用于医学磁共振影像的稀土上转换发光纳米材料
孟宪福,刘艳颜,步文博
用于医学磁共振影像的稀土上转换发光纳米材料
孟宪福,刘艳颜,步文博*
(华东师范大学化学与分子工程学院上海市绿色化学与化工过程绿色化重点实验室,上海 200062)
上转换发光纳米颗粒是一类遵循反斯托克斯原理的新型发光材料,具有发光强度高、发光稳定、无组织背景荧光、无光漂白、低毒性以及较好的生物相容性等优点,其激发光为红外或者近红外光,活体组织穿透深度高,在生物医学检测、诊断以及疾病治疗等方面均具有潜在的应用价值。磁共振成像是目前医学临床常用的影像检测手段之一,具有软组织成像质量高、空间分辨率高、无辐射、无损伤等优点,在心脑血管、肿瘤等疾病的影像诊断方面具有重要作用。本文将聚焦于近年来稀土上转换发光纳米材料在磁共振影像方面的研究进展,通过介绍磁共振成像机理、磁共振造影剂的构建、上转换发光纳米材料的设计及在磁共振医学影像、疾病治疗等方面的应用,并结合我们课题组基于UCNP医学磁共振多模态影像的相关研究进展,对上转换发光纳米颗粒在磁共振成像方面的应用研究进行探讨和展望。
上转换发光纳米颗粒;磁共振成像;多模态影像;疾病治疗
1 引 言
上转换发光纳米颗粒(Upconversion nanoparticle,UCNP)是一类特殊的稀土发光材料,自Bloembergen首次提出上转换发光的激发态吸收原理以来[1],人们对UCNP的认识和研究不断深入。与传统发光材料不同,UCNP发光机理遵循反斯托克斯(Anti-Stokes)定律,通过吸收两个或多个低能光子,发射出一个高能光子,即将低频率低能量的激发光转化为高频高能的发射光[2]。这一特殊的发光机理赋予了上转换发光纳米材料诸多优势:(1)激发光一般为红外或者近红外光,活体组织穿透深度高且光损伤小;(2)无光漂白现象,能够有效地避免自发荧光干扰,提高诊断检测的信噪比与灵敏度;(3)激发谱与发射谱带窄,发光稳定性好、强度高,材料毒性低、生物相容性良好等[3-9]。因此,UCNP在生物标记、检测以及疾病(如肿瘤)的影像诊断、治疗等方面具有巨大的应用潜力[10-11]。
当今社会,癌症作为影响人类健康的重大杀手,已经严重影响了人们的健康。国际学术期刊CA Cancer JClin发表的中国2015年度癌症统计数据显示,中国2015年约有430万肿瘤新发病例,约280万人次死于癌症[12]。然而,癌症不是不可治愈的,早期癌症患者中大约有百分之八十的患者可以完全治愈,反之,晚期癌症患者存活率较低。因此,人们对其早期诊断越来越重视。磁共振成像(Magnetic resonance imaging,MRI)是目前临床上常用的一种影像检查手段,可对人体任何部位进行成像,其软组织成像质量高,可用于精准发现体内病灶区,且具有无辐射、无损伤等优点,在医学影像诊断方面具有重要作用,尤其对于肿瘤筛查。但目前MRI在肿瘤的早期诊断方面依然存在非常大的困难。为了提高早期肿瘤的诊断率,早发现早治疗,医务工作者通常会借助于MRI造影剂来增强病变部位的显像,最大限度地提高病灶区与正常组织的对比度,实现对病变区灵敏精确的诊断检测。目前,临床上主要使用的MRI造影剂为马根维显(Magnevist),它可快速增强病变区显影,提高病变区信号,在T1序列下呈现出高亮的信号图像,主要用于心脑血管疾病以及肿瘤等疾病的检查诊断。然而遗憾的是,马根维显血液半衰期短,体内代谢快,不能提供较长时间的影像效果,且其纵向弛豫率较低,临床应用时需要较大的用量,会增加肾代谢的负担[13]。
随着纳米科学技术的进步,弛豫率高、血液半衰期长、生物相容性好的MRI纳米造影剂不断涌现。同时,MRI技术发展也由单一的结构影像(T1/T2)逐步向结构影像与功能影像(如:弥散加权成像(DWI)、化学交换饱和转移成像(CEST)、磁共振波谱成像(MRS)等)融合;MRI检查手段逐步由单一模态向多模态(如:电子计算机断层扫描(CT)、正电子发射计算机断层扫描(PET)、荧光成像、超声成像(US)以及光声影像等手段)影像手段发展,从而可以更加精准、及时地发现早期病变,判断病变区的临床周期,更好地指导临床用药,及时消灭恶性肿瘤等病变。多模态纳米影像探针在实现肿瘤等疾病高效诊断的同时,还可结合新型高效的肿瘤治疗手段(如光热治疗、光动力学治疗以及化学动力学治疗等),用以构建诊疗一体化纳米探针,实现对肿瘤影像介导下的高效精准治疗。因此,发展新一代多功能MRI纳米探针对于医学临床诊断和治疗尤为重要。
UCNP作为一类新颖的发光纳米材料,通过对其精确的组分调控及结构设计,可将荧光、MRI、CT以及PET等多种影像技术融为一体,结合化疗、放疗以及光热、光动力等新型治疗手段,可构建高性能诊疗一体化纳米探针。本文将主要聚焦于近年来UCNP在MRI方面的研究进展,通过介绍磁共振成像机理、磁共振造影剂的构建、UCNP的设计以及在磁共振生物医学等方面的应用,并结合我们课题组的相关研究进行综述,对UCNP在磁共振影像学的应用研究进行探讨和展望。
2 磁共振成像
2.1 MRI原理
磁共振成像的原理首先要追溯到核磁共振(Nuclear magnetic resonance,NMR)的发现。随着NMR理论的发展和完善,磁共振成像技术如雨后春笋般涌现并迅速发展。1970年,来自美国纽约州立大学的瑞蒙·达马迪安(Raymond Damadian),通过对患有恶性肿瘤的大鼠进行NMR测试时,发现了正常组织与肿瘤组织核磁信号有明显差别,且当受激时,可以发现两种不同的信号,即T1/T2,并首次实现了人体活组织T1测量,这使得MRI在医学应用方面具有开拓性意义。随着时间的推移,保罗·劳特伯(Paul Lauterbur)与彼得·曼斯菲尔德(Peter Mansfield)先后对MRI技术优化改进,促使实时磁共振成像技术的诞生,使得图像质量更好、成像过程更快。二人也因对MRI的巨大贡献共享了2003年诺贝尔生理学或医学奖。MRI技术理论的不断完善,磁共振成像设备的不断优化,使得MRI在生物医学领域中具有举足轻重的作用。
MRI成像原理离不开弛豫(Relaxation)。所谓弛豫是指低能原子核受外加射频脉冲激发后,以非辐射跃迁的方式回到低能态的过程[14-15]。自旋核的弛豫一般分为两个过程:自旋-晶格弛豫(Spin-lattice relaxation)与自旋-自旋弛豫(Spinspin relaxation)。自旋-晶格弛豫是指高能态的受激核附近有能使其跃迁到低能态的磁场,受激核将自身能量以转动、振动或者平移的热能传递方式传递给低能磁场,自身弛豫到低能态。自旋-晶格弛豫过程可以看作为自旋核与环境能量交换的过程。在该过程中,自旋核从共振激发恢复到平衡态时所用时间为自旋-晶格弛豫时间,用T1来表示;T1还可以看作成磁化强度矢量M的纵向分量Mz弛豫(纵向弛豫)至稳态值63%所需时间,如图1(a)所示。自旋-自旋弛豫过程,是指高能态核将能量传递给低能态核,本身跃迁到低能态,而后者则跃迁到高能态,整个体系总能量并未变化。因此,自旋-自旋弛豫可以看成是一个自旋核与另一个自旋核能量交换的过程。该过程中,自旋核能量交换所用时间成为自旋-自旋弛豫时间,用T2表示;T2又可以看作磁化强度矢量M的横向分量Mxy弛豫(横向弛豫)至最大值37%所需时间,如图1(b)所示[16-17]。两种弛豫机理不同,造成在一般生物体内通常T1>T2。

图1 纵向弛豫和横向弛豫曲线Fig.1 Curves of longitudinal relaxation and transverse relaxation
质子在顺磁性环境中纵向弛豫率R1与横向弛豫率R2可用经典的S-B公式表示[18]:

式中,S是电子-自旋量子数;γ是磁旋比;ωi与ωs分别是核与电子的自旋角频率;r是弛豫中的氢核到顺磁性物质中心的距离;τc与τe分别是偶极-偶极耦合与标量耦合相关时间;A、h、β均为物理常数。
而其中的 τe可以定义为[19]:
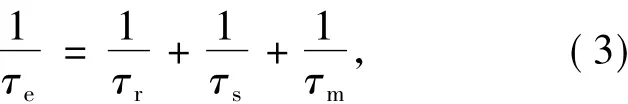
式中,τr、τs与 τm分别为转动时间、电子-自旋弛豫时间以及化学变化相关时间(水分子滞留时间)。
顺磁性物质中未成对电子可以改变上述τr、τs与τm以及结合水的个数,进而可以改变纵向弛豫率R1与横向弛豫率R2的大小,达到对比增强的目的。
一般来说,T1-MRI成像机理用内球水机理、中间层球水机理与外球水机理来解释[20-21]。紧密结合在顺磁性物质上的水分子受到的弛豫为内球水弛豫机理,中间层水分子通过氢键等作用力与顺磁性物质结合在一起导致的弛豫为中间层球水弛豫原理,外围自由扩散水分子受到顺磁性中心的弛豫成为外球水机理。增加结合水个数,延长τr,优化 τs与 τm可以增强造影剂的 T1弛豫性能,其中将顺磁性离子固定在纳米粒子表面或者形成螯合剂是一般常用的增强T1的手段[22-26]。
对于T2-MRI来说,根据经典的量子力学外球理论[27],T2造影剂会改变磁场的均匀性,使得水分子在磁场中发生相位变化,进而影响氢核弛豫时间。因此,R2也可以表示为:
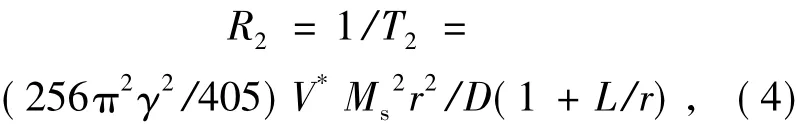
其中,Ms与r分别为磁性粒子的磁化强度与半径,γ为旋磁比,V*是体积分数,D与L分别为水分子的扩散率及磁性核外层不透水的壳层厚度。因此,可以通过优化磁化强度、壳层厚度、粒子半径与体积等参数来增强T2造影性能。
此外,MRI在成像过程中一开始得到的并不是图像,而是包含空间编码信息的原始数据。这些原始数据可以通过K空间来描绘,K空间实际则是傅里叶变换的频率空间,通过傅里叶变换及K空间不但可以把NMR采集的数据运算简单化,还可以有效地将数据转变为人们直观肉眼可见的黑白图像,因此,MRI技术与傅里叶变换原理密不可分[15]。
2.2 MRI造影剂
