Lanthanide-doped Upconversion Nano-bioprobes for In-vitro Detection of Tumor Markers
YU Li-hua,LIU Yong-sheng ,CHEN Xue-yuan*
(1.Key Laboratory of Design and Assembly of Functional Nanostructures of Chinese Academy of Sciences,Fujian Key Laboratory of Nanomaterials,Fujian Institute of Research on The Structure of Matter,Chinese Academy of Sciences,Fuzhou 350002,China.2.College of Materials Science and Engineering,Fujian Normal University,Fuzhou 350007,China)*Corresponding Authors,E-mail:xchen@firsm.ac.cn;liuysh@fjirsm.ac.cn
Lanthanide-doped Upconversion Nano-bioprobes for In-vitro Detection of Tumor Markers
YU Li-hua1,2,LIU Yong-sheng1*,CHEN Xue-yuan1,2*
(1.Key Laboratory of Design and Assembly of Functional Nanostructures of Chinese Academy of Sciences,Fujian Key Laboratory of Nanomaterials,Fujian Institute of Research on The Structure of Matter,Chinese Academy of Sciences,Fuzhou 350002,China.2.College of Materials Science and Engineering,Fujian Normal University,Fuzhou 350007,China)*Corresponding Authors,E-mail:xchen@firsm.ac.cn;liuysh@fjirsm.ac.cn
Sensitive and specific biodetection of tumor markers is essential for early-stage cancer diagnosis and therapy,and will ultimately increase the patient survival rate.As a new generation of luminescent nano-bioprobes,trivalent lanthanide(Ln3+)-doped upconversion(UC)nanoparticles(NPs)possess exceptional properties such as nearinfrared triggered anti-Stokes luminescence and long excited-state lifetime,and thus are considered to be the most promising alternative to conventional probes such as organic dyes,lanthanide chelates and quantum dots for early cancer theranostics.In this review,we provide a brief overview of the most recent advances in the development of Ln3+-doped UCnano-bioprobes,which covers from their chemical and physical fundamentals to biodetection including the controlled synthesis,surface modification,optical spectroscopy and their in-vitro UC luminescent bioassay of tumor markers.Some future prospects and challenges in this rapidly growing field are finally envisioned.
lanthanide ion;upconversion;tumor maker;nanoparticle;bioassay
1 Introduction
Cancer is a word used to describe a large family of diseases that involve cell growth out of control with the potential to invade or spread to other parts of the normal body.The most common types of cancer in males are lung,prostate and stomach cancers,while for the females the most common types of cancer are breast, colorectal, lung and cervical cancers.These cancers were the leading causes of morbidity and mortality worldwide,with approximately 91 million new cases and 8.8 million cancer related deaths in 2015[1].There is no doubt that the ultrasensitive detection and accurate analysis of tumor markers in fluid samples of cancerous patients like serum,saliva and urine at the earliest stage possible are of crucial importance for early cancer diagnosis and therapy and consequently increases their survival rates.
For this purpose,some tumor marker-detecting methods[2-5]such as time-resolved(TR)fluoroimmunoassay(TRFIA),dissociation-enhanced lanthanide fluoroimmunoassay(DELFIA),enzyme-linked immunosorbent assay(ELISA),Frster resonance energy transfer(FRET)and TR-FRET assays have been well developed in the past decades by using organic dyes,lanthanide chelates and quantum dots(QDs)as luminescent bioprobes[6-11].However,the use of these conventional luminescent bioprobes somewhat suffers from disadvantages like the severe background noise and photodamage to biological samples under ultraviolet(UV)excitation,poor photochemical stability,potential long-term toxicity and high cost-to-use[12].Therefore,it is particularly urgent to develop a new generation of luminescent bioprobes to circumvent above limitations of traditional ones.In contrast to these conventional bioprobes,trivalent lanthanide(Ln3+)-doped upconversion(UC)nanoparticles(NPs)(UCNPs)possess exceptional nonlinear optical properties,namely large anti-Stokes shifts,sharp emission spectra and long-lived lifetimes,and thus are considered to be the most promising alternative to conventional probes for early cancer theranostics[13-14].Notably,these UCNPs utilizes near-infrared(NIR)excitation rather than ultraviolet(UV)excitation,which can minimize the unwanted background noises,photobleaching and photodamage to biological specimens,thereby enabling the ultrasensitive detection of tumor markers in fluid samples[15-20].
