导致减数第1次分裂恢复延迟[18];敲减泛素结合酶2C(UBE2C)导致第一极体排出受阻及染色体分离紊乱[19]。研究UPP在动物配子生成中的生物学功能及作用机制,不但能推进对生殖过程的进一步了解,对治疗人类不孕不育及提高经济动物繁殖性能也具有重要意义。本文综述了泛素-蛋白酶体通路在动物生殖细胞减数分裂过程及配子生成中的信号传导及调节机制,以期为后续的相关研究提供参考。
1 UPP概述
UPP由泛素、泛素激活酶(ubiquitin-activating enzyme,E1)、泛素结合酶(ubiquitin-conjugating enzyme,E2)、泛素-蛋白质连接酶(ubiquitin-protein ligase,E3)及蛋白酶体(proteasomes)组成。泛素分子在3种酶的共同作用下与底物蛋白共价结合的过程称为泛素化[20],其中多聚泛素化在蛋白质降解及信号转导过程中发挥重要作用。泛素分子含7个赖氨酸残基(K6、 K11、 K27、 K29、K33、K48及 K63),每个残基都能与泛素连接,从而形成至少7种不同的多聚泛素连接[21]。泛素化受三酶级联反应调控。E1以ATP依赖性激活泛素后,其硫基与泛素C端的羧基连接形成硫酯键;随后通过交酯化过程将活化泛素从E1转移至E2,形成E2-泛素中间物;最后E3募集底物蛋白并与E2-泛素中间物结合,催化泛素转移至底物蛋白的赖氨酸残基上,从而形成泛素异肽链[22]。靶蛋白在3种酶的作用下共价连接几个泛素分子,被26S蛋白酶体识别后水解[22]。UPP调控不同信号通路蛋白质的表达,从而在不同生物过程中发挥作用。UPP组件在生殖细胞发育各阶段表达,在调控减数分裂及雌雄配子生成方面具有重要作用。
2 UPP与雌性生殖细胞减数分裂
2.1 卵母细胞减数第1次分裂恢复
哺乳动物胚胎期卵原细胞分化为初级卵母细胞后,细胞周期休止于减数第1次分裂(meiosis Ⅰ,M Ⅰ)前期,直到进入青春期后,卵母细胞恢复减数分裂进行细胞发育形成具有受精能力的卵子。研究证实,后期促进复合物/细胞周期体(anaphase-promoting complex/cyclosome,APC/C)在卵母细胞M Ⅰ恢复中具有重要作用。APC/C为Cullin-RING连接酶(Cullin-RING ligases,CRL),APC/C通过与适配蛋白——细胞分裂周期蛋白20同源蛋白1(Cdc20 homolog 1,Cdh1)和细胞分裂周期蛋白20(cell division cycle protein 20,Cdc20)结合,形成APC/CCdh1或APC/CCdc20复合体,从而发挥E3活性,其中Cdh1和Cdc20与底物结合并将其呈递至APC/C[23]。细胞周期蛋白Cyclin B和分离酶抑制蛋白(securin)为APC/C主要底物,卵母细胞减数分裂休止期必须避免Cyclin B和securin的过早积累。青春期时,卵母细胞恢复减数分裂,该阶段卵母细胞Cyclin B积累并通过激酶/磷酸酶激活Cyclin B与细胞周期蛋白依赖性激酶1(cyclin-dependent kinase 1,Cdk1)构成的复合物——促成熟因子(maturation promoting factor,MPF),进而使卵母细胞核膜破裂并退出M Ⅰ前期,而这一过程与APC/CCdh1的微调作用密切相关[13,24]。研究显示,APC/CCdh1为哺乳动物卵母细胞M Ⅰ前期和前中期的主要形式,APC/CCdh1活性对于减缓M Ⅰ前期Cyclin B积累及维持securin和Cyclin B平衡必不可少[25],进而在维持卵母细胞减数分裂休止中发挥重要作用[26]。此外,研究显示,属于E2的UBE2C、泛素结合酶2S(UBE2S)、泛素结合酶2D(UBE2D)调节小鼠卵母细胞减数分裂中APC/C活性,促进卵母细胞染色体分离,并与纺锤体的形成相关。这些酶的缺失导致M Ⅰ胞质分裂水平降低50%,而其过表达导致胞质分裂速率提高2倍,高活性的UBE2C导致M Ⅰ的恢复[27]。
Cullin蛋白家族成员CUL4在卵母细胞M Ⅰ过程中也发挥着重要生理作用。Cullin蛋白内无完整的RING结构域,但其C端可通过结合RING家族的小蛋白如RBX1/2(ROC1/2)等构成CRL类E3复合物,负责与E2结合,Cullin蛋白N端则通过桥接蛋白与不同底物识别亚基结合,从而负责不同底物蛋白的募集,进而在不同生物学过程中发挥作用[28]。CUL4的C端及N端分别与RBX1及桥接蛋白——DNA损伤结合蛋白1(damaged DNA binding protein 1,DDB1)结合,构成DDB1-CUL4-RBX1 E3复合物[29],DDB1通过结合含WD40重复结构域的底物识别亚基DCAF1(DDB1-CUL4 associated factor 1)/VPRBP,进而结合并降解特异底物。研究显示,DCAF1能识别蛋白磷酸酶2A亚基(PP2A-A),使其聚泛素化并靶向蛋白酶体降解。作为细胞周期调节因子,PP2A-A在卵母细胞减数分裂过程中发挥作用[18],PP2A-A为维持MⅠ前期生发泡(germinal vesicle,GV)期卵母细胞正常休止所必需,生发泡破裂(germinal vesicle break down,GVBD)期卵母细胞PP2A-A蛋白水平开始降低,小鼠卵母细胞特异性缺失DDB1或DCAF1会发生PP2A-A的积累及减数第1次分裂恢复的延迟,CRL4缺失也会使卵母细胞同源染色体分离受到抑制[18]。