目前,常用的成像原子核有1H、19F、31P等。临床中最常用的为1H谱,是因为人体中含有许多水分,具有大量的氢质子。影响体内氢质子弛豫的有诸多因素,如结合水效应(蛋白质等生物大分子会结合具有极性的水分子,进而使水分子的运动频率下降接近拉莫尔频率)、顺磁性或者超顺磁性物质(如铁、锰、钆与钬等稀土元素以及自由基等)以及脂类分子等[28]。体内的许多病变也会影响磁共振信号,如癌症、脑卒中等疾病[29-30]。肿瘤早期的病变组织与正常组织很难区分,单纯MRI影像效果差,这就给医生们在肿瘤早期诊断时带来了困惑。因此,医务工作者借助磁共振造影剂来对比增强二者之间的差别,进而可以更精准区分病变组织和正常组织,做到早发现、早治疗。
磁共振造影剂,也叫作磁共振对比度增强剂(Contrast enhancement agents),是能够增强磁共振图像信息的药物制剂,在提高组织分辨率、反映组织血流血供情况以及改变组织特征参数等方面具有重要作用[31-32]。造影剂可以分为阳性造影剂(正增强,图像变亮,T1造影剂)和阴性造影剂(负增强,图像变暗,T2造影剂)。顺磁性物质可以影响质子的弛豫,诸多金属离子具有明显的顺磁性,表1列举了部分金属离子的结构与性质。其中,金属离子对 MRI的作用可以归为:(1)Gd3+、Fe3+、Mn2+等可以作为弛豫增强剂;(2)Dy3+、Ho3+、Eu2+等可以作为共振频率偏移剂;(3)Gd3+、Dy3+、Ho3+等常用作磁化率增强剂。临床上常用的磁共振造影剂为钆剂(二亚乙基三胺五乙酸钆,Gd-DTPA),可显著缩短组织的T1,增强组织显像。然而,其体内代谢快,血液半衰期短,不能提供较长时间的影像效果;其次,其纵向弛豫率较低,在临床应用时需要较大的用量,进而会增加肾代谢的负担。
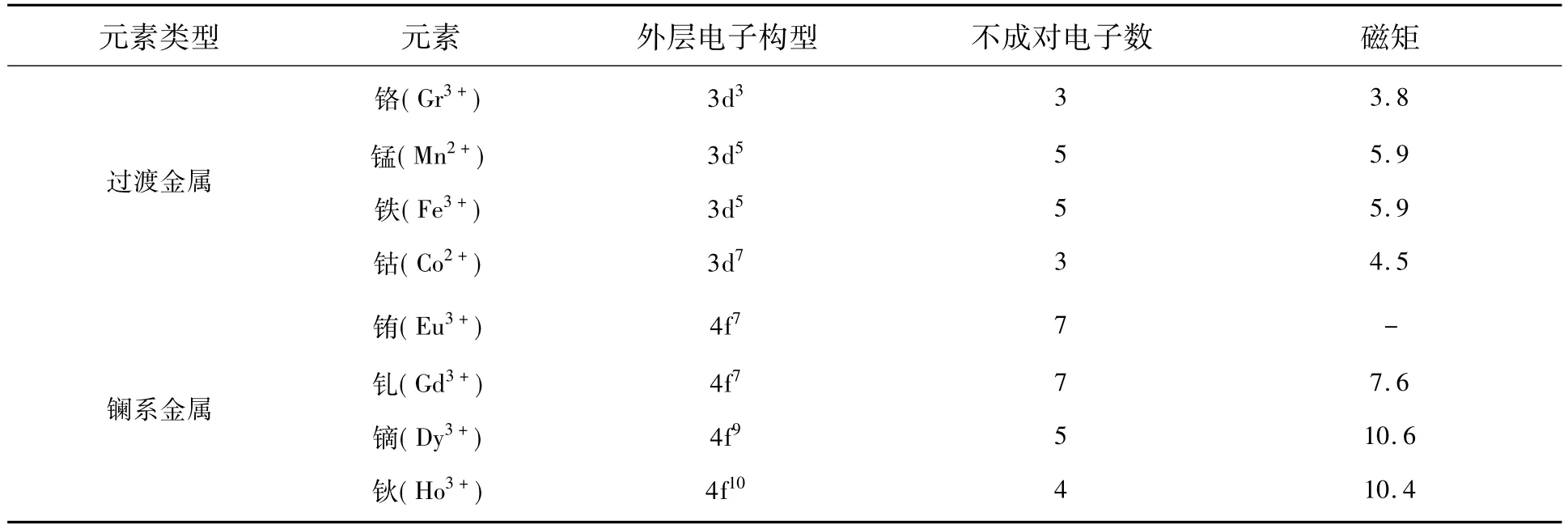
表1 部分金属离子的结构与性质Tab.1 Structures and properties of metal ions
纳米磁共振造影剂是近年来人们关注较多的一类对比剂,纳米材料由于具有特殊的量子限域效应、大的比表面积等特点,在MRI造影剂方面具有极大的潜力。纳米MRI造影剂血液半衰期长,表面易于改性修饰,具有毒性低、生物相容性好等优点;其次,通过将钆、镝、钬等具有顺磁性离子掺杂进纳米材料中,或者直接合成铁磁性、亚铁磁性以及超顺磁性的磁性纳米颗粒,可以获得较大的弛豫率,进而在较少的对比剂用量下获得较高的对比度;最后,纳米MRI造影剂可以通过修饰改性嫁接上靶向分子、药物分子或者结合一些如放疗、光热、光动力学治疗等手段,再通过与CT/PET/荧光、超声等影像手段有机结合,可以构建多模态影像介导下的诊疗一体化纳米探针,用于疾病的早期精确诊断和高效治疗。
3 上转换纳米材料用于磁共振造影剂
3.1 UCNP的发光机理、组成与制备
UCNP是一类特殊的发光材料,其发光遵循anti-Stokes机理[33],一般激发光为低能的红外或者近红外光,UCNP通过吸收一个或者多个低频率光子,发射出一个高频率的光子,如图2所示。目前,人们一般认为上转换发光机理可以分为激发态吸收、能量传递以及光子雪崩等[34]。其中,又以能量传递的方式不同为原则,分为伴随能量传递的激发态吸收、连续能量传递、交叉驰豫、协同敏化与协同增强等[34]。

图2 上转换发光示意图与UCNP分别掺杂Yb3+、Er3+与Tm3+发光能级示意图[35]。Fig.2 Luminescence scheme of UCNP and the energy level scheme of Yb3+,Er3+,Tm3+doped UCNP[35].
稀土离子具有丰富的4f电子能级,较长的荧光寿命,因此,UCNP一般是稀土离子掺杂的纳米材料,由基质、敏化剂与激活剂构成。基质一般为光学惰性的磷酸物、氧化物或者氟化物构成,由于稀土离子中Y3+与La3+无4f电子,Gd3+4f电子层为半充满,Lu3+4f电子层为全充满,它们是光学惰性的,因而常用作基质材料的掺杂离子;Yb3+由于具有较大的吸收截面,只有一个激发态,对近红外光吸收效率高,可以将吸收的能量有效地转移给激活剂,因此,Yb3+是UCNP中常见的敏化剂;具有多能级结构的发光中心,如Nd3+、Pr3+、Tb3+、Sm3+、Er3+、Tm3+、Ho3+等拥有丰富的发光能级,由于4f能级的电子屏蔽效应使其发光寿命较长,因而常用作激活剂[36-41]。到目前为止,NaYF4[42]与 NaLuF4[43]被认为是上转换发光效率较高的理想发光基质,尤其是对于Yb3+-Tm3+与Yb3+-Er3+体系而言[35]。
目前,制备UCNP的方法有很多种,主要分为共沉淀法、溶胶凝胶法、热分解法以及水/溶剂热法。
共沉淀法是将含有一种或者几种离子的可溶性盐溶液加入沉淀剂生成难溶性盐,再经过加热煅烧等后处理手段,得到纳米级的材料。Martin等首先利用共沉淀法,在低温下合成了NaYF4∶Yb,Pr上转换纳米材料[44]。最初得到的材料为立方相(α),当反应时间从24 h延长到240 h后,材料可由α相完全转化为六方相(β)。王猛等利用络合能力极强的络合剂二乙三胺五乙酸(DTPA)作为沉淀剂,合成了粒径可调的NaYF4∶Yb,Er上转换纳米粒子[45]。共沉淀法的反应条件温和,操作简单,成本低,然而合成的纳米粒子经常会遭遇严重团聚,不利于后续生物应用。
溶胶凝胶法是利用有机金属盐或者卤化物为原料,首先形成溶胶,再聚合成凝胶,通过干燥煅烧等步骤得到所需纳米粒子。A Biswas等利用溶胶凝胶法合成了Er3+/Yb3+共掺杂的LaF3-SiO2纳米粒子[46]。虽然该方法操作简单,但是反应时间长,需要高温煅烧,导致纳米粒子形貌、粒径等不可控,难以进一步修饰。
热分解法是由北京大学严纯华教授课题组发明的一种以稀土三氟乙酸盐为原料,制备上转换纳米粒子的新方法。他们采用该方法合成了形貌均一、粒径可控的LaF3纳米晶[47]。该方法要求条件苛刻,需高温无水无氧操作,得到的粒子为油溶性,需要进一步改性修饰才能应用于生物研究。
溶剂热或者水热法制备UCNP,其原理相通,均为高温高压下裂解稀土前驱体,是目前常用的合成方法[40,48-52]。溶剂热法与水热法的区别在于二者溶剂不同,一种为油溶性溶剂,一般为油酸和十八烯体系;一种为水相体系,反应溶剂多为水或者水与其他溶剂的混合。溶剂热法合成的UCNP形貌、粒径可控,工艺较成熟,可以大量合成;缺点是反应条件苛刻,需高温无水无氧,合成粒子为油溶性,如若应用于生物医学,需要进一步改性。水热法合成的UCNP,反应条件温和,操作简单,污染较低;其缺点为合成粒径不可控,容易团聚。
综合以上制备方法,考虑各种因素,一般常采用水热/溶剂热法或者热分解法合成UCNP,再经过进一步离子掺杂、改性修饰等,得到具有MRI性能的UCNP用于生物医学研究。
3.2 金属离子掺杂的UCNP用于MRI
目前,公认的上转换发光效率较高的基质为NaYF4与NaLuF4,通过掺杂激活剂与敏化剂等金属离子,赋予UCNP良好的发光性能。La系稀土离子位于同主族,最外层电子结构相似,半径相近,经常被用来掺杂到 UCNP中。Gd3+、Dy3+、Ho3+、Eu2+等具有顺磁性,Gd3+、Eu2+[53]常用作T1造影剂[54-57],Dy3+、Ho3+具有较大的磁矩,常用作T2造影剂[58-60]。其中,Gd3+离子由于外层 7个单电子,具有最高的单电子数,较强的顺磁性,经常掺杂到UCNP或者基质中赋予其良好的T1性能。另外,由表1可知,过渡金属Fe、Mn、Co等金属离子也具有不同的单电子数,经常作为顺磁性或者铁磁性离子掺杂到UCNP中,以提高其上转换发光(UCL)与MRI性能。
3.2.1 Gd3+掺杂的 UCNP
由于Gd3+4f电子层具有7个单电子,具有较强的顺磁性,与水分子的配位点最多,因而广泛被用作T1加权MRI造影剂[61-71]。UCNP中 Gd3+的掺杂,不但对UCNP的发光有较大影响,还会赋予其MRI性能。但是体相钆和表面钆的掺杂会对UCNP的MRI性能有不同影响。在这方面,我们课题组做了系统研究。首先,我们构建了体相钆与表面钆掺杂的UCNP模型,详细地研究了二者在T1加权成像上的区别,得知影响UCNP中MRI的关键因素为表面钆,而体相钆对MRI的作用几乎可以忽略不计。此外,钆的掺杂对于UCNP的UCL与MRI具有正负晶格屏蔽效应,这对于合理构建所需磁共振荧光探针具有重要的指导意义,如图3(a)所示[72]。约翰逊(Johnson)等合成了不同粒径的超小NaGdF4基质,通过调控其粒径大小可以将其弛豫率由3.0 mmol-1·L·s-1提升到7.2 mmol-1·L·s-1,得出了可以通过调控纳米粒子粒径尺寸来改善造影剂性能的结论,如图3(b)[73]。本课题组也通过热解法合成了~2 nm NaGdF4,通过在外层包裹磷脂PEG并嫁接DTPA用来防止Gd3+的泄露,成功实现了兔子动脉粥样硬化斑块磁共振造影显像[74],如图3(c)所示。
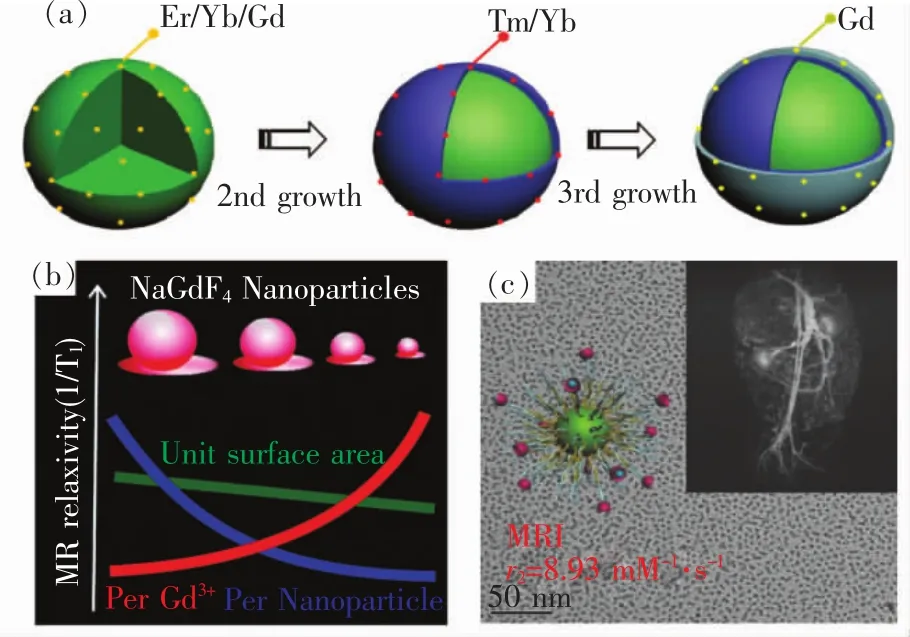
图3 (a)体相钆与表面钆掺杂对于UCNP上转换发光和MRI正负晶格屏蔽效应研究[72];(b)超小NaGdF4基质的粒径对于 MRI性能的影响[73];(c)超小NaGdF4用于兔子动脉粥样硬化斑块的研究[74]。Fig.3 (a)Positive and negative lattice shielding effects co-existing in Gd(Ⅲ) ionsdoped upconversion nanoprobes[72].(b)MRI study of ultra-small NaGdF4[73].(c)Ultrasmall NaGdF4 nanodots for efficient MR angiography and atherosclerotic plaque imaging[74].