With the rapid development in Ln3+-doped UCNPs,many significant advances have recently been achieved in the structure/morphology control,surface modification and functionalization,and optical performance optimization of these UC nano-bioprobes for a diversity of biomedical applications such as biological imaging,anti-counterfeiting,thermal sensing,full-color volumetric three dimensional displays and cancer theranostics[21-26].Although the research on Ln3+-doped UCNPs as UC luminescence(UCL)nano-bioprobes for cancer theranostics is still in its infancy,impressive results are already emerging[27].In this review,we focus on the most recent advances towards the development of Ln3+-doped UCNPs as UCL nano-bioprobes and their promising application for the sensitive in-vitro detection of diverse tumor markers(Fig.1).We start with a brief introduction to the commonly used tumor markers in clinical practice(Section 2),followed by an overview of the chemical synthesis and surface functionalization of UCL nano-bioprobes in Section 3.Next we give a brief description of the physical fundamentals of UCL nanobioprobes with an emphasis on the most successful approaches to achieve highly enhanced UCL(Section 4).In Section 5,the promising use of Ln3+-doped UCNPs as UCL nano-bioprobes for the sensitive biodetection of diverse tumor markers in vitro is highlighed.Finally,some important emerging trends and future efforts towards this active field are also envisioned.

Fig.1 Schematic illustration of Ln3+-doped UCL nano-bioprobes for the detection of tumor markers
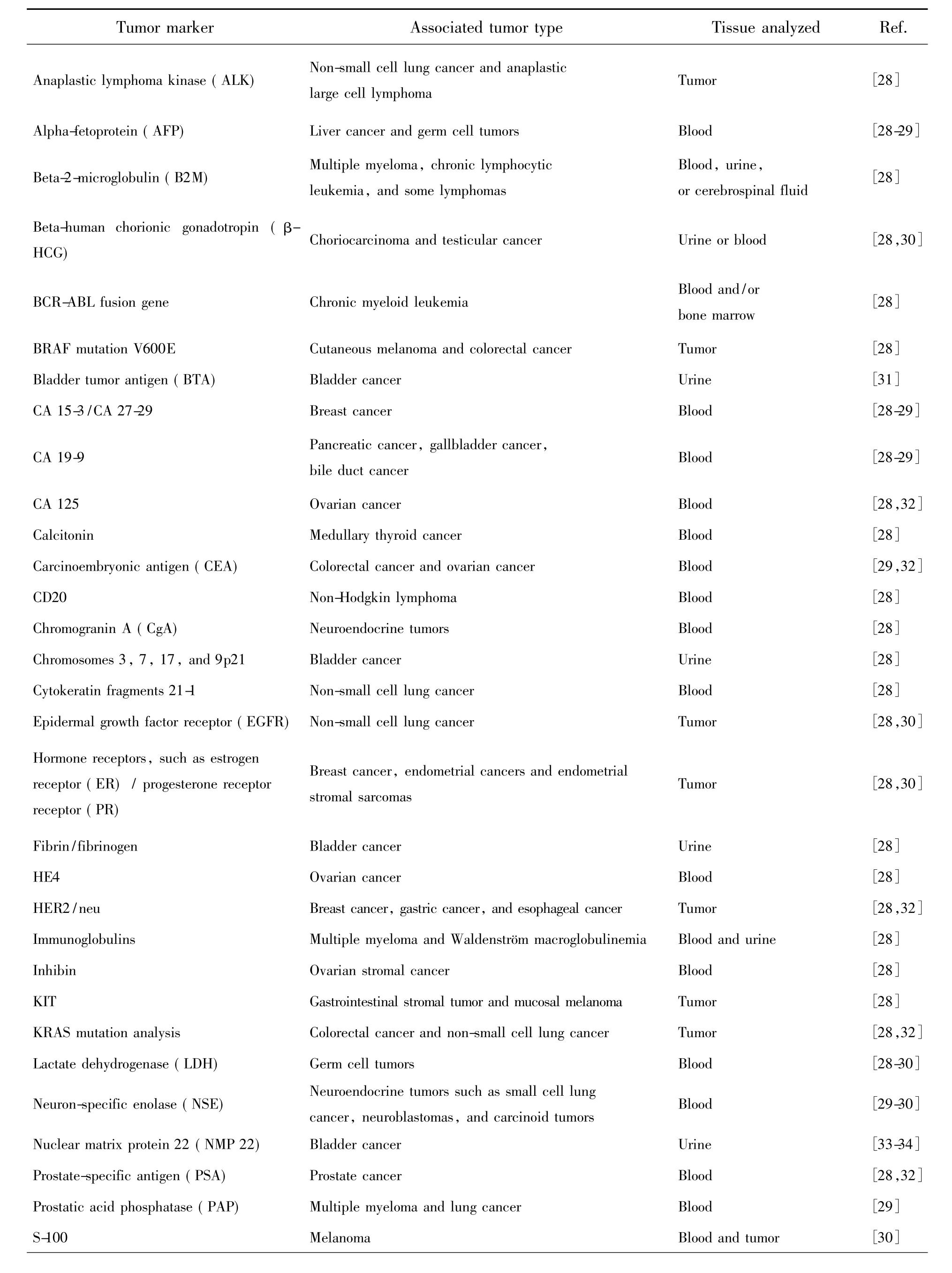
Tab.1 List of the commonly used tumor markers along with their associated tumor types and tissue analyzed in clinical application(Reprinted with permission from Ref.[12]Copyright 2015,Royal Society of Chemistry)

Tab.1(Continued)
2 Commonly Used Tumor Markers in Clinical Practice
Tumor markers are substances that are made by the cancer tissue itself or sometimes by the body in response to cancer growth[12,35]. These substances can be found in the blood,urine,stool,tumor tissue,or bodily fluids of some cancerous patients and are playing an important role in cancer detection and management.As such,these tumor markers can be used as reliable indicators to help detect and diagnose some types of cancer,predict and monitor a person's response to certain treatments,and detect recurrence of cancer.In asymptomatic subjects,tumor markers are potentially used in screening for early malignancy.While in symptomatic patients,tumor markers may be helpful in the differential diagnosis of benign and malignant diseases.Following diagnosis and surgical removal of a malignancy,these markers may be tracked for assessing prognosis,postoperative surveillance,therapy prediction and monitoring response to systemic therapy.