2.2 第一极体排出
卵母细胞M Ⅰ完成的标志为第一极体的排出,APC/CCdc20活性与该过程密切相关。研究显示,APC/CCdh1在M Ⅰ前期和前中期先被激活,而M Ⅰ后期Cdk1介导激活的APC/CCdc20则为主要活性形式,APC/CCdc20靶向securin和Cyclin B1降解,进而调控染色体的浓缩分离及第一极体的排出[14,30]。Cdk1蛋白酶抑制剂导致卵母细胞第1次分裂Cdk1失活及第一极体排出,而去除抑制剂的卵母细胞不能进入减数第2次分裂(meiosis Ⅱ,M Ⅱ)。纺锤体组装检查点(spindle assembly checkpoint,SAC)监管机制使染色体着丝粒正确附着于微管,也是调节APC/CCdc20活性的机制。在中期之前,SAC保持活性,并可能通过SAC组件——有丝分裂阻滞缺陷蛋白2(mitotic arrest deficient 2,Mad2)与Cdc20结合,阻碍APC/C与Cdc20之间互作,从而抑制APC/CCdc20活性[31]。当所有染色体正确连接时,SAC关闭而APC/CCdc20活性达到峰值,APC/CCdc20通过降解Cyclin B1和securin,分离酶得以切开连接染色体的环状黏连蛋白(cohesin)促使染色体分离,对随后的第一极体的排出具有关键作用。此外,SCFβ-TrCP-EMI1-APPC/C通路在小鼠卵母细胞MⅠ进程及第一极体排出中同样具有重要作用。与DDB1-CUL4-RBX1泛素连接酶复合体类似,SCF(Skp1-Cullin-F-box)同为CRL类E3,不同的是SCF的支架蛋白为Cullin1,Cullin1的C端同样与RBX1结合,N端则以Skp1为桥接蛋白与F-box蛋白连接,进而识别特异性底物。SCF泛素连接酶的底物——有丝分裂早期抑制蛋白1(early mitotic inhibitor-1,Emi1)为APC/C抑制剂,能抑制APC/C活性。SCF重要组成部分——RBX1在卵母细胞成熟过程中围绕并随着纺锤体和浓缩染色体迁移,用相应的小干扰RNA(siRNA)敲低Rbx1导致卵母细胞第一极体排出率降低且大多数细胞阻滞于M Ⅰ中期,下调Rbx1的表达也导致Emi1的积累以及securin和Cyclin B1的表达明显增加[32]。
泛素连接形式及蛋白酶体活性对第一极体的排出同样具有重要影响。研究发现K-11泛素连接为第一极体排出的必要信号[19,29]。通过对小鼠GV期卵母细胞显微注射泛素突变体以阻断泛素链的延伸的试验研究发现,卵母细胞注射被精氨酸取代的K-11突变泛素分子,其第一极体的排出被显著干扰,染色体分离过程也严重受损[19]。相较于野生型泛素注射控制组,微注射K-11突变多聚泛素链组卵母细胞出现染色体分离失败及第一极体排出显著减少[29]。蛋白酶体的催化活性对于MPF活性降低及第一极体的排出是必不可少。卵母细胞恢复减数分裂后,蛋白酶体转移到纺锤体,蛋白酶体抑制剂——MG132处理大鼠卵母细胞出现cyclin B积累及MPF活性增强,且细胞阻遏于M Ⅰ中期,阻碍第一极体的排出[33]。此外,敲减UBE2C、UBE2S及UBE2D中任一E2,均显示出第一极体排出障碍、纺锤体形成及染色体分离紊乱[19]。但APC/C在哺乳动物雄性生殖细胞减数分裂中的作用机制还有待进一步研究。
综述所述,UPP在卵子发生中的作用见图1。
3 UPP与雄性生殖细胞减数分裂
3.1 性染色体失活
哺乳动物雄性生殖细胞减数分裂过程中,M I前期精母细胞X和Y染色体于拟常染色体区联会浓缩形成XY小体(XY body),XY小体具有转录沉默现象,称为减数分裂性染色体失活(meiotic sex chromosome inactivation,MSCI)[34],该过程与减数分裂进程密切相关,且能阻遏抑制精子发生基因的表达,对精子发生至关重要[35]。XY小体富含泛素化组蛋白H2A(uH2A),其水平在精母细胞粗线期达到峰值,为性染色体转录沉默标志[36]。研究显示,果蝇及哺乳动物中uH2A与基因阻遏相关[37],对MSCI可能具有重要意义。属于E3的UBR2与属于E2的HR6B互作,介导H2A泛素化,UBR2缺失小鼠M Ⅰ精母细胞表现出H2A泛素化及MSCI缺失,而MSCI缺失可能激活粗线期检查点(check point)机制并导致M Ⅰ阻滞[38]。