为获得高弛豫率钆(Gd3+)基无机纳米造影剂,目前常用的方法主要包括:调控造影剂的粒径和形貌、造影剂表面修饰和包裹等;然而遗憾的是,这些调控技术对提高钆基无机纳米造影剂的磁共振成像性能效果有限,在临床3 T仪器上该类造影剂的r1值依然较低。近期,我们课题组将“缺陷调控技术”新概念创新性地引入到钆基无机纳米造影剂磁共振性能调控研究中,通过调控钆掺杂乌青铜纳米颗粒(NaxGdWO3-x)的氧空位缺陷,成功实现了造影剂磁共振性能的调控和优化[75]。我们利用氧空位对氧原子天然的亲和力,赋予氧空位独特的结合水分子能力,进而显著影响水分子与Gd3+的相互作用;系统研究了不同氧空位浓度对Gd3+的弛豫时间的内在影响,发现氧空位的引入可以显著提高钆基无机纳米颗粒的弛豫率,在 3 T 下,弛豫率高达 32 mmol-1·L·s-1,是临床钆剂的8倍。荷瘤鼠实验表明,该新型造影剂可以实现低剂量下高效血池造影成像与肿瘤的精准影像诊断。值得一提的是,该研究提出的“缺陷调控技术”(氧空位)不但可以调控磁共振影像性能,同时可以利用氧空位诱导产生极化子所具有的极佳光热性能,将近红外光高效地转为热量“烧死”肿瘤细胞,实现了基于MRI精准影像介导下的肿瘤高效光热治疗(PTT)。这类新型的“缺陷调控技术”将为高性能无机纳米诊疗剂的设计提供借鉴性研究思路。
3.2.2 Mn2+掺杂的 UCNP
Mn2+最外层电子数为5,具有较强的顺磁性,因此,Mn基纳米材料常用作T1加权磁共振造影剂[76]。Mn2+掺杂到UCNP中,不仅可以改善其发光性能[77-81],还能给 UCNP带来优良的 MRI性能[82]。林君教授等采用溶剂热法,合成了Mn2+掺杂的中空CaF2∶Yb3+/Er3+上转换纳米颗粒,通过共价嫁接Pt前药来响应肿瘤的还原环境,实现了UCL/MRI/CT三模式介导下的肿瘤治疗[83],如图4(a)。Yang等设计合成了核壳结构的Fe3O4@Mn2+-doped NaYF4∶Yb/Tm纳米粒子,Mn2+的掺杂赋予材料优异的T1-MRI加权性能,再结合内核Fe3O4的T2-MRI加权性能以及来自UCNP的上转换发光,得到了兼具UCL以及T1/T2双加权的MRI影像造影剂[84],如图4(b)所示。

图4 (a)Mn2+掺杂的中空UCNP用于UCL/MRI/CT三模式介导下的肿瘤治疗[83];(b)T1/T2-MRI以及UCL成像造影剂的制备[84]。Fig.4 (a)Mn2+doped hollow UCNP with UCL/MRI/CT trimodality imaging for tumor therapy[83].(b)Preparation of UCL/T1/T2-MRI contrast agents[84].
3.2.3 Dy3+与 Ho3+掺杂的 UCNP
Dy3+与Ho3+同属于La系元素,具有稀土元素丰富的4f电子能级,虽然二者4f轨道单电子较少,但是二者有较大的磁矩以及相对较短的电子弛豫时间,通过改变局部磁场的均一性,使得质子失相位,产生不同的拉莫尔频率,进而缩短T2弛豫时间[85]。
Ho3+由于具有丰富的4f电子能级,常被用来作为UCNP的激活剂[86-89],同时由于其较大的磁矩,可以用做T2加权成像造影剂[90-92]。我们课题组通过对UCNP化学组分的调控,制备出Ho3+掺杂的NaYbF4上转换纳米颗粒,并首次对UCNP中掺杂的敏化剂Yb3+和激活剂Ho3+二者的T2-MRI性能进行了系统性研究。此外,Yb3+和Ho3+由于重金属元素特性,同时具有良好的CT造影性能,实现了UCNP中MR/UCL/CT三模态协同增强影像,并对荷瘤鼠原位脑胶质瘤实现了精确MRI影像诊断[93],如图5(a)所示。
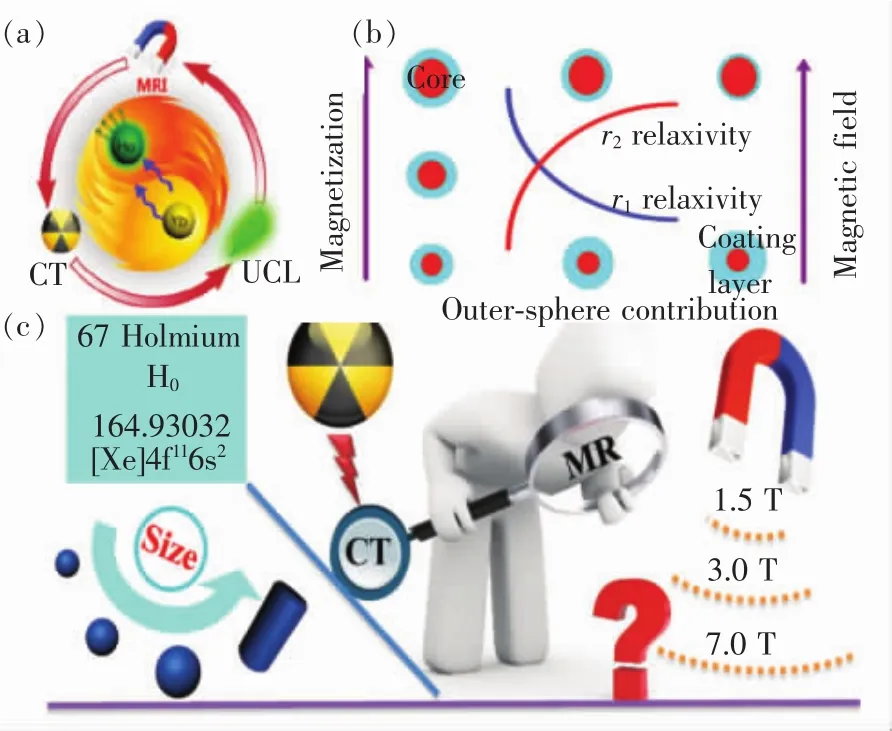
图5 (a)Ho3+掺杂的NaYbF4上转换纳米颗粒用于MR/UCL/CT三模成像以及荷瘤鼠原位脑胶质瘤的 MRI精确影像诊断[93];(b)NaDyF4与 NaHoF4纳米颗粒用于高场磁共振成像[98];(c)不同粒径的NaHoF4纳米颗粒其磁化率与场强的关系[59]。Fig.5 (a)Single Ho3+-doped upconversion nanoparticles for high-performance T2-weighted brain tumor diagnosis and MR/UCL/CT multimodal imaging[93].(b)NaDyF4 and NaHoF4 for high field magnetic resonance imaging[98].(c)PEGylated NaHoF4 nanoparticles as contrast agents for both X-ray computed tomography and ultra-high field magnetic resonance imaging[59].
为满足医学科技发展的需要,提高场强是改善MRI图像质量和信噪比的重要手段之一。目前,临床所用磁场场强多为3.0 T,愈来愈多的高场强7.0 T甚至超高场强9.4 T磁共振设备已开发,因而对于高场强下适用的MRI造影剂愈发需求。由于Ho3+与Dy3+具有较大磁矩和较小横向弛豫时间,其弛豫率r2随着场强的增高而增加,适用于高场甚至超高场T2造影剂的制备[69,94-97]。Zhang等详细研究了NaDyF4与NaHoF4纳米颗粒粒径、形状、表面修饰以及Zeta电位在超高场强9.4 T情况下与弛豫率的关系[98],如图5(b)所示;结果发现,两种造影剂均具有较大的r2/r1(分别为410和781),适合作为T2造影剂。通过调控粒径与表面修饰,再加上理论计算得出r1在较高频率下几乎不受粒径等因素影响,只有质子拉莫尔频率在1 MHz左右时才会受影响,而r2在高场强下随着粒径等参数的变化会显著改变。我们课题组也制备了不同粒径的NaHoF4纳米颗粒,系统研究了粒径在超高磁场情况下对磁化率的影响[59];通过实验和理论研究,发现r2确实随着场强的增大而增大,且当纳米粒子粒径小于7.4 nm时,居里机制占据主导作用,此时粒子粒径增加,r2值急剧减小;当NaHoF4纳米颗粒粒径大于7.4 nm时,极化机制占据主导作用,随着粒子粒径的增大,r2逐渐增大,如图5(c)所示。
3.2.4 UCNP与其他纳米材料结合构建复合纳米材料用于MRI
除了将金属离子掺杂赋予UCNP磁共振影像的功能外,还可以将其他纳米材料与UCNP结合构建复合纳米材料,以获得良好的MRI性能。铁基的纳米材料一般具有顺磁性、超顺磁性或者铁磁性,生物相容性好、毒性低,被广泛应用于MRI领域[99-112]。因此,将UCNP与铁基纳米材料复合构建纳米材料可以实现UCL/MRI等多模成像造影剂,扩大其在生物医学领域的应用价值。
复旦大学李富友教授等合成了中空核壳结构的Fe3O4@NaLuF4∶Yb,Er/Tm纳米复合物。其中,Fe3O4具有超顺磁性,可用于 MRI造影剂;UCNP具有优异的UCL发光性能,且由于高原子序数Lu的引入,进而赋予了该材料良好的CT影像功能。通过巧妙地构建复合纳米材料,可以实现同一探针 UCL/MRI/CT三模成像功能[113],如图6(a)所示。我们课题组也利用“颈部融合”方法,合成了上转换荧光/磁共振成像双模式复合新型探针,将上转换荧光与磁共振成像高效结合[114]。所用工艺简单,产物可达克级量产,重复性好,可以实现肿瘤细胞及小鼠活体UCL/MRI双模成像,如图6(b)所示。进一步,我们深入研究了Fe3O4纳米颗粒对UCNP发光性能的影响,通过实验发现当二者摩尔比在很小的范围内时,Fe3O4纳米颗粒不但不会猝灭UCNP的发光,反而会增强其发光;另外,通过引入SiO2层,可以更好地防止Fe3O4纳米颗粒对UCNP荧光猝灭[115],如图6(c)所示。Yang等也构建了 Fe3O4@g-C3N4-UCNPs-PEG纳米复合材料,其中UCNPs内核中掺杂Gd3+,赋予体系T1加权MRI成像性能,Fe3O4的顺磁性也赋予体系T2影像功能;为了防止Fe3O4猝灭UCNPs的发光,在二者夹层中间引入C3N4,形成了三明治结构。半导体C3N4不但可以抑制Fe3O4对UCNPs的猝灭,其本身可以吸收UCNPs发出的光,进而产生活性氧,实现了对肿瘤的光动力治疗。该体系融合磁靶向与UCL,成功构建了多功能影像介导的光动力学治疗体系[116],如图 6(d)所示。

图6 (a)Fe3 O4@NaLuF4∶Yb,Er/Tm用于UCL/MRI/CT三模成像[113];(b)通过“颈部融合”的方法构建上转换荧光/磁共振成像双模式复合新型探针[114];(c)研究Fe3O4对于UCNP荧光性能的影响[115];(d)核壳-卫星结构的Fe3O4@g-C3N4-UCNPs-PEG纳米复合材料用于T1/T2双模影像介导的磁靶向光动力治疗[116]。Fig.6 (a)Fe3 O4@NaLuF4∶Yb,Er/Tm for UCL/MRI/CT trimodality imaging[113].(b)A“neck-formation”strategy for UCL/MRI bimodal cancer probe[114].(c)Investigation of which Fe3 O4 affects the luminescence of UCNP[115].(d) “Core-satellite”Fe3 O4@g-C3N4-UCNPs-PEG nanomaterials for T1/T2-MRI guided photodynamic cancer therapy[116].