With the rapid development of genomics,proteomics and molecular pathology,a large amount of tumor markers have been characterized and used in clinical application[36].Generally speaking,tumor markers can be roughly divided into four broad classifications on the basis of their chemical nature,namely proteins,conjugated proteins,peptides and carbohydrates(Tab.1).As shown in Tab.1,there exist many different tumor markers,some are specific for a particular type of cancer,whereas others are associated with two or more cancer types.We would like to emphasize that hitherto no“universal”tumor marker that can detect any type of cancer has been found.For instance,alpha-fetoprotein(AFP)is a glycoprotein of 591 amino acids and a carbohydrate moiety,which is produced by the yolk sac and the liver during fetal or cancer development.As a result,AFPcan be used as a tumor marker to help detect and diagnose cancers of the liver,testes,and ovaries in males or non-pregnant females.The normal value of AFP is generally less than 40 ng·mL-1,and a level above 200 ng·mL-1in adults can be indicative of hepatocellular carcinoma(HCC),germ cell tumors,and metastatic cancers of the liver[28-31,33,37-39]. Carcinoembryonic antigen(CEA)depicts a set of highly related glycoproteins involved in cell adhesion.CEA is generally yielded in gastrointestinal tissue during fetal development,but the production stops before birth.Therefore,CEA is usually presented only at very low levels in the blood of healthy adults.However,the serum levels will be raised in some types of cancers such as colon and rectal cancer,which means that it can be used as a tumor marker in clinical tests.Human chorionicβ-gonadotropin(β-HCG)is a glycoprotein hormone produced rapidly by placental trophoblasts during pregnancy.Elevated expression ofβ-HCG in serum,urine or tumor tissue is a strong indicator of adverse prognosis in many nontrophoblastic tumors[28-30,40].Prostate-specific antigen(PSA),a serine protease belonging to the human kallikrein family,is best known as a prostate cancer biomarker.The expression of PSA is highly restricted to prostate epithelial cells in men and thus PSA is used extensively as a biomarker to screen for prostate cancer,to detect recurrence after definitive therapy,and to follow response to treatment[28,30,32].