MSCI另一显著标志为组蛋白H3第4位赖氨酸(H3K4)二甲基化降低[39],这一过程由HR6B与另一属于E3的RAD18互作介导。HR6B或RAD18功能缺失导致M Ⅰ双线期X、Y染色体H3K4二甲基化水平增加及沉默基因去阻遏现象的发生[40]。研究显示,RAD18通过结合重组酶RAD51C介导DNA损伤后的同源重组修复,Rad18沉默小鼠的精母细胞M Ⅰ粗线期X、Y染色体联会失败、H3K4二甲基化增加及相应的X连锁基因去阻遏,同时小鼠表现出生育力低下、体重降低及睾丸体积减少[41]。研究证实,属于E3的环指蛋白8(RING finger protein 8,RNF8)通过磷酸激酶ATM(ataxia telangiectasia mutated kinase)途径介导H2AX(H2A的变体)第139位丝氨酸的磷酸化(γ-H2AX)[42],Rnf8基因缺失小鼠性染色体出现正常的γ-H2AX的积聚及MSCI的启动,但XY小体不发生泛素化[13]。此外,泛素连接酶Ret finger蛋白(RFP)为转录抑制因子,能与核基质结合蛋白及双链DNA相互作用,于精母细胞M Ⅰ期性染色体异步联会至关重要[43]。
3.2 减数分裂进程
UPP组件中属于E1的UBA6为减数分裂引发因子,与其他组织相比,人和小鼠UBA6 mRNA及蛋白表达水平在睾丸组织中最高。除了在新生小鼠生殖细胞表达,UBA6在PND10(postnatal day)的精原细胞和M Ⅰ前细线期精母细胞细胞质中表达最高,可能在启动M Ⅰ方面发挥作用[44],而PND20时则在精母细胞M Ⅰ细线期和偶线期细胞核中表达。除了参与卵母细胞M Ⅰ及第一极体的排出过程,属于E3的SCFβ-TrCP也参与雄性生殖细胞减数分裂。β-转导重复相容蛋白(beta-transducin repeats-containing proteins,β-TrCP)为Skp1-Cullin-F-box泛素连接酶复合物结构中的F-box蛋白,β-TrCP缺失小鼠出现M Ⅰ中期精母细胞生成增加、精子细胞生成减少。β-TrCP缺失小鼠睾丸表现出SCFβ-TrCP底物——Emi1、细胞周期蛋白Cyclin A等细胞周期调节因子水平的增加,可能为其导致减数分裂缺陷的分子机制[45]。此外,维持睾丸自由泛素单体平衡重要基因——聚泛素基因Ubi-p63E,于果蝇精母细胞正常染色体凝聚及M Ⅰ中G2期向M期转变至关重要,Ubi-p63E突变导致M Ⅰ期阻滞[46]。
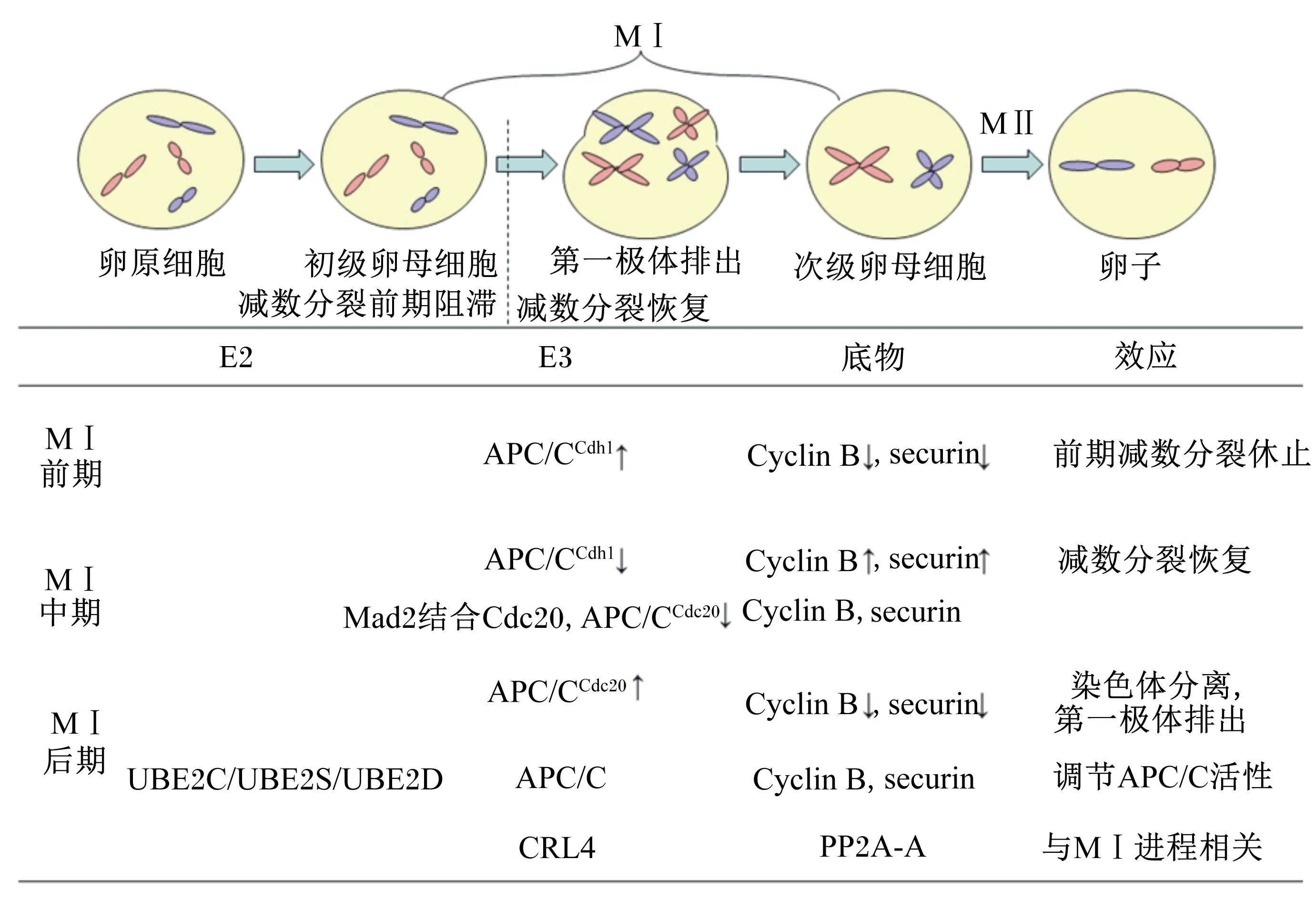
M Ⅰ:减数第1次分裂 meiosis Ⅰ;M Ⅱ:减数第2次分裂 meiosis Ⅱ;PBI:第一极体 the first polar body;E2:泛素结合酶 ubiquitin-conjugating enzyme;E3:泛素-蛋白质连接酶 ubiquitin-protein ligase;UBE2C:泛素结合酶2C ubiquitin-conjugating enzyme 2C;UBE2S:泛素结合酶2S ubiquitin-conjugating enzyme 2S;UBE2D:泛素结合酶2D ubiquitin-conjugating enzyme 2D;APC/C:后期促进复合物/细胞周期体 anaphase-promoting complex/cyclosome;Cdc20:细胞分裂周期蛋白20 cell division cycle protein 20;Cdh1:Cdc20同源蛋白1 CDC20 homolog 1;CRL4:Cullin-RING连接酶4 Cullin-RING ligase 4;Mad2:有丝分裂阻滞缺陷蛋白2 mitotic arrest deficient 2; PP2A-A:蛋白磷酸酶2A亚基 protein phosphatase 2A。
“↑”表示表达量升高,“↓”表示表达量降低。
“↑” represented the increase of expression, and the “↓” represented the decrease of expression.
图1UPP在卵子发生中的作用
Fig.1 The role of UPP during oogenesis[14,18,23-27,30-31]
属于E3的CUL4在雄性生殖细胞减数分裂进程中同样具有重要生理作用。RNA干扰失活秀丽隐杆线虫CUL4,导致DNA复制起始蛋白CDT-1降解失败。基因打靶小鼠Cul4导致CDT-1、磷酸化p53和错配修复蛋白MLH1积聚增加及M Ⅱ粗线期及双线期细胞死亡率增加[29]。尽管有正常的联会复合体及DNA双链断裂(DNA double-strand breaks,DSBs)修复,但小鼠M Ⅱ双线期精母细胞重组小结MLH1解聚发生延迟,可能为导致M Ⅱ发生中断的分子机制。中华绒螯蟹(Eriocheirsinensis)CUL4、细胞增殖核抗原(proliferating cell nuclear antigen,PCNA)及细胞周期蛋白p21、p27、p53在初级精母细胞阶段具有高转录水平,在精子生成阶段则水平降低(除p27之外),p53介导的生殖细胞自发凋亡可能为消除生殖细胞缺陷的质量监控机制,而CRL4复合物可能通过维持初级精母细胞内p53、p21及p27平衡进而调控雄性生殖细胞M Ⅱ进程[47]。
综上所述,UPP在精子发生中的作用见图2。
4 UPP与生殖细胞减数分裂重组