3.3 UCNP的表面修饰改性用于MRI
溶剂热法或者热裂解法合成的UCNP具有粒径可控、发光强等优点,然而最初合成的UCNP水溶性差,若想UCNP进一步应用于生物医学MRI上,需先对其表面进行功能化修饰,使其变为水溶性及生物相容性良好的材料。UCNP的表面改性不仅可以改善其生物相容性,还可以改善其MRI性能以及发光等特性,促使其在生物医学中发挥更大的作用。
目前,UCNP常用的改性方法有:(1)表面硅层修饰法[117],包括实心二氧化硅包覆[118-119]、介孔二氧化硅包覆[120-122]以及空心二氧化硅包覆[123]等;(2) 有机配体修饰,包括 PEG[124-125]、PEI[126]、聚多巴胺[127-128]以及磷脂分子[129-130]等。以上两种改性方法在改善UCNP生物相容性的同时或可增强UCNP发光性能,或影响其MRI性能,或赋予 UCNP新的影像、治疗等功能[131],从而满足现代医学发展要求[132-133]。
3.3.1 UCNP的表面硅层修饰改性用于MRI
二氧化硅生物相容性好,易于可控合成与修饰,被广泛应用于无机纳米粒子的改性[134-138]。UCNP表面的硅烷化修饰,包括用经典Stober方法或者反微乳液法,在其外层包覆一层介孔或者非介孔二氧化硅[93,139-142],在改善 UCNP 生物相容性的同时,一定程度上还可以影响其MRI性能。
Jin等合成了实心二氧化硅包覆NaYF4∶Yb,Tm@NaGdF4,通过共价嫁接光敏剂分子竹红霉素以及靶向分子叶酸,赋予了UCNP靶向光动力学治疗的性能。由于UCNP外层Gd3+的掺杂,赋予体系磁共振成像性能,从而可以实现UCL/MRI双模成像介导下的肿瘤光动力靶向治疗,如图7(a)所示[143]。我们课题组系统地研究了实心二氧化硅与介孔二氧化硅包覆UCNP对于MRI性能的影响,如图7(b)所示。通过构建不同厚度实心与介孔二氧化硅包裹的UCNP以及无表面配体的钆掺杂UCNP结构模型,研究了MRI成像机理与Gd3+掺杂的UCNP表面硅修饰的关系。由实验得知,对于无表面配体的钆掺杂UCNP来说,由于表面钆离子具有直接螯合水分子的能力,其成像原理由外球水和内球水机理共同主导,且二者贡献相当;对于表面硅层修饰的UCNP,由于表面的Gd3+被Si—O键螯合,极大地减小了螯合水分子的能力,致使此时的UCNP的MRI成像原理为外球水所主导[144]。

图7 (a)NaYF4∶Yb,Tm@NaGdF4@d-SiO2用于UCL/MRI介导的光动力学治疗[143];(b)Gd3+掺杂的UCNP弛豫机理的探究及灵敏度优化[144]。Fig.7 (a)NaYF4∶Yb,Tm@NaGdF4@d-SiO2 for UCL/MRI guided photodynamic therapy[143].(b)Gd3+-ion-doped upconversion nanoprobes:relaxivity mechanism probing and sensitivity optimization[144].
3.3.2 有机配体修饰UCNP用于MRI
有机配体对于无机纳米材料的表面修饰至关重要[145-146],对于将油溶性的UCNP改为水溶性更是起到不可或缺的作用[147-149]。UCNP表面有机配体修饰方法,主要有配体吸附法[51]、配体交换法[150-151]、配体组装法[152]和配体氧化法[153-154]等。
Adah Almutairi等通过磷脂聚乙二醇(DSPEPEG)胶束包裹在超小NaGdF4外层,将油溶性的NaGdF4转变为水相,引导水分子可以顺利透过接触到表面的Gd3+并被束缚,缩短质子弛豫时间,在临床1.5 T磁共振仪器上测得单个Gd3+的T1弛豫率高达 80 mmol-1·L·s-1,是临床钆剂的近25倍,如图8(a)所示。此外,他们还通过控制磷脂PEG的分子量来调控材料的弛豫率[25]。北京大学严纯华教授等通过NaGdF4外层包裹聚丙烯酸(PAA),展示了优于聚乙烯亚胺(PEI)以及聚乙二醇(PEG)修饰的弛豫率,其原因主要是因为PAA对水分子具有更强的氢键束缚作用,有效缩短质子的弛豫时间,导致r2/r1的比值非常接近于1,可以用来作为T1加权磁共振造影剂[155],如图8(b)。在此基础上,他们又合成了含Gd量子点,在0.5 T磁共振仪器下,r1高达47 mmol-1·L·s-1,即使在超高场 7 T 磁场下,r1也可达到 25.2 mmol-1·L·s-1。

图8 (a)NaGdF4@DSPE-PEG用于超高T1弛豫率磁共振造影剂研究[25];(b)NaGdF4表面修饰PAA通过超强氢键来增强弛豫率[155]。Fig.8 (a)High T1 relaxivity of NaGdF4@DSPE-PEG[25].z(b)Hydrogen bonds of NaGdF4@PAA for enhancing relaxivity[155].
4 UCNP作为 MRI造影剂的生物应用
UCNP作为新一代发光材料,在诸如生物示踪标记、生物影像以及癌症治疗等方面具有重要的研究意义和临床价值[153,156-167]。MRI作为临床影像诊断中重要的手段之一,具有较高的软组织分辨率以及无放射性、无损伤等优点[168-169]。因此,将UCNP作为磁共振造影剂应用于生物检测、影像诊断以及疾病治疗等方面具有重要的意义。
4.1 UCNP用于生物影像
UCNP具有毒性低、发光稳定性好、强度高等优点,其激发光一般为红外或近红外光,组织穿透深度高,无光漂白,具有较高的灵敏度和信噪比,广泛用于细胞成像与活体成像。另外,通过合理的组分调控、结构设计以及恰当的表面修饰,可以赋予 UCNP 其他影像功能,如 MRI、PET、CT、US以及PAI(光声成像)等,进而可以构建基于UCNP的多模态影像探针用于生物医学影像。
我们课题组基于UCNP构建了具有MRI/UCL双模态影像功能的探针UCNP@hmSiO2,用于监控抗癌药物DOX的释放[170]。首先,制备摇铃结构的纳米探针,将Gd3+掺杂的UCNP与药物DOX分别置于空腔与介孔孔道内,借助于发光共振能量转移(LRET)与T1加权磁共振成像技术,达到了实时、可视地监控在NIR光照下DOX释放的动态变化过程,如图9(a)所示。Tang等将UCNP与金属有机框架材料(MOF)结合,合成了具有核壳结构的纳米材料UCNP@Fe-MIL-101-NH2。再通过PEG的修饰改性以及共价嫁接叶酸(FA)分子,得到了特异性靶向肿瘤KB细胞的UCL/T2-MRI双模影像探针,成功实现了荷KB瘤小鼠的体内双模成像,为UCNP作为多模影像探针用于肿瘤影像提供了新的指导思路[171],如图9(b)所示。Li等首先合成了 NaLuF4∶Yb,Tm 的发光内核,再在外层包覆了一层 Sm3+掺杂的NaGdF4惰性层,不但提高了发光效率(减少了表面缺陷),还由于Gd3+和Sm3+的引入,赋予材料MRI以及单电子发射CT影像(SPECT)功能。此外,内核基质NaLuF4可以增强对X射线吸收,实现CT成像。因此,通过对UCNP合理的设计,可以构建集UCL/CT/MRI/SPECT四模成像于一体的多功能纳米探针,实现荷瘤鼠体内成像[172],如图9(c)所示。Nie等设计合成了由800 nm激光激发的具有多层壳结构的UCNP∶NaYF4∶Yb∶Er@NaYF4∶Yb@NaNdF4∶Yb@NaYF4@NaGdF4。构建多层壳的目的是增强体系的上转换发光,减少Er与Nd的能量交换以及溶剂猝灭效应,同时可以实现材料的MRI功能;再通过嫁接吲哚菁绿(ICG),赋予了材料较强的光声成像能力,如图9(d)。该复合纳米材料不但具有较高的上转换量子效率、较强的PAI活体组织影像功能,还具有小鼠全身高分辨率MRI影像功能,可以提供更加详细的小鼠组织结构信息,如对小鼠微血管以及肿瘤边界的观察[173]。
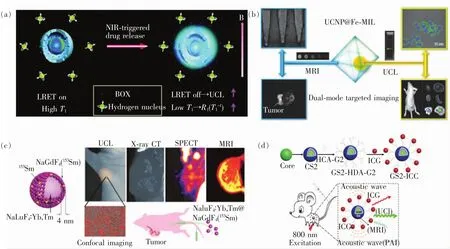
图9 (a)UCL/MRI双模态探针UCNP用于监控药物释放[170];(b)UCNP@MOF用于荷KB瘤小鼠的UCL/MRI双模成像[171];(c)基于UCNP的UCL/CT/MRI/SPECT四模影像探针的构建[172];(d)构建高效的上转换纳米复合物用于 UCL/MRI/PAI多模成像[173]。Fig.9 (a)UCNPwith UCL/MRIdual-modal imaging for monitoring the releasing of DOX[170].(b)UCL/MRI of UCNP@MOF for KB tumor-bearing mice[171].(c)Construction of UCL/CT/MRI/SPECT four-modal probes based on UCNP[172].(d)Upconversion nanomaterials of high performance for UCL/MRI/PAI[173].
4.2 UCNP用于构建影像介导的多功能诊疗剂
UCNP不但具有影像功能,还由于其发光特性可以结合光敏剂或者光热材料,进而实现光动力学治疗 (PDT)[54,174-176]或者光热治疗(PTT)[177-179];UCNP通过组分调控可作为新一代放疗增敏剂(RT)[180-181],表面再经过合理的修饰改性,可以作为化疗药物载体[182-183]。因此,以UCNP为基础可以构建集影像诊断、治疗于一体的多功能纳米诊疗剂。
苏州大学的刘庄教授等构建了基于UCNP的多模式介导下的光热诊疗探针[183]。首先,他们通过层层自组装的方法将Fe3O4包裹在UCNP外面,再通过种子生长法在复合材料外层生长Au壳,形成三明治结构,有效防止Au壳吸收UCNP的上转换荧光。随后,在材料外层表面修饰PEG与叶酸分子,成功实现了UCL/MRI双模式下叶酸靶向与磁靶向双靶向介导的肿瘤光热治疗,如图10(a)所示。Wang等构建了一种多功能生物安全性高的纳米诊疗剂,具有5种特定功能:UCL/MRI/CT 与 PTT、化疗[182]。该诊疗剂组成为:UCNP@PDA5-PEG-DOX,其中,UCNP可以提供 UCL/MRI/CT三模态成像,聚多巴胺(PDA)在808 nm激光照射下可以产生明显的光热效果,过热温度杀伤肿瘤的同时促进化疗药物DOX的释放,进一步加强DOX对肿瘤的杀伤作用,从而实现UCL/MRI/CT多模式影像介导的肿瘤PTT与化疗协同治疗,如图10(b)所示。
光动力学治疗是一种新型治疗肿瘤方法,光敏剂通过吸收合适波长的光后与周围的含氧物质(氧气、水以及过氧化氢等)结合,使其转化为称作活性氧的一类物质,用以高效杀死肿瘤[54,185-188]。Xu 等通过巧妙的设计,构建了金纳米棒二聚体与UCNP异质结合的核-卫星状纳米复合物[189],如图10(c)所示。二聚体的金纳米棒在808 nm激光的照射下,会迅速产生高热用来杀死肿瘤,实现PTT效果;UCNP负载光敏剂Ce6,通过与DNA的点击反应与金纳米棒结合,当980 nm激光照射时,Ce6吸收UCNP发出的红光再结合周围的氧分子,产生单线态氧来杀伤肿瘤。此外,金纳米棒与UCNP组成的纳米复合结构还提供了UCL/MRI/CT/PAI等多模影像手段,这为UCNP构建有效的多功能纳米诊疗剂提供了有力的支撑。
我们课题组在以UCNP为基础构建多功能纳米诊疗剂方面也做了一些研究。光热治疗可以通过高热快速杀死肿瘤,然而受限于激发光源在活体组织中的穿透深度,目前只能治疗体表肿瘤,对于深部肿瘤治疗效果较差[190]。放疗不受组织穿透深度限制,是目前临床上治疗癌症的主要手段之一。为了在放疗时对肿瘤达到高效治疗并降低对正常组织的伤害,一般采取低剂量X射线,再通过纳米重金属增敏剂来增强治疗效果[191-192]。因此,我们课题组构建了一种多功能“核-卫星”纳米诊疗剂,如图10(d)所示,其中,内核UCNP不但具有UCL/MRI/CT三模态成像的功能,同时由于UCNP中含有高原子序数元素,显著增强了X射线在病灶区的能量沉积,在低剂量X射线照射下可以高效杀伤肿瘤,起到放疗增敏的效果。另外,在UCNP外层点缀上卫星状CuS,经近红外光照射下产生大量的热,过热的温度可以进一步杀死肿瘤,实现UCL/MRI/CT介导下的放疗与光热治疗协同治疗[180]。
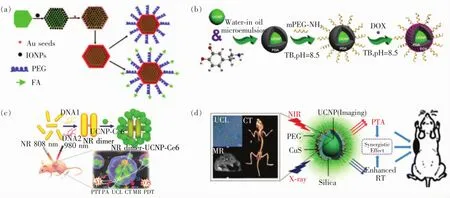
图10 (a)UCNP在UCL/MRI双模式下用于双靶向光热治疗肿瘤[184];(b)UCNP在UCL/MRI/CT三模介导下的光热治疗与化疗协同治疗癌症[182];(c)通过DNA链构建金纳米棒与UCNP的纳米复合材料用于UCL/MRI/CT/PAI等多模影像手段介导下的肿瘤光治疗[189];(d)基于UCNP构建“核-卫星”结构的纳米诊疗剂,实现UCL/MRI/CT多模式影像手段介导下的肿瘤放疗与光热治疗协同治疗[180]。Fig.10 (a)UCNPfor UCL/MRIguided dual-targeted photothermal cancer therapy[184].(b)UCNPfor UCL/MRI/CTguided chemophotothermal synergistic therapy of cancer[182].(c)Hierarchical plasmonic nanorods and upconversion core-satellite nanoassemblies for multimodal imaging-guided combination phototherapy[189].(d)A core/satellite multifunctional nanotheranostic based on UCNPfor in vivo UCL/MRI/CT imaging and tumor eradication by radiation/photothermal synergistic therapy[180].