Although high tumor marker levels are usually considered to be a strong sign of cancer,this alone is not enough to diagnose cancer.Sometimes,some tumor marker levels may be high in people without cancer,and a condition or disease other than cancer can also increase tumor marker levels.Even for some specific cancer patients,the level of a tumor marker may not rise until the cancer worsens.This is not helpful for early detection,screening,or watching for recurrence.Some cancers do not make tumor markers that are found in the blood.This includes cancers with no known tumor markers.Also,some patients do not have higher tumor marker levels even if the type of cancer they have usually makes tumor markers.Therefore,measurements of tumor markers are usually combined with other tests such as biopsies to diagnose cancer.Tumor marker levels may be measured before treatment to help doctor's plan the appropriate therapy.
3 General Protocols for The Fabrication of UCL nano-bioprobes
For Ln3+-doped UCNPs to be utilized as a promising UCL nano-bioprobe,one major challenge is to devise methods for making them with tunable size and shape,high UCL efficiency as well asdesirable surface characteristics that are compatible with biological environments.To meet these requirements,many efforts have recently been dedicated to the chemical synthesis and surface functionalization of Ln3+-doped UCNPs.In this section,some typical synthesis and surface modification strategies for the fabrication of Ln3+-doped UCL nano-bioprobes will be overviewed as follows.
3.1 Controlled Synthesis
To obtain high-quality UCNPs,many efforts have been devoted to developing facile synthetic methods such as thermal decomposition[41-44],hightemperature coprecipitation[45-46],hydro(solvo)thermal synthesis[47-50],sol-gel procedure[51-52],microwave-assisted synthesis,cation exchange and ionic liquid-based synthesis[53].The availability of these versatile,readily scalable methods builds up the solid foundation necessary to implement the commercialization of UCNPs.As a result,a large amount of UCNPs ranging from oxides to oxyfluorides and fluorides have been synthesized and studied as host materials,among which hexagonal-phase NaYF4was acknowledged as one of the most efficient UC host materials for Ln3+ions doping[25-26]. For instance,through thermal decomposition and high-temperature coprecipitation methods in the presence of oleic acid(OA),oleylamine(OM)and 1-octadecene(ODE),we have synthesized a number of high-quality lanthanide fluoride and oxyfluoride NPs with intense UC luminescence including NaYF4[54],NaGdF4[55],Na-ScF4[56],LiYF4[57],LiLuF4[58],LiYbF4[59],KYF4[60],KLaF4[61], CaF2[62], Lu6O5F8[63], Sr2YF7[64]and KSc2F7[65],to name a few.
Particularly,by using a layer-by-layer(LBL)oriented epitaxial growth protocol of thermal decomposition,we have recently fabricated monodisperse and uniform multilayer-structured KSc2F7nanorods comprising different UC sensitizer/activator pairs of Yb3+/X3+(X=Tm,Ho and Er)separately incorporated layers and thickness-tunable pure KSc2F7interlayers to achieve a highly enhanced UC luminescence with an intensity~70 times higher than codoped conventional UCNPs(Fig.2(a) - (c))[65].Based on the optimized interlayer thickness,we fabricated a myriad of KSc2F7nanorods doped with different Yb3+/X3+pairs free of negative effect of cross-relaxation type ETs(CR-ETs).By adjusting the Yb3+/X3+doping combinations and locations in multilayer-structured nanorods,in all,64 kinds of KSc2F7nanorods were obtained with tunable UC emission outputs spanning from blue to red(Fig.2(d)-(k)).Considering the diversity and flexibility of the multilayer nanostructures we specially designed,these KSc2F7nanorods exhibited great promise in applications as a new class of single-nanocrystal-based UC barcodes for advanced anti-counterfeiting,which can greatly circumvent the deficiencies of those previously reported microcrystal-based UC barcodes utilizing merely two tips of NaYF4microrods[66]for encoding and thus are expected to be more difficult to replicate in practical anti-counterfeiting applications(Fig.2(a) - (o)).