DSBs及DSBs修复为M Ⅰ前期重要事件,同源重组(homologous recombination,HR)为DSBs修复途径之一,减数分裂重组是确保M Ⅰ前期生殖细胞遗传物质高效交换及保持基因组完整性的关键步骤。研究显示,酵母中属于E2的RAD6与属于E3的BRE1互作调控组蛋白H2B的K123位发生单泛素化[48]。RAD6突变导致孢子生成及DNA修复缺陷[49],失活BRE1或突变H2B残基导致减数分裂中DSBs的减少,H2B的K123位发生突变也导致细胞分裂停滞于M Ⅰ期,表明RAD6/BRE1调控的H2B单泛素化为DSBs形成机制[50]。哺乳动物中属于E2的HR6A及HR6B(与酵母RAD6同源)介导DNA损伤修复[51]。Hr6b基因敲除小鼠不育,在第一精子发生波中表现出初级精母细胞凋亡增加、M Ⅰ粗线期联会复合体较长、近端粒区出现联会复合体蛋白缺失及MLH1含量的增加,表明HR6B可能具有抑制减数分裂重组的作用[52-53]。研究表明,HR6A/HR6B与属于E3的UBR2互作[54],在维持基因组完整性及同源重组修复(homologous recombination repair,HRR)双键断裂中具有重要作用[55]。Ubr2基因缺失小鼠睾丸细胞缺乏完整的联会复合体(synaptonemal complex),造成细胞凋亡,进而导致小鼠不育。此外,Ubr2基因单核苷酸多态性分析表明其与男性非梗阻性无精子症相关[56]。

M Ⅰ:减数第1次分裂 meiosis Ⅰ;M Ⅱ:减数第2次分裂 meiosis Ⅱ;E1:泛素激活酶 ubiquitin-activating enzyme;E2:泛素结合酶 ubiquitin-conjugating enzyme;E3:泛素-蛋白质连接酶 ubiquitin-protein ligase;β-TrCP:β-转导重复相容蛋白 beta-transducin repeats-containing proteins;MDC1:mediator of DNA damage checkpoint 1;Emi1:有丝分裂早期抑制蛋白1 early mitotic inhibitor-1;DSBs:DNA双链断裂 DNA double-strand breaks;MSCI:减数分裂性染色体失活 meiotic sex chromosome inactivation;CUL4A:Cullin4A;SCF:Skp1-Cullin-F-box;RNF4:环指蛋白4 RING finger protein 4;RNF8:环指蛋白8 RING finger protein 8;RNF168:环指蛋白168 RING finger protein 168。
图2UPP在精子发生中的作用
Fig.2 The role of UPP during spermatogenesis[11,16,29,38-41,44-45,47-48]
DNA双键断裂后,γ-H2AX被标记到DSBs损伤位点,其磷酸化的第139位丝氨酸与MDC1(mediator of DNA damage checkpoint 1)蛋白作用并将MDC1招募到损伤位点,进而激活RNF8-RNF168介导的泛素化通路[57]。属于E3的RNF8和RNF168与属于E2的UBC13互作,催化损伤染色体的组蛋白H2A及H2AX[58]发生K-63连接的多聚泛素化,进而促进DNA损伤应答蛋白——P53结合蛋白(P53 binding protein 1,53BP1)和乳腺癌易感蛋白1(breast cancer susceptibility gene 1,BRCA1)对损伤位点的识别与结合[59],BRCA1 N端的环指结构域与BRCA1相关环状蛋白(BRCA1-associated RING domain,BARD1)结合形成的异源二聚体——BRCA1/BARD1具有E3活性,通过与属于E2的UBCH5C互作催化K-6连接的多聚泛素链合成,介导DSBs修复[60]。然而,有研究显示,环指蛋白RNF169与53BP1和BRCA1竞争性结合uH2A,负反馈调节同源重组修复[61]。而SUMO依赖的E3(SUMO-dependent ubiquitin ligase)RNF4能泛素化SUMO化修饰的DNA损伤检查点蛋白1(mediator of DNA damage checkpoint protein 1,MDC1)及BRCA1,与同源重组修复蛋白RAD51互作参与DSBs修复[11],RNF4缺失小鼠精母细胞凋亡增加并出现精子发生缺陷,小鼠胚胎成纤维细胞Rnf4hypo/hypo电离辐射后发生DNA永久性损伤[11]。
CUL4在生殖细胞减数分裂重组中也发挥重要作用。CUL4的C端与含环指结构域小蛋白ROC1/RNF75结合,其N端则与DNA损伤结合蛋白1(damaged DNA binding protein 1,DDB1)结合构成DDB1-CUL4-ROC1 E3复合物,其中DDB1负责特定靶蛋白的募集,而ROC1则与E2结合,介导靶蛋白泛素化[62]。哺乳动物含2种Cul4基因:Cul4A(常染色体)和Cul4B(X染色体)[16]。研究表明,Cul4A截断表达小鼠不发生ROC1连接而表现出不育;Cul4A-/-第4~8外显子缺失未检测到截短蛋白,粗线期精母细胞发生永久性DSBs,出现同源重组缺陷,雄性小鼠睾丸细胞出现显著的凋亡及M Ⅰ前期初级精母细胞缺乏,表现出严重的精子发生障碍及不育[16]。