5 总结与展望
本文主要通过介绍MRI机理、磁共振造影剂的构建、UCNP的设计以及其在磁共振生物医学等方面的应用,并结合我们课题组相关研究进行综述。通过对UCNP合理的组分调控和结构设计以及恰当的表面修饰,构建高性能MRI影像探针,并联合其他影像模式以及治疗模式构建基于UCNP的多功能诊疗一体化纳米探针。可以预见,由于UCNP极好的发光特性、较低的生物毒性以及多功能影像等特点,其在未来的生物医学领域一定会大放异彩。
然而,若想使UCNP更好地从研究领域尽快转化到临床医学应用上,还需要更进一步的系统研究:(1)对比其他无机造影剂如Fe3O4[193]等,UCNP作为MRI多功能造影剂应用于生物医学领域之前,应详细研究其生物相容性、体内代谢、组织血液的分布情况,以及在大型动物如灵长类动物身上的短期、长期毒性病理评价;(2)磁共振结构影像是指在MRI下可以反映组织结构信息的图像,如我们常说的T1加权成像以及T2&T2*加权成像;磁共振功能影像则是可以反映体内器官、组织的功能状态,揭示生物体内的生理学信息,如弥散加权成像(DWI)、脑功能成像(fMRI)、磁共振波谱成像(MRS)、灌注加权成像(PWI)以及化学交换饱和位移成像(CEST)等[194-196]。目前,UCNP用于MRI的研究多聚焦于MRI结构影像,对于MRI功能影像涉及甚少。因此,研究设计基于UCNP的MRI造影剂用于MRI结构成像与功能成像,实现MRI多模态同机融合技术检测体内生理病变信息以及精细结构信息十分必要[197];(3)针对医学领域实行个体化治疗方案,可以研究基于UCNP多功能纳米探针的个体化肿瘤治疗,针对不同种类肿瘤采用不同的UCNP基多功能纳米诊疗剂,发挥其最大的应用价值;(4)开发可以量产、制备简单、容易改性、低毒性、高MRI造影性能的UCNP合成工艺,优化组分调控、结构设计以及表面修饰来提高UCNP的量子效率及其他影像、治疗功能,开发新型发光基质解决光源体内穿透深度低的限制等[198]。
[1 ]BLOEMBERGEN N.Solid state infrared quantum counters[J].Phys.Rev.Lett.,1959,2(3):84-85.
[2]WU S,HAN G,MILLIRON D J,et al..Non-blinking and photostable upconverted luminescence from single lanthanidedoped nanocrystals[J].Proc.National Acad.Sci.,2009,106(27):10917-10921.
[3]CHAN E M.Combinatorial approaches for developing upconverting nanomaterials:high-throughput screening,modeling,and applications[J].Chem.Soc.Rev.,2015,44(6):1653-1679.
[4]FAN W,HUANG P,CHEN X.Overcoming the Achilles'heel of photodynamic therapy[J].Chem.Soc.Rev.,2016,45(23):6488-6519.
[5]LI X,ZHANG F,ZHAO D.Lab on upconversion nanoparticles:optical properties and applications engineering via designed nanostructure[J].Chem.Soc.Rev.,2015,44(6):1346-1378.
[6]PARK Y I,LEE K T,SUH Y D,et al..Upconverting nanoparticles:a versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging[J].Chem.Soc.Rev.,2015,44(6):1302-1317.
[7]SEDLMEIER A,GORRISH H.Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications[J].Chem.Soc.Rev.,2015,44(6):1526-1560.
[8]SHANMUGAM V,SELVAKUMAR S,YEH C S.Near-infrared light-responsive nanomaterials in cancer therapeutics[J].Chem.Soc.Rev.,2014,43(17):6254-6287.
[9]YANG D,MA P A,HOU Z,et al..Current advances in lanthanide ion(Ln3+)-based upconversion nanomaterials for drug delivery[J].Chem.Soc.Rev.,2015,44(6):1416-1448.
[10]CHEN F,BU W,CAI W,et al..Functionalized upconversion nanoparticles:versatile nanoplatforms for translational research [J].Curr.Mol.Med.,2013,13(10):1613-1632.
[11]RAMASAMY P,CHANDRA P,RHEE SW,et al..Enhanced upconversion luminescence in NaGdF4∶Yb,Er nanocrystals by Fe3+doping and their application in bioimaging[J].Nanoscale,2013,5(18):8711-8717.
[12]CHEN W,ZHENG R,BAADE PD,et al..Cancer statistics in China,2015[J].CACancer J.Clin.,2016,66(2):115-132.
[13]PENFIELD J G,REILLY JR R F.What nephrologists need to know about gadolinium[J].Nat.Rev.Nephrol.,2007,3(12):654.
[14]LEE SH,KIM B H,NA H B,et al..Paramagnetic inorganic nanoparticles as T1MRI contrast agents[J].Wiley Interdiscip.Rev.:Nanomed.Nanobiotechnol.,2014,6(2):196-209.
[15]赵喜平.磁共振成像系统的原理及其应用[M].北京:科学出版社,2000.ZHAO X P.The Principle and Application of Magnetic Resonance Imaging System[M].Beijing:Science Press,2000.(in Chinese)
[16]赵喜平.磁共振成像[M].北京:科学出版社,2004.
ZHAO X P.Magnetic Resonance Imaging[M].Beijing:Science Press,2004.(in Chinese)
[17]黄继英.磁共振成像原理[M].西安:陕西科学技术出版社,1998.HUANG JY.The Principle of Magnetic Resonance Imaging[M].Xi'an:Shaanxi Science and Technology Press,1998.(in Chinese)
[18]AIME S,BOTTA M,FASANOM,et al..Lanthanide(Ⅲ)chelates for NMR biomedical applications[J].Chem.Soc.Rev.,1998,27(1):19-29.
[19]DEBROYE E,PARAC-VOGT T N.Towards polymetallic lanthanide complexes as dual contrast agents for magnetic resonance and optical imaging[J].Chem.Soc.Rev.,2014,43(23):8178-8192.
[20]CARAVAN P,ELLISON JJ,MCMURRY T J,et al..Gadolinium(Ⅲ)chelates as MRI contrast agents:structure,dynamics,and applications[J].Chem.Rev.,1999,99(9):2293-352.
[21]熊国欣,李立本.核磁共振成像原理[M].北京:科学出版社,2007.XIONG G X,LI L B.Principle of Nuclear Magnetic Resonance Imaging[M].Beijing:Science Press,2007.(in Chinese)
[22]NICHOLLSF J,ROTZ M W,GHUMAN H,et al..DNA-gadolinium-gold nanoparticles for in vivo T1 MR imaging of transplanted human neural stem cells[J].Biomaterials,2016,77:291-306.
[23]RAMMOHAN N,MACRENARIS K W,MOORE L K,et al..Nanodiamond-gadolinium(Ⅲ)aggregates for tracking cancer growth in vivo at high field [J].Nano Lett.,2016,16(12):7551-7564.
[24]YANG CT,PADMANABHAN P,GULY S B Z.Gadolinium(Ⅲ)based nanoparticles for T1-weighted magnetic resonance imaging probes[J].RSC Adv.,2016,6(65):60945-60966.
[25]JOHNSON NJ,HES,NGUYENHUUV A,et al..Compact micellization:a strategy for ultrahigh T1magnetic resonance contrast with gadolinium-based nanocrystals[J].ACSNano,2016,10(9):8299-8307.
[26]RAMMOHAN N.Modular Carbon and Gold Nanoparticles for High Field MR Imaging and Theranostics[D].Evanston:Northwestern University,2016.
[27]TONGS,HOU S,ZHENGZ,et al..Coating optimization of superparamagnetic iron oxide nanoparticles for high T2relaxivity[J].Nano Lett.,2010,10(11):4607-13.
[28]SHOKROLLAHI H.Contrast agents for MRI[J].Mater.Sci.Eng.C,2013,33(8):4485-4497.
[29]龚洪翰.MRI磁共振成像原理与临床应用[M].南昌:江西科学技术出版社,2006.GONG H H.The Principle and Clinical Application of MRIMagnetic Resonance Imaging[M].Nanchang:Jiangxi Science and Technology Press,2006.(in Chinese)
[30]余小多.磁共振成像原理及肿瘤方面应用[J].抗癌之窗,2014(3):6-10.YU X D.The principle of magnetic resonance imaging and the application of tumor[J].Anti-cancer Window,2014(3):6-10.(in Chinese)
[31]许乙凯.磁共振造影剂及临床应用[M].北京:人民卫生出版社,2003.XU Y K.Magnetic Resonance Contrast Agent and Its Clinical Application[M].Beijing:People's Health Press,2003.(in Chinese)
[32]VAN BOCHOVE G S,PAULIS L E,SEGERS D,et al..Contrast enhancement by differently sized paramagnetic MRI contrast agents in mice with two phenotypes of atherosclerotic plaque [J].Contrast Media Mol.Imaging,2011,6(1):35.
[33]AUZEL F.Upconversion and anti-Stokes processes with f and d ions in solids[J].Chem.Rev.,2004,104(1):139-174.
[34]JOUBERT M F.Photon avalanche upconversion in rare earth laser materials[J].Opt.Mater.,1999,11(2):181-203.
[35]GREBENIK E A,KOSTYUK A B,DEYEV SM.Upconversion nanoparticles and their hybrid assemblies for biomedical applications[J].Russ.Chem.Rev.,2016,85(12):1277-1296.
[36]GAMELIN D R,GDEL H U.Design of luminescent inorganic materials:new photophysical processes studied by optical spectroscopy[J].ACC Chem.Res.,2000,33(4):235-242.
[37]GAMELIN D R,GUDEL H U.Transition Metal and Rare Earth Compounds[M].Berlin:Springer.2001:1-56.
[38]YANGY,ZHAOQ,FENGW,et al..Luminescent chemodosimeters for bioimaging[J].Chem.Rev.,2013,113(1):192-270.
[39]ZHOU J,LIU Z,LI F.Upconversion nanophosphors for small-animal imaging[J].Chem.Soc.Rev.,2012,41(3):1323-1349.
[40]WANG F,HANY,LIM CS,et al..Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping[J].Nature,2010,463(7284):1061.
[41]WANG F,LIU X.Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals[J].Chem.Soc.Rev.,2009,38(4):976-89.
[42]KR MER K W,BINER D,FREI G,et al..Hexagonal sodium yttrium fluoride based green and blue emitting upconversion phosphors[J].Chem.Mater.,2004,16(7):1244-1251.
[43]LIU Q,SUN Y,YANGT,et al..Sub-10 nm hexagonal lanthanide-doped NaLuF4upconversion nanocrystals for sensitive bioimaging in vivo[J].J.Am.Chem.Soc.,2011,133(43):17122-17125.
[44]MARTIN N,BOUTINAUD P,MAHIOU R,et al..Preparation of fluorides at 80℃ in the NaF-(Y,Yb,Pr)F3system[J].J.Mater.Chem.,1999,9(1):125-128.
[45]王猛,密丛丛,王单,等.NaYF4∶Yb,Er上转换荧光纳米颗粒的共沉淀法合成及表征[J].光谱学与光谱分析,2009,29(12):3327-3331.WANG M,MICC,WANGD,et al..Synthesis and characterization of NaYF4∶Yb,Er upconversion fluorescent nanoparticles by coprecipitation method [J].Spectrosc.Spect.Anal.,2009,29(12):3327-3331.(in Chinese)
[46]BISWASA,MACIEL G,FRIEND C,et al..Upconversion properties of a transparent Er3+-Yb3+co-doped LaF3-SiO2glass-ceramics prepared by sol-gel method [J].J.Non-Cryst.Solids,2003,316(2):393-397.
[47]ZHANG Y W,SUN X,SI R,et al..Single-crystalline and monodisperse LaF3triangular nanoplates from a single-source precursor[J].J.Am.Chem.Soc.,2005,127(10):3260-3261.
[48]WANG F,LIU X.Upconversion multicolor fine-tuning:visible to near-infrared emission from lanthanide-doped NaYF4nanoparticles[J].J.Am.Chem.Soc.,2008,130(17):5642-5643.
[49]WANG F,DENG R,WANG J,et al..Tuning upconversion through energy migration in core-shell nanoparticles[J].Nat.Mater.,2011,10(12):968.