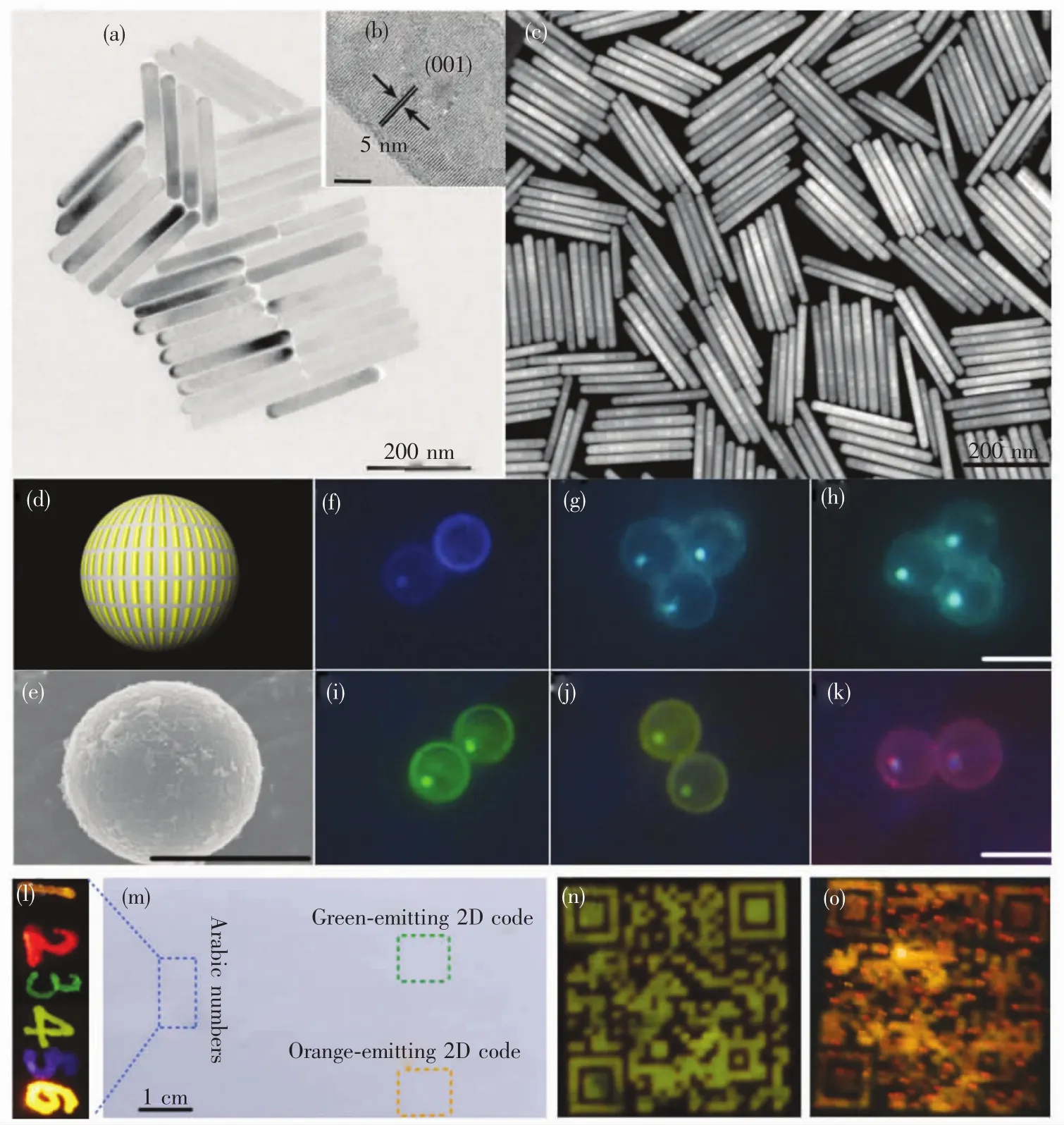
Fig.2 Typical(a)low-resolution and(b)high-resolution TEM images for multilayer-structured KSc2F7 nanorods.(c)Representative high angle annular dark-field scanning TEM(HAADF-STEM)image of the multilayer-structured KSc2 F7 nanorods.(d)Schematic illustration and(e)typical SEM image of polystyrene microbeads loaded with diverse multilayerstructured KSc2 F7 nanorods,demonstrating the diversity of the UCcolor outputs from(f- k)blue to red under 980-nm diode laser irradiation(scale bar is 10 μm).UC photographs of the 2D code,multicolored sketch and Arabic numbers“123456”that were printed or handwritten on a piece of A4 paper by using blue-,green-,yellow-,orange-and redemitting multilayer-structured KSc2 F7 nanorods as UC security ink at(m)daylight(or upon irradiation with a 365 nm UV lamp)and(l- o)upon 980-nm diode laser irradiation in the dark.(Reprinted with permission from Ref.[65].Copyright 2017,Royal Society of Chemistry.)