5 去泛素酶(deubiquitinating enzymes,DUBs)在减数分裂中的作用
DUBs通过水解底物蛋白上的多聚泛素链逆转蛋白泛素化,以维持蛋白质代谢平衡。研究显示,DUBs在动物生殖细胞减数分裂过程中发挥关键作用。例如,DUBs中的泛素羧基末端水解酶家族(ubiquitin C-terminal hydrolases,UCHs)通过调节卵母细胞发育及纺锤体形成,于哺乳动物卵母细胞成熟至关重要。Uchl-1和Uchl-3 mRNA在小鼠及恒河猴卵母细胞GV期和M Ⅱ中期高表达,其中,Uchl-1与卵母细胞皮质有关,Uchl-3则与减数分裂纺锤体形成相关。GV期卵母细胞微注射UCHs抑制剂——泛素醛(ubiquitin-aldehyde,UBAL)导致大部分细胞不能进入M Ⅰ中期,并出现纺锤体和第一极体排出的异常,注射Uchl-3抗体影响卵母细胞成熟并导致纺锤体形态异常及减数分裂异常[63]。此外,UCHs缺乏卵母细胞受精率降低,且突变体胚胎无法形成囊胚[64]。UCHs在精子形成中也发挥重要生理作用[65],例如,Uchl-1在精原干细胞有丝分裂增殖中发挥作用,Uchl-3则在精母细胞减数分裂及附睾精子成熟发挥作用[65]。研究显示Uchl-1对精子发生过程中细胞凋亡的调节具有重要作用,小鼠睾丸过表达Uchl-1导致精母细胞凋亡数量增加,出现精母细胞粗线期阻滞[66],敲除Uchl-1导致PND7-14减数分裂前生殖细胞数量及凋亡蛋白TRP53、Bax及caspase-3等的水平增加[67]。
6 小 结
UPP在生殖细胞减数分裂过程不可或缺,为保障生殖细胞基因组完整性及雌雄配子正常发育的遗传基础,其相关组件缺失及突变导致减数分裂重组、MSCI及细胞周期进程阻滞等缺陷。此外,UPP在精子顶体形成、精子尾部发育、精卵结合、合子中父系线粒体降解及母系蛋白降解等过程发挥重要生理调控作用。研究显示,众多UPP组件在生殖细胞不同发育阶段发挥作用,同一UPP组件在雌性生殖细胞和雄性生殖细胞发育中具有不同的调控作用,同一E2与不同的E3结合也发挥不同生理作用,UPP这一复杂而精确的调控机制是保障生殖细胞正常减数分裂过程并产生具有受精/授精能力的配子的基础。目前,针对UPP对生殖细胞减数分裂影响的研究主要集中在小鼠和人类中,而在猪生殖过程中的研究相对较少。因此,对UPP在猪生殖细胞减数分裂过程中的生化特性、亚细胞定位、相关底物及其在猪配子生成过程中发挥的生物学功能和具体作用机制的研究,能进一步推进对其生殖功能的了解,对提高其繁殖性能具有十分重要的意义。
[1] LI C H,XIAO Z X.Regulation of p63 protein stability via ubiquitin-proteasome pathway[J].BioMed Research International,2014,2014:175721.
[2] HAO Y H,DOYLE J M,RAMANATHAN S,et al.Regulation of WASH-dependent actin polymerization and protein trafficking by ubiquitination[J].Cell,2013,152(5):1051-1064.
[3] PETERS J M,HARRIS R,FINLEY D.Ubiquitin and the biology of the cell[M].New York:Springer Science & Business Media,2013:230.
[4] TU Y Q,CHEN C,PAN J R,et al.The ubiquitin proteasome pathway (UPP) in the regulation of cell cycle control and DNA damage repair and its implication in tumorigenesis[J].International Journal of Clinical & Experimental Pathology,2012,5(8):726-738.
[5] YOSHIZAWA T,KARIM M F,SATO Y,et al.SIRT7 controls hepatic lipid metabolism by regulating the ubiquitin-proteasome pathway[J].Cell Metabolism,2014,19(4):712-721.