[50]LI Z,ZHANG Y,JIANG S.Multicolor core/shell-structured upconversion fluorescent nanoparticles[J].Adv.Mater.,2008,20(24):4765-4769.
[51]YI G S,CHOW G M.Water-soluble NaYF4∶Yb,Er(Tm)/NaYF4/polymer core/shell/shell nanoparticles with significant enhancement of upconversion fluorescence[J].Chem.Mater.,2007,19(3):341-343.
[52]WANG L,LI Y.Na(Y1.5Na0.5)F6single-crystal nanorods as multicolor luminescent materials[J].Nano Lett.,2006,6(8):1645-1649.
[53]GARCIA J,KUDAWEDAGEDARA A N,ALLEN M J.Physical properties of Eu2+-containing cryptates as contrast agents for ultrahigh-field magnetic resonance imaging[J].Eur.J.Inorg.Chem.,2012,2012(12):2135-2140.
[54]PARK Y I,KIM H M,KIM J H,et al..Theranostic probe based on lanthanide-doped nanoparticles for simultaneous in vivo dual-modal imaging and photodynamic therapy[J].Adv.Mater.,2012,24(42):5755-5761.
[55]CHEN F,ZHANG S,BU W,et al..A uniform sub-50 nm-sized magnetic/upconversion fluorescent bimodal imaging agent capable of generating singlet oxygen by using a 980 nm laser[J].Chem.AEur.J.,2012,18(23):7082-7090.
[56]NACCACHE R,CHEVALLIER P,LAGUEUX J,et al..High relaxivities and strong vascular signal enhancement for NaGdF4nanoparticles designed for dual MR/optical imaging[J].Adv.Healthcare Mater.,2013,2(11):1478-1488.
[57]KUMAR R,NYK M,OHULCHANSKYY T Y,et al..Combined optical and MR bioimaging using rare earth ion doped NaYF4nanocrystals[J].Adv.Funct.Mater.,2009,19(6):853-859.
[58]ZHANG X,BLASIAK B,MARENCO A J,et al..Design and regulation of NaHoF4and NaDyF4nanoparticles for highfield magnetic resonance imaging[J].Chem.Mater.,2016,28(9):3060-3072.
[59]NI D,ZHANGJ,BU W,et al..PEGylated NaHoF4nanoparticles as contrast agents for both X-ray computed tomography and ultra-high field magnetic resonance imaging[J].Biomaterials,2016,76:218-225.
[60]DASG K,JOHNSON N J,CRAMEN J,et al..NaDyF4nanoparticles as T2contrast agents for ultrahigh field magnetic resonance imaging[J].J.Phys.Chem.Lett.,2012,3(4):524-529.
[61]HU F,ZHAO Y S.Inorganic nanoparticle-based T1and T1/T2magnetic resonance contrast probes[J].Nanoscale,2012,4(20):6235-6243.
[62]XIAOQ,BU W,REN Q,et al..Radiopaque fluorescence-transparent TaOxdecorated upconversion nanophosphors for in vivo CT/MR/UCL trimodal imaging[J].Biomaterials,2012,33(30):7530-7539.
[63]LIU C,GAO Z,ZENG J,et al..Magnetic/upconversion fluorescent NaGdF4∶Yb,Er nanoparticle-based dual-modal molecular probes for imaging tiny tumors in vivo[J].ACSNano,2013,7(8):7227.
[64]SUPKOWSKI R M,HORROCKSW D.Displacement of inner-sphere water molecules from Eu3+analogues of Gd3+MRI contrast agents by carbonate and phosphate anions:dissociation constants from luminescence data in the rapid-exchange limit[J].Inorg.Chem.,1999,38(24):5616-5619.
[65]NIVOROZHKIN A L,KOLODZIEJA F,CARAVAN P,et al..Enzyme-activated Gd3+magnetic resonance imaging contrast agents with a prominent receptor-induced magnetization enhancement[J].Angew.Chem.Int.Ed.,2001,40(15):2903-2906.
[66]SITHARAMAN B,KISSELL K R,HARTMAN K B,et al..Superparamagnetic gadonanotubes are high-performance MRI contrast agents[J].Chem.Commun.,2005,31:3915-3917.
[67]MI P,KOKURYO D,CABRAL H,et al..Hydrothermally synthesized PEGylated calcium phosphate nanoparticles incorporating Gd-DTPA for contrast enhanced MRI diagnosis of solid tumors[J].J.Control.Release,2014,174:63-71.
[68]KIRCHER M F,DE LA ZERDA A,JOKERST JV,et al..A brain tumor molecular imaging strategy using a new triplemodality MRI-photoacoustic-Raman nanoparticle[J].Nat.Med.,2012,18(5):829-834.
[69]KATTEL K,PARK JY,XU W,et al..A facile synthesis,in vitro and in vivo MR studies of D-glucuronic acid-coated ultrasmall Ln2O3(Ln=Eu,Gd,Dy,Ho,and Er)nanoparticles as a new potential MRI contrast agent[J].ACSAppl.Mater.Interf.,2011,3(9):3325-3334.
[70]KIM T,MOMIN E,CHOI J,et al..Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T1contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells[J].J.Am.Chem.Soc.,2011,133(9):2955-2961.
[71]ALRIC C,TALEB J,DUC G L,et al..Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging[J].J.Am.Chem.Soc.,2008,130(18):5908-5915.
[72]CHEN F,BU W,ZHANG S,et al..Positive and negative lattice shielding effects co-existing in Gd(Ⅲ)ion doped bifunctional upconversion nanoprobes[J].Adv.Funct.Mater.,2011,21(22):4285-4294.
[73]JOHNSON N J,OAKDEN W,STANISZ G J,et al..Size-tunable,ultrasmall NaGdF4nanoparticles:insights into their T1 MRI contrast enhancement[J].Chem.Mater.,2011,23(16):3714-3722.
[74]XING H,ZHANGS,BU W,et al..Ultrasmall NaGdF4nanodots for efficient MR angiography and atherosclerotic plaque imaging[J].Adv.Mater.,2014,26(23):3867-3872.
[75]NI D,ZHANGJ,WANGJ,et al..Oxygen vacancy enables markedly enhanced magnetic resonance imaging-guided photothermal therapy of a Gd3+-doped contrast agent[J].ACSNano,2017,11(4):4256.
[76]THUEN M,BERRY M,PEDERSEN T B,et al..Manganese-enhanced MRI of the rat visual pathway:acute neural toxicity,contrast enhancement,axon resolution,axonal transport,and clearance of Mn2+[J].J.Magn.Reson.Imaging,2008,28(4):855-865.
[77]DONG H,SUN L D,YAN CH.Basic understanding of the lanthanide related upconversion emissions[J].Nanoscale,2013,5(13):5703-5714.
[78]LI Z P,DONGB,HE Y Y,et al..Selective enhancement of green upconversion emissions of Er3+∶Yb3Al5O12nanocrystals by high excited state energy transfer with Yb3+-Mn2+dimer sensitizing[J].J.Lumin.,2012,132(7):1646-168.
[79]LI X,XUE Z,LIU H.Hydro-thermal synthesis of PEGylated Mn2+dopant controlled NaYF4∶Yb/Er up-conversion nanoparticles for multi-color tuning[J].J.Alloys Compd.,2016,681:379-383.
[80]庄宇,毛春生,赵丽君.Mn2+掺杂NaFY4∶Yb,Er晶体的形貌及发光性能研究[J].电子显微学报,2013,32(6):474-478.ZHUANG Y,MAOCS,ZHAOL J.Study on the morphology and luminescence properties of Mn2+doped NaFY4∶Yb,Er crystal[J].J.Electron Microsc.,2013,32(6):474-478.(in Chinese)
[81]SHARMA N,SHARMA V,BOHRA R,et al..Functionalized upconversion nanoparticles:versatile nanoplatforms for translational research [J].Cur.Mol.Med.,2013,13(10):1613-1632.
[82]TIAN G,GU Z,ZHOU L,et al..Mn2+dopant-controlled synthesis of NaYF4∶Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery[J].Adv.Mater.,2012,24(9):1226-1231.
[83]DENG X,DAI Y,LIU J,et al..Multifunctional hollow CaF2∶Yb3+/Er3+/Mn2+-poly(2-aminoethyl methacrylate)microspheres for Pt(Ⅳ)pro-drug delivery and tri-modal imaging[J].Biomaterials,2015,50(Supplement C):154-163.
[84]LUO Y,DU S,ZHANG W,et al..Core@shell Fe3O4@Mn2+-doped NaYF4∶Yb/Tm nanoparticles for triple-modality T1/T2-weighted MRI and NIR-to-NIR upconversion luminescence imaging agents [J].RSC Adv.,2017,7(60):37929-37937.
[85]SOESBE T C,RATNAKAR SJ,MILNE M,et al..Maximizing T2-exchange in Dy3+DOTA-(amide)X chelates:finetuning the water molecule exchange rate for enhanced T2contrast in MRI[J].Magn.Reson.Med.,2014,71(3):1179-1185.
[86]TYMINSKI A,GRZYB T,LISS.RE VO4-based nanomaterials(RE=Y,La,Gd,and Lu)as hosts for Yb3+/Ho3+,Yb3+/Er3+,and Yb3+/Tm3+ions:structural and up-conversion luminescence studies[J].J.Am.Ceram.Soc.,2016,99(10):3300-3308.
[87]CHEN D,LIU L,HUANG P,et al..Nd3+-sensitized Ho3+single-band red upconversion luminescence in coreshell nanoarchitecture[J].J.Phys.Chem.Lett.,2015,6(14):2833-2840.
[88]CHEN B,LIU Y,XIAOY,et al..Amplifying excitation-power sensitivity of photon upconversion in a NaYbF4∶Ho nanostructure for direct visualization of electromagnetic hotspots[J].J.Phys.Chem.Lett.,2016,7(23):4916-4921.
[89]ZHAN Q,QIAN J,LIANGH,et al..Using 915 nm laser excited Tm3+/Er3+/Ho3+-doped NaYbF4upconversion nanoparticles for in vitro and deeper in vivo bioimaging without overheating irradiation [J].ACS Nano,2011,5(5):3744-3757.
[90]郑晓宇,时朔,孙聆东,等.应用于高磁场强度磁共振成像的稀土纳米颗粒造影剂[C].中国化学会第29届学术年会摘要集,北京,2014.ZHENG X Y,SHI S,SUN L D,et al..Rare earth nanoparticle contrast agent used in high magnetic field intensity magnetic resonance imaging[C].Summary of The Twenty-ninth Annual Conference of The Chinese Chemical Association,Beijing,China,2014.(in Chinese)
[91]LIH,LIU G,WANGJ,et al..Hydrothermal synthesis,down-/enhanced up-converting,color tuning luminescence,energy transfer and paramagnetic properties of Ln3+(Ln=Eu/Dy,Yb/Ho)-doped Ba2GdF7multifunctional nanophosphors[J].New J.Chem.,2017,41(4):1609-1617.
[92]FENG Y,XIAO Q,ZHANGY,et al..Neodymium-doped NaHoF4nanoparticles as near-infrared luminescent/T2-weighted MR dual-modal imaging agents in vivo[J].J.Mater.Chem.B,2017,5(3):504-510.
[93]NI D,BU W,ZHANG S,et al..Single Ho3+-Doped upconversion nanoparticles for high-performance T2-weighted brain tumor diagnosis and MR/UCL/CT multimodal imaging[J].Adv.Funct.Mater.,2014,24(42):6613-6620.
[94]NOREK M,KAMPERT E,ZEITLER U,et al..Tuning of the size of Dy2O3nanoparticles for optimal performance as an MRI contrast agent[J].J.Am.Chem.Soc.,2008,130(15):5335-5340.
[95]KATTEL K,PARK JY,XU W,et al..Paramagnetic dysprosium oxide nanoparticles and dysprosium hydroxide nanorods as T2MRI contrast agents[J].Biomaterials,2012,33(11):3254-3261.
[96]NOREK M,PEREIRA GA,GERALDESCF,et al..NMR transversal relaxivity of suspensions of lanthanide oxide nanoparticles[J].J.Phys.Chem.C,2007,111(28):10240-10246.
[97]DASG K,ZHANG Y,D'SILVA L,et al..Single-phase Dy2O3∶Tb3+nanocrystals as dual-modal contrast agent for high field magnetic resonance and optical imaging[J].Chem.Mater.,2011,23(9):2439-2446.
[98]ZHANG X,BLASIAK B,MARENCO A J,et al..Design and regulation of NaHoF4and NaDyF4nanoparticles for highfield magnetic resonance imaging[J].Chem.Mater.,2016,28(9):3060-3072.
[99]ARSALANI N,FATTAHI H,NAZARPOOR M.Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4nanoparticles as an MRI contrast agent[J].Express Polym.Lett.,2010,4(6):329-338.
[100]ZHANG Y,LIU J Y,MA S,et al..Synthesis of PVP-coated ultra-small Fe3O4nanoparticles as a MRI contrast agent[J].J.Mater.Sci.:Mater.Med.,2010,21(4):1205-1210.
[101]ZENG J,JING L,HOU Y,et al..Anchoring group effects of surface ligands on magnetic properties of Fe3O4nanoparticles:towards high performance MRI contrast agents[J].Adv.Mater.,2014,26(17):2694-2698.
[102]HU F,WEI L,ZHOU Z,et al..Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer[J].Adv.Mater.,2006,18(19):2553-2556.
[103]WANG C,CHEN J,TALAVAGE T,et al..Gold nanorod/Fe3O4nanoparticle“nano-pearl-necklaces”for simultaneous targeting,dual-mode imaging,and photothermal ablation of cancer cells[J].Angew.Chem.Int.Ed.,2009,48(15):2759-2763.
[104]CHEN F,BU W,CHEN Y,et al..A sub-50-nm monosized superparamagnetic Fe3O4@SiO2T2-weighted MRI contrast agent:highly reproducible synthesis of uniform single-loaded core-shell nanostructures[J].Chem.An Asian J.,2009,4(12):1809-1816.
[105]CHEN F,BU W,LU C,et al..Hydrothermal synthesis of a highly sensitive T2-weigthed MRI contrast agent:zinc-doped superparamagnetic iron oxide nanocrystals[J].J.Nanosci.Nanotechnol.,2011,11(12):10438-10443.
[106]XIE J,CHEN K,LEE H Y,et al..Ultrasmall c(RGDyK)-coated Fe3O4nanoparticles and their specific targeting to integrin αvβ3-rich tumor cells[J].J.Am.Chem.Soc.,2008,130(24):7542-7543.
[107]TIAN Q,HU J,ZHU Y,et al..Sub-10 nm Fe3O4@Cu2-xScore-shell nanoparticles for dual-modal imaging and photothermal therapy[J].J.Am.Chem.Soc.,2013,135(23):8571-8577.
[108]YANGH,ZHUANGY,SUN Y,et al..Targeted dual-contrast T1-and T2-weighted magnetic resonance imaging of tumors using multifunctional gadolinium-labeled superparamagnetic iron oxide nanoparticles[J].Biomaterials,2011,32(20):4584-4593.
[109]PANKHURST Q A,CONNOLLY J,JONESSK,et al..Applications of magnetic nanoparticles in biomedicine[J].J.Phys.D,2003,36(13):R167-R181.
[110]FY C,CH S,YSY,et al..Characterization of aqueous dispersions of Fe3O4nanoparticles and their biomedical applications[J].Biomaterials,2005,26(7):729.
[111]ZENG H,LI J,WANG Z L,et al..Bimagnetic core/shell FePt/Fe3O4nanoparticles[J].Nano Lett.,2015,4(1):187-190.
[112]XIE J,XU C,KOHLER N,et al..Controlled PEGylation of monodisperse Fe3O4nanoparticles for reduced non-specific uptake by macrophage cells[J].Adv.Mater.,2007,19(20):3163-3166.
[113]ZHU X,ZHOU J,CHEN M,et al..Core-shell Fe3O4@NaLuF4∶Yb,Er/Tm nanostructure for MRI,CT and upconversion luminescence tri-modality imaging[J].Biomaterials,2012,33(18):4618-4627.
[114]CHEN F,ZHANG S,BU W,et al..A“neck-formation”strategy for an antiquenching magnetic/upconversion fluorescent bimodal cancer probe[J].Chem.A Eur.J.,2010,16(37):11254-11260.
[115]CHEN F,BU W,ZHANG L,et al..Is black iron oxide nanoparticle always a light absorber? [J].J.Mater.Chem.,2011,21(22):7990-7995.
[116]FENG L,DAN Y,FEIH,et al..A core-shell-satellite structured Fe3O4@g-C3N4-UCNPs-PEGfor T1/T2-weighted dualmodal MRI-guided photodynamic therapy[J].Adv.Healthcare Mater.,2017,32(5):1265-1271.
[117]LIU JN,BUWB,SHIJL.Silica coated upconversion nanoparticles:a versatile platform for the development of efficient theranostics[J].ACC Chem.Res.,2015,48(7):1797-1805.
[118]LIU F,ZHAO Q,YOU H,et al..Synthesis of stable carboxy-terminated NaYF4∶Yb3+,Er3+@SiO2nanoparticles with ultrathin shell for biolabeling applications[J].Nanoscale,2013,5(3):1047-1053.
[119]XIA A,CHEN M,GAOY,et al..Gd3+complex-modified NaLuF4-based upconversion nanophosphors for trimodality imaging of NIR-to-NIR upconversion luminescence,X-ray computed tomography and magnetic resonance[J].Biomaterials,2012,33(21):5394-5405.
[120]CHEN Y Y,MA PA,YANGD M,et al..Multifunctional core-shell structured nanocarriers for synchronous tumor diagnosis and treatment in vivo[J].Chem.An Asian J.,2014,9(2):506-513.
[121]HAN R,SHIJ,LIUZ,et al..Fabrication of mesoporous silica-coated upconverting nanoparticles with ultrafast photosensitizer loading and 808 nm NIR light triggering capability for photodynamic therapy[J].Chem.An Asian J.,2017,12(3):613-621.
[122]XU Y,LI H,MENGX,et al..Rhodamine-modified upconversion nanoprobe for distinguishing Cu2+from Hg2+and live cell imaging[J].New J.Chem.,2016,40(4):3543-3551.
[123]ZHAOL,PENGJ,CHEN M,et al..Yolk-shell upconversion nanocomposites for LRETsensing of cysteine/homocysteine[J].ACSAppl.Mater.Interf.,2014,6(14):11190-11197.
[124]何璐.多层双聚合物修饰的稀土上转换发光纳米材料用于多模态成像及血清存在下提高转染效率的研究[D].苏州:苏州大学,2014.HE L.Multilayered Double Polymer Modified Up Conversion Luminescent Nanomaterials for Multimodal Imaging and The Improvement of Transfection Efficiency in The Presence of Serum[D].Suzhou:Suzhou University,2014.(in Chinese)
[125]WANG C,CHENGL,LIUY,et al..Biomedical applications:imaging-guided pH-sensitive photodynamic therapy using charge reversible upconversion nanoparticles under near-infrared light[J].Adv.Funct.Mater.,2013,23(24):3077-3086.
[126]王欣.稀土上转换发光纳米材料用于近红外光激发的光动力治疗联合肿瘤基因治疗的研究[D].苏州:苏州大学,2015.WANG X.Study of Rare-earth Upconversion Luminescent Nanomaterials for Photodynamic Therapy Combined with Tumor Gene Therapy Excited by Near Infrared Light[D].Suzhou:Suzhou University,2015.(in Chinese)
[127]LIU F,HE X,LEI Z,et al..Cancer theranostics:facile preparation of doxorubicin-loaded upconversion@polydopamine nanoplatforms for simultaneous in vivo multimodality imaging and chemophotothermal synergistic therapy [J].Adv.Healthcare Mater.,2015,4(4):559.
[128]LIU B,LI C,XING B,et al..Multifunctional UCNPs@PDA-ICG nanocomposites for upconversion imaging and combined photothermal/photodynamic therapy with enhanced antitumor efficacy[J].J.Mater.Chem.B,2016,4(28):1232-1238.
[129]李颖.多功能稀土上转换发光纳米材料的合成及生物成像应用[D].上海:上海师范大学,2016.LI Y.Synthesis and Bioimaging Application of Multifunction Rare Earth Upconversion Luminescent Nanomaterials[D].Shanghai:Shanghai Normal University,2016.(in Chinese)
[130]LIU Y,CHEN M,CAO T,et al..A cyanine-modified nanosystem for in vivo upconversion luminescence bioimaging of methylmercury[J].J.Am.Chem.Soc.,2013,135(26):9869-9876.
[131]GAI S,LI C,YANG P,et al..Recent progress in rare earth micro/nanocrystals:soft chemical synthesis,luminescent properties,and biomedical applications[J].Chem.Rev.,2013,114(4):2343-2389.
[132]CHEN G,GREN H,OHULCHANSKYY T Y,et al..Light upconverting core-shell nanostructures:nanophotonic control for emerging applications[J].Chem.Soc.Rev.,2015,44(6):1680-1713.
[133]SUN L D,WANG Y F,YAN C H.Paradigms and challenges for bioapplication of rare earth upconversion luminescent nanoparticles:small size and tunable emission/excitation spectra[J].ACC Chem.Res.,2014,47(4):1001-1009.
[134]LI W,ZHAO D.Extension of the Stber method to construct mesoporous SiO2and TiO2shells for uniform multifunctional core-shell structures[J].Adv.Mater.,2013,25(1):142-149.
[135]ZHANG F,BRAUN G B,SHI Y,et al...Fabrication of Ag@SiO2@Y2O3∶Er nanostructures for bioimaging:tuning of the upconversion fluorescence with silver nanoparticles[J].J.Am.Chem.Soc.,2010,132(9):2850-2851.
[136]ZHANG S,WEN L,YANG J,et al..Facile fabrication of dendritic mesoporous SiO2@CdTe@SiO2fluorescent nanoparticles for bioimaging[J].Particle Particle Systems Charact.,2016,33(5):261-270.
[137]CHEN F,BU W,CHEN Y,et al..A sub-50-nm monosized superparamagnetic Fe3O4@SiO2T2-weighted MRI contrast agent:highly reproducible synthesis of uniform single-loaded core-shell nanostructures[J].Chem.Asian J.,2009,4(12):1809.
[138]CHEN Y,CHEN H,MA M,et al..Double mesoporous silica shelled spherical/ellipsoidal nanostructures:synthesis and hydrophilic/hydrophobic anticancer drug delivery[J].J.Mater.Chem.,2011,21(14):5290-5298.
[139]MA J,HUANGP,HEM,et al..Folic acid-conjugated LaF3∶Yb,Tm@SiO2nanoprobes for targeting dual-modality imaging of upconversion luminescence and X-ray computed tomography[J].J.Phys.Chem.B,2012,116(48):14062-14070.
[140]WANG F,ZHAI D,WU C,et al..Multifunctional mesoporous bioactive glass/upconversion nanoparticle nanocomposites with strong red emission to monitor drug delivery and stimulate osteogenic differentiation of stem cells[J].Nano Res.,2016,9(4):1193-1208.
[141]陈颖,李菲菲,李春光,等.稀土上转换发光材料标记抗体的制备及在免疫组化中的应用[J].高等学校化学学报,2013,34(4):788-793.CHENY,LIFF,LICG,et al..Preparation and application of rare-earth up-conversion luminescent material labeled antibody and its application in immunohistochemistry[J].Chem.J.Chin.Univ.,2013,34(4):788-793.(in Chinese)
[142]丁晓英,范慧俐,徐晓伟,等.SiO2包覆上转换发光材料 NaY0.57Yb0.39Er0.04F4的研究[J].发光学报,2006,27(3):353-357.DING H Y,FAN H L,XU X W,et al..Study on NaY0.57Yb0.39Er0.04F4of SiO2coated upconversion luminescent material[J].Chin.J.Lumin.,2006,27(3):353-357.(in Chinese)
[143]YANG C,LIU Q,HE D,et al..Dual-modal imaging and photodynamic therapy using upconversion nanoparticles for tumor cells[J].Analyst,2014,139(24):6414-6420.
[144]CHEN F,BU W,ZHANGS,et al..Gd3+-ion-doped upconversion nanoprobes:relaxivity mechanism probing and sensitivity optimization [J].Adv.Funct.Mater.,2013,23(3):298-307.
[145]ZHANGC,BU W,NID,et al..A polyoxometalate cluster paradigm with self-adaptive electronic structure for acidity/reducibility-specific photothermal conversion[J].J.Am.Chem.Soc.,2016,138(26):8156-8164.
[146]ZHANG C,BU W,NI D,et al..Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction[J].Angew.Chem.,2016,128(6):2141-2146.
[147]LI P,LIU L,ZHOU J,et al..pH-sensitive polymer functionalized upconversion nanoparticles(UCNPs)as biomarkers[J].J.Control.Release,2017,259:e106.
[148]LIU B,DENG X,XIE Z,et al..Thiol-ene click reaction as a facile and general approach for surface functionalization of colloidal nanocrystals[J].Adv.Mater.,2017,29(36):1604878.
[149]XIE Z,DENG X,LIU B,et al..Construction of hierarchical polymer brushes on upconversion nanoparticles via NIR-light-initiated RAFT polymerization [J].ACSAppl.Mater.Interf.,2017,9(36):30414.
[150]ZHANGQ,SONGK,ZHAOJ,et al..Hexanedioic acid mediated surface-ligand-exchange process for transferring NaYF4∶Yb/Er(or Yb/Tm)up-converting nanoparticles from hydrophobic to hydrophilic [J].J .Colloid Interf.Sci.,2009,336(1):17117-17125.
[151]YI G S,CHOW G M.Synthesis of hexagonal-phase NaYF4∶Yb,Er and NaYF4∶Yb,Tm nanocrystals with efficient upconversion fluorescence[J].Adv.Funct.Mater.,2006,16(18):2324-2329.