[6] BHAT M,KALAM R,QADRI S S Y H,et al.Vitamin D deficiency-induced muscle wasting occurs through the ubiquitin proteasome pathway and is partially corrected by calcium in male rats[J].Endocrinology,2013,154(11):4018-4029.
[7] HAMILTON A M,ZITO K.Breaking it down:the ubiquitin proteasome system in neuronal morphogenesis[J].Neural Plasticity,2013,3:196848.
[8] AL-QUSAIRI L,PROKIC I,AMOASII L,et al.Lack of myotubularin (MTM1) leads to muscle hypotrophy through unbalanced regulation of the autophagy and ubiquitin-proteasome pathways[J].The FASEB Journal,2013,27(8):3384-3394.
[9] ZHANG L P,TANG H,KOU Y,et al.MG132-mediated inhibition of the ubiquitin-proteasome pathway ameliorates cancer cachexia[J].Journal of Cancer Research and Clinical Oncology,2013,139(7):1105-1115.
[10] CHEN F Z,ZHAO X K.Ubiquitin-proteasome pathway and prostate cancer[J].Oncology Research and Treatment,2013,36(10):592-596.
[11] VYAS R,KUMAR R,CLERMONT F,et al.RNF4 is required for DNA double-strand break repairinvivo[J].Cell Death and Differentiation,2013,20(3):490-502.
[12] AN J Y,KIM E A,JIANG Y,et al.UBR2 mediates transcriptional silencing during spermatogenesis via histone ubiquitination[J].Proceedings of the National Academy of Sciences of the United States of America,2010,107(5):1912-1917.
[13] OH J S,HAN S J,CONTI M.Wee1B,Myt1,and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption[J].The Journal of Cell Biology,2010,188(2):199-207.
[14] POMERANTZ Y,DEKEL N.Molecular participants in regulation of the meiotic cell cycle in mammalian oocytes[J].Reproduction,Fertility and Development,2013,25(3):484-494.
[15] SHIN S W,SHIMIZU N,TOKORO M,et al.Mouse zygote-specific proteasome assembly chaperone important for maternal-to-zygotic transition[J].Biology Open,2012,2(2):170-182.
[16] KOPANJA D,ROY N,STOYANOVA T,et al.Cul4A is essential for spermatogenesis and male fertility[J].Developmental Biology,2011,352(2):278-287.
[17] WYKES S M,KRAWETZ S A.The structural organization of sperm chromatin[J].Journal of Biological Chemistry,2003,278(32):29471-29477.
[18] YU C,JI S Y,SHA Q Q,et al.CRL4-DCAF1 ubiquitin E3 ligase directs protein phosphatase 2A degradation to control oocyte meiotic maturation[J].Nature Communications,2015,6:8017.