[152]WANG L,YAN R,HUO Z,et al..Fluorescence resonant energy transfer biosensor based on upconversion-luminescent nanoparticles[J].Angew.Chem.Int.Ed.Engl.,2005,44(37):6054.
[153]CHEN Z,CHEN H,HU H,et al..Versatile synthesis strategy for carboxylic acid-unctionalized upconverting nanophosphors as biological labels[J].J.Am.Chem.Soc.,2008,130(10):3023-3029.
[154]HU H,YU M,LI F,et al..Facile epoxidation strategy for producing amphiphilic up-converting rare-earth nanophosphors as biological labels[J].Chem.Mater.,2008,20(22):7003-7009.
[155]ZHENG X Y,ZHAO K,TANG J,et al..Gd-dots with strong ligand-water interaction for ultrasensitive magnetic resonance renography[J].ACSNano,2017,11(4):3642-3650.
[156]ZHOU J,LIU Z,LI F.Upconversion nanophosphors for small-animal imaging[J].Chem.Soc.Rev.,2012,41(3):1323.
[157]LIU J,CHENG J,ZHANG Y.Upconversion nanoparticle based LRET system for sensitive detection of MRSA DNA sequence[J].Biosens.Bioelectron.,2013,43(1):252.
[158]WANG F,BANERJEE D,LIU Y,et al..Upconversion nanoparticles in biological labeling,imaging,and therapy[J].Analyst,2010,135(8):1839-1854.
[159]WANG C,CHENG L,LIU Z.Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy[J].Biomaterials,2011,32(4):1110.
[160]GU Z,YAN L,TIAN G,et al..Recent advances in design and fabrication of upconversion nanoparticles and their safe theranostic applications[J].Adv.Mater.,2013,25(28):3758-3779.
[161]JIANG S,ZHANG Y.Use of IR-to-visible upconversion fluorescent nanoparticles for tracking of siRNA delivery[C].Proceedings of The Sixth IASTED International Conference on Biomedical Engineering,Innsbruck,Austria,2008:368-371.
[162]IDRISNM,GNANASAMMANDHAN M K,ZHANGJ,et al..In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers[J].Nat.Med.,2012,18(10):1580-1585.
[163]LI Z,LIANG T,LV S,et al..A rationally designed upconversion nanoprobe for in vivo detection of hydroxyl radical[J].J.Am.Chem.Soc.,2015,137(34):11179-11185.
[164]WANG C,CHENG L,LIU Z.Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics[J].Theranostics,2013,3(5):317-330.
[165]CHEN Z,ZHEN L,LI Z,et al..Upconversion nanoprobes for efficiently in vitro imaging reactive oxygen species and in vivo diagnosing rheumatoid arthritis[J].Biomaterials,2015,39:15-22.
[166]ZHOU L,CHEN Z,DONG K,et al..DNA-mediated construction of hollow upconversion nanoparticles for protein harvesting and near-infrared light triggered release[J].Adv.Mater.,2014,26(15):2424-2430.
[167]DING X,LIU J,LIU D,et al..Multifunctional core/satellite polydopamine@Nd3+-sensitized upconversion nanocomposite:a single 808 nm near-infrared light-triggered theranostic platform for in vivo imaging-guided photothermal therapy[J].Nano Res.,2017,10(10):3434-3446.
[168]BASSER PJ,PIERPAOLI C.Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI[J].J.Magn.Reson.,2011,213(2):560.
[169]CLIFFORD R J,RONALD C P,YUE C X,et al..Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease[J].Neurology,1997,49(3):786-794.
[170]LIU J,BU J,BU W,et al..Real-time in vivo quantitative monitoring of drug release by dual-mode magnetic resonance and upconverted luminescence imaging[J].Angew.Chem.Int.Ed.,2014,53(18):4551-4555.
[171]LI Y,TANG J,HE L,et al..Core-shell upconversion nanoparticle@metal-organic framework nanoprobes for luminescent/magnetic dual-mode targeted imaging[J].Adv.Mater.,2015,27(27):4075-4080.
[172]SUN Y,ZHU X,PENG J,et al..Core-shell lanthanide upconversion nanophosphors as four-modal probes for tumor angiogenesis imaging[J].ACSNano,2013,7(12):11290-11300.
[173]LIU Y,KANG N,LV J,et al..Deep photoacoustic/luminescence/magnetic resonance multimodal imaging in living subjects using high-efficiency upconversion nanocomposites[J].Adv.Mater.,2016,28(30):6411-6419.
[174]ZHAO Z,HAN Y,LIN C,et al..Multifunctional core-shell upconverting nanoparticles for imaging and photodynamic therapy of liver cancer cells[J].Chem.An Asian J.,2012,7(4):830-837.
[175]GUAN M,DONG H,GE J,et al..Multifunctional upconversion-nanoparticles-trismethylpyridylporphyrin-fullerene nanocomposite:a near-infrared light-triggered theranostic platform for imaging-guided photodynamic therapy[J].NPG Asia Mater.,2015,7:e205.
[176]WANG C,CHENG L,LIU Y,et al..Imaging-guided pH-sensitive photodynamic therapy using charge reversible upconversion nanoparticles under near-infrared light[J].Adv.Funct.Mater.,2013,23(24):3077-3086.
[177]CHENG L,YANGK,LI Y,et al..Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy[J].Biomaterials,2012,33(7):2215-2222.
[178]GE W,CHEN T,LI Z,et al..Plasmonic-enhanced and Nd3+-sensitized upconversion nanoparticles for magnetically targeted MRI/UCL dual-mode imaging and photothermal therapy[J].Nanosci.Nanotechnol.Lett.,2017,9(4):416-424.
[179]CHEN Q,WEN J,LI H,et al..Recent advances in different modal imaging-guided photothermal therapy[J].Biomaterials,2016,106:144-166.
[180]XIAO Q,ZHENG X,BU W,et al..A core/satellite multifunctional nanotheranostic for in vivo imaging and tumor eradication by radiation/photothermal synergistic therapy[J].J.Am.Chem.Soc.,2013,135(35):13041-13048.
[181]FAN W,SHEN B,BU W,et al..Rattle-structured multifunctional nanotheranostics for synergetic chemo-/radiotherapy and simultaneous magnetic/luminescent dual-mode imaging[J].J.Am.Chem.Soc.,2013,135(17):6494-6503.
[182]LIU F,HE X,LEI Z,et al..Facile preparation of doxorubicin-loaded upconversion@polydopamine nanoplatforms for simultaneous in vivo multimodality imaging and chemophotothermal synergistic therapy [J].Adv.Healthcare Mater.,2015,4(4):559-568.
[183]DAI Y,XIAO H,LIU J,et al..In vivo multimodality imaging and cancer therapy by near-infrared light-triggered transplatinum pro-drug-conjugated upconverison nanoparticles[J].J.Am.Chem.Soc.,2013,135(50):18920-18929.
[184]CHENGL,YANGK,LI Y,et al..Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy[J].Angew.Chem.,2011,123(32):7523-7528.
[185]LIU Y,ZHANGJ,ZUOC,et al..Upconversion nano-photosensitizer targeting into mitochondria for cancer apoptosis induction and cytc fluorescence monitoring[J].Nano Res.,2016,9(11):3257-3266.
[186]LIU Y,LIU Y,BU W,et al..Hypoxia induced by upconversion-based photodynamic therapy:towards highly effective synergistic bioreductive therapy in tumors[J].Angew.Chem.Int.Ed.,2015,54(28):8105810.
[187]LIU X,ZHENG M,KONGX,et al..Separately doped upconversion-C60 nanoplatform for NIR imaging-guided photodynamic therapy of cancer cells[J].Chem.Commun.,2013,49(31):3224-3226.
[188]WANG C,CHENG L,LIU Z.Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics[J].Theranostics,2013,3(5):317.
[189]SUN M,XU L,MA W,et al..Hierarchical plasmonic nanorods and upconversion core-satellite nanoassemblies for multimodal imaging-guided combination phototherapy[J].Adv.Mater.,2016,28(5):898-904.
[190]ALVAREZ-LORENZOC,BROMBERGL,CONCHEIROA.Light-sensitive intelligent drug delivery systems[J].Photochem.Photobiol.,2009,85(4):848-860.
[191]CHITHRANI D B,JELVEH S,JALALI F,et al..Gold nanoparticles as radiation sensitizers in cancer therapy[J].Radiat.Res.,2010,173(6):719-728.
[192]KOBAYASHI K,USAMI N,PORCEL E,et al..Enhancement of radiation effect by heavy elements[J].Mutat.Res./Rev.Mutation Res.,2010,704(1):123-131.
[193]LU Y,XU Y J,ZHANG G B,et al..Iron oxide nanoclusters for T1magnetic resonance imaging of non-human primates[J].Nat.Biomed.Eng.,2017,1(8):637.
[194]王小玲,赵振华,王伯胤,等.MRI功能成像对肝动脉化疗栓塞治疗肝细胞肝癌的疗效评价[J].临床放射学杂志,2017(5):700-704.WANG X L,ZHAO Z H,WANG B Y,et al..Evaluation of MRI functional imaging for hepatic arterial chemoembolization in the treatment of hepatocellular carcinoma[J].J.Clin.Radiol.,2017(5):700-704.(in Chinese)
[195]袁红梅,余建群.MRI功能成像在乳腺良恶性肿瘤诊断中的应用[J].生物医学工程学杂志,2009(2):421-424.YUAN H M,YU JQ.The application of MRI functional imaging in the diagnosis of benign and malignant breast tumors[J].Biomed.Eng.J.,2009(2):421-424.(in Chinese)
[196]岳倩倩,王新怡.MRI功能成像在小肝癌诊断中的应用进展[J].中华消化病与影像杂志(电子版),2016(4):180-183.YUE Q Q,WANGX Y.Progress in the application of MRIfunctional imaging in the diagnosis of small hepatocellular carcinoma[J].Chin.J.Digest.Imaging(Electron.Ed.),2016(4):180-183.(in Chinese)
[197]NI D,SHEN Z,ZHANG J,et al..Integrating anatomic and functional dual-mode magnetic resonance imaging:design and applicability of a bifunctional contrast agent[J].ACSNano,2016,10(3):3783-3790.
[198]ZHANG C,ZHAO K,BU W,et al..Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence[J].Angew.Chem.Int.Ed.,2015,54(6):1770-1774.
Rare-earth Upconversion Nanomaterials for Medical Magnetic Resonance Imaging
MENG Xian-fu,LIU Yan-yan,BU Wen-bo*
(Shanghai Key Laboratory of Green Chemistry and Chemical Processes,College of Chemistry and Molecular Engineering,East China Normal University,Shanghai 200062,China)*Corresponding Author,E-mail:wbbu@chem.ecnu.edu.cn
Upconversion nanoparticle(UCNP)following anti-Stokes principle is a novel type of luminescence materials,which possesses many unique merits such as high luminescence intensity,luminous stability,no background fluorescence,no bleaching,low toxicity and good biocompatibility.The infrared or near infrared excitation light endows UCNPwith deep penetration in living tissues for potential applications in biomedical detection,diagnose and treatment.Magnetic resonance imaging(MRI),as one of the commonly used techniques in clinical,has many special advantages like high quality of soft tissue imaging,high spatial resolution,no radiation and no damage,which plays a significant role in diagnosis of cardiovascular and cerebrovascular diseases.In this review,we focus on the recent researches in regard to rare-earth upconversion nanomaterials for MRI diagnosis and application.The mechanism of magnetic resonance imaging,the construction of MRI contrast agents,and the design and synthesis of UCNP-based multi-functional nanomaterials for MRI diagnosis and disease therapy have been introduced in detail,on the other hand,by means of the relevant researches reported by our group,which are based on UCNP for medical MRI and multimodal imaging,the prospects of UCNP in the application of MRI have also been discussed in this review.
2017-10-12;
2017-12-10
国家杰出青年科学基金(51725202);上海市优秀学术带头人计划(16XD1404000)资助项目Supported by China National Funds for Distinguished Young Scientists(51725202);Shanghai Excellent Academic Leaders Program(16XD1404000)
upconversion nanoparticle;magnetic resonance imaging(MRI);multi-modality imaging;disease therapy
O482.31
A
10.3788/fgxb20183901.0069
1000-7032(2018)01-0069-23

孟宪福(1991-),男,河北泊头人,博士研究生,2016年于上海大学获得硕士学位,主要从事新型无机纳米探针用于肿瘤影像诊断的研究。
E-mail:861380023@qq.com

步文博(1973-),男,山东成武人,教授,博士生导师,2012年于南京工业大学获得博士学位,主要从事稀土多功能材料的结构设计、化学可控合成及其生物医学应用的研究。
Email:wbbu@chem.ecnu.edu.cn

刘艳颜(1988-),女,山东德州人,博士,讲师,2016于上海硅酸盐研究所获得博士学位,主要从事稀土功能纳米材料的研究
E-mail:244212898@qq.com