[19] KIRENBERG I,SHAHAR-POMERANTZ Y,ELBAZ J,et al.New insights into the ubiquitin-proteasome pathway in oocytes resuming meiosis[J].Biology of Reproduction,2012,87(Suppl.1):126.
[20] KLEIGER G,MAYOR T.Perilous journey:a tour of the ubiquitin-proteasome system[J].Trends in Cell Biology,2014,24(6):352-359.
[21] IKEDA F,DIKIC I.Atypical ubiquitin chains:new molecular signals.Protein modifications[J].Croatian Medical Journal,2008,9(6):536-542.
[22] NIR I,HUTTNER D,MELLER A.Direct sensing and discrimination among ubiquitin and ubiquitin chains using solid-state nanopores[J].Biophysical Journal,2015,108(9):2340-2349.
[23] 沈夷清.细胞周期调控中APC/C降解机制的研究[D].硕士学位论文.天津:河北工业大学,2014:57-58.
[24] JESSUS C.MPF and the control of meiotic divisions:old problems,new concepts[J].Oogenesis:The Universal Process,2010:227-265.
[25] MARANGOS P,CARROLL J.Securin regulates entry into M-phase by modulating the stability of cyclin B[J].Nature Cell Biology,2008,10(4):445-451.
[26] MAGANGOS P,VERSCHUREN E W,CHENH R,et al.Prophase Ⅰ arrest and progression to metaphase I in mouse oocytes are controlled by Emi1-dependent regulation of APCCdh1[J].The Journal of Cell Biology,2007,176(1):65-75.
[27] BEN-ELIEZER I,POMERANTZ Y,GALIANI D,et al.Appropriate expression of Ube2C and Ube2S controls the progression of the first meiotic division[J].The FASEB Journal,2015,29(11):4670-4681.
[28] 刘相元,胡弘历,欧阳华芳,等.CRL E3泛素连接酶复合体研究进展[J].中国细胞生物学学报,2014,36(2):157-168.
[29] YIN Y,LIN C X,KIM S T,et al.The E3 ubiquitin ligase Cullin 4A regulates meiotic progression in mouse spermatogenesis[J].Developmental Biology,2011,356(1):51-62.
[30] REIS A,MADGWICK S,CHANG H Y,et al.Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes[J].Nature Cell Biology,2007,9(10):1192-1198.
[31] DEANTONI A,SALA V,MUSACCHIO A.Explaining the oligomerization properties of the spindle assembly checkpoint protein Mad2[J].Philosophical Transactions of the Royal Society of London B:Biological Sciences,2005,360(1455):637-648.
[32] ZHOU L,YANG Y,ZHANG J J,et al.The role of RING box protein 1 in mouse oocyte meiotic maturation[J].PLoS One,2013,8(7):e68964.
[33] JOSEFSBERG L B Y,GALIANI D,DANTES A,et al.The proteasome is involved in the first metaphase-to-anaphase transition of meiosis in rat oocytes[J].Biology of Reproduction,2000,62(5):1270-1277.
[34] CLOUTIER J M,TURNER J M A.Meiotic sex chromosome inactivation[J].Current Biology,2010,20(22):R962-R963.
[35] TURNER J M A.Meiotic sex chromosome inactivation[J].Development,2007,134(10):1823-1831.
[36] BAARENDS W M,HOOGERBRUGGE J W,ROEST H P,et al.Histone ubiquitination and chromatin remodeling in mouse spermatogenesis[J].Developmental Biology,1999,207(2):322-333.
[37] WANG H,WANG L J,ERDJUMENT-BROMAGE H,et al.Role of histone H2A ubiquitination in polycomb silencing[J].Nature,2004,431(7010):873-878.
[38] AN J Y,KIM E,ZAKRZEWSKA A,et al.UBR2 of the N-end rule pathway is required for chromosome stability via histone ubiquitylation in spermatocytes and somatic cells[J].PLoS One,2012,7(5):e37414.
[39] VASKOVA E A,PAVLOVA S V,SHEVCHENKO A I,et al.Meiotic inactivation of sex chromosomes in mammals[J].Russian Journal of Genetics,2010,46(4):385-393.
[40] INAGAKI A,SCHOENMAKERS S,BAARENDS W M.DNA double strand break repair,chromosome synapsis and transcriptional silencing in meiosis[J].Epigenetics,2010,5(4):255-266.
[41] INAGAKI A,SLEDDENS-LINKELS E,WASSENAAR E,et al.Meiotic functions of RAD18[J].Journal of Cell Science,2011,124(16):2837-2850.
[42] MA T,KELLER J A,YU X C.RNF8-dependent histone ubiquitination during DNA damage response and spermatogenesis[J].Acta Biochimica et Biophysica Sinica,2011,43(5):339-345.
[43] GILLOT I,MATTHEWS C,PUEL D,et al.Ret finger protein:an E3 ubiquitin ligase juxtaposed to the XY body in meiosis[J].International Journal of Cell Biology,2009,2009:524858.
[44] HOGARTH C A,MITCHELL D,EVANOFF R,et al.Identification and expression of potential regulators of the mammalian mitotic-to-meiotic transition[J].Biology of Reproduction,2011,84(1):34-42.
[45] GUARDAVACCARO D,KUDO Y,BOULAIRE J,et al.Control of meiotic and mitotic progression by the F box protein β-Trcp1invivo[J].Developmental Cell,2003,4(6):799-812.
[46] LU C,KIM J,FULLER M T.The polyubiquitin gene Ubi-p63E is essential for male meiotic cell cycle progression and germ cell differentiation in Drosophila[J].Development,2013,140(17):3522-3531.
[47] WANG Y L,LI D,YANG H D,et al.The E3 ubiquitin ligase CRL4 regulates proliferation and progression through meiosis in Chinese mitten crabEriocheirsinensis[J].Biology of Reproduction,2001,94(3):65.
[48] TURCO E,GALLEGO L D,SCHNEIDER M,et al.Monoubiquitination of histone H2B is intrinsic to the Bre1 RING domain-Rad6 interaction and augmented by a second Rad6-binding site on Bre1[J].Journal of Biological Chemistry,2015,290(9):5298-5310.
[49] GAME J C,CHERNIKOVA S B.The role of RAD6 in recombinational repair,checkpoints and meiosis via histone modification[J].DNA Repair,2009,8(4):470-482.
[50] YAMASHITA K,SHINOHARA M,SHINOHARA A.Rad6-Bre1-mediated histone H2B ubiquitylation modulates the formation of double-strand breaks during meiosis[J].Proceedings of the National Academy of Sciences of the United States of America,2004,101(31):11380-11385.
[51] KIM J,GUERMAH M,MCGINTY R K,et al.RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells[J].Cell,2009,137(3):459-471.
[52] BAARENDS W M,WASSENAAR E,HOOGERBRUGGE J W,et al.Increased phosphorylation and dimethylation of XY body histones in the Hr6b-knockout mouse is associated with derepression of the X chromosome[J].Journal of Cell Science,2007,120(11):1841-1851.
[53] BAARENDS W M,WASSENAAR E,HOOGERBRUGGE J W,et al.Loss of HR6B ubiquitin-conjugating activity results in damaged synaptonemal complex structure and increased crossing-over frequency during the male meiotic prophase[J].Molecular and Cellular Biology,2003,23(4):1151-1162.
[54] TASAKI T,KWON Y T.The mammalian N-end rule pathway:new insights into its components and physiological roles[J].Trends in Biochemical Sciences,2007,32(11):520-528.
[55] KWON Y T,XIA Z X,AN J Y,et al.Female lethality and apoptosis of spermatocytes in mice lacking the UBR2 ubiquitin ligase of the N-end rule pathway[J].Molecular and Cellular Biology,2003,23(22):8255-8271.
[56] MIYAMOTO T,TSUJIMURA A,MIYAGAWA Y,et al.Single nucleotide polymorphism in theUBR2 gene may be a genetic risk factor for Japanese patients with azoospermia by meiotic arrest[J].Journal of Assisted Reproduction and Genetics,2011,28(8):743-746.
[57] CUI L,LI W.Role of ubiquitination in meiotic recombination repair[J].Science China Life Sciences,2010,53(4):447-454.
[58] PINATO S,SCANDIUZZI C,ARNAUDO N,et al.RNF168,a new RING finger,MIU-containing protein that modifies chromatin by ubiquitination of histones H2A and H2AX[J].BMC Molecular Biology,2009,10(1):55.
[59] PANIER S,DUROCHER D.Regulatory ubiquitylation in response to DNA double-strand breaks[J].DNA Repair,2009,8(4):436-443.
[60] POLANOWSKA J,MARTIN J S,GARCIA-MUSE T,et al.A conserved pathway to activate BRCA1-dependent ubiquitylation at DNA damage sites[J].The EMBO Journal,2006,25(10):2178-2188.
[61] POULSEN M,LUKAS C,LUKAS J,et al.Human RNF169 is a negative regulator of the ubiquitin-dependent response to DNA double-strand breaks[J].The Journal of Cell Biology,2012,197(2):189-199.
[62] POMERANTZ Y,ELBAZ J,BEN-ELIEZER I,et al.From ubiquitin-proteasomal degradation to CDK1 inactivation:requirements for the first polar body extrusion in mouse oocytes[J].The FASEB Journal,2012,26(11):4495-4505.
[63] MTANGO N R,SUTOVSKY M,VANDEVOORT C A,et al.Essential role of ubiquitin C-terminal hydrolases UCHL1 and UCHL3 in mammalian oocyte maturation[J].Journal of Cellular Physiology,2012,227(5):2022-2029.
[64] MTANGO N R,LATHAM K E,SUTOVSKY P.Deubiquitinating enzymes in oocyte maturation,fertilization and preimplantation embryo development[M]//SUTOVSKY P.Posttranslational protein modifications in the reproductive system.New York:Springer,2014:45-48.
[65] KWON J,WANG Y L,SETSUIE R,et al.Developmental regulation of ubiquitin C-terminal hydrolase isozyme expression during spermatogenesis in mice[J].Biology of Reproduction,2004,71(2):515-521.
[66] WANG Y L,LIU W Z,SUN Y J,et al.Overexpression of ubiquitin carboxyl-terminal hydrolase L1 arrests spermatogenesis in transgenic mice[J].Molecular Reproduction and Development,2006,73(1):40-49.
[67] KWON J,MOCHIDA K,WANG Y L,et al.Ubiquitin C-terminal hydrolase L-1 is essential for the early apoptotic wave of germinal cells and for sperm quality control during spermatogenesis[J].Biology of Reproduction,2005,73(1):29-35.
*Corresponding author, professor, E-mail: chenbin7586@126.com