3.2 Surface Modification and Bioconjugation
Ln3+-doped UCNPs are usually synthesized by reacting lanthanide precursors in organic media in the presence of capping ligands such as OA and OM for the benefit of size and shape uniformity.These UCNPs are often heavily aggregated in aqueous solutions owing to the hydrophobic nature of the capping ligands,which thereby limits their biomedical applications.To this end,diverse surface modification techniques including ligand exchange[67-69],ligand oxidation[70-71],ligand-free synthesis[72],ligand attraction[73-74], electrostatic layer-by-layer assembly[75-76]and surface silanization[77-78]have been employed to render the nanocrystals hydrophilic and amenable for further bioconjugation over the past decade(Fig.3)[79].Through these surface modification approaches,some functional groups such as carboxylic acids,amines and maleimides can be anchored on the surface of these UCNPs for further bioconjugation with specific biomolecules such as antibody,aptamer and DNA fragments that can specifically recognize typical tumor markers.
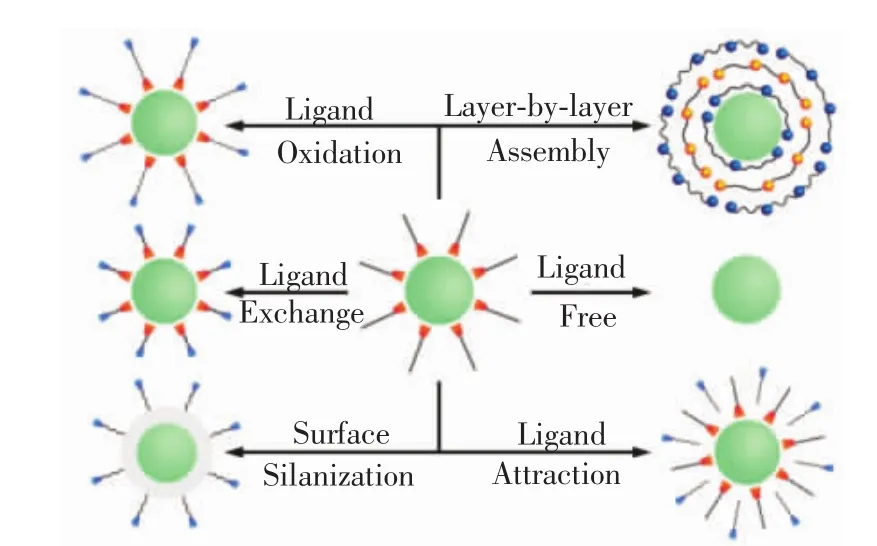
Fig.3 Typical surface modification strategies to render the hydrophobic Ln3+-doped NPs hydrophilic and simultaneously endow themwith designed functional groups.(Reprinted with permission from Ref.[79].Copyright 2015,Royal Society of Chemistry.)
Bioconjugation of specific biomolecules to the surface of these surface modified Ln3+-doped UCNPs has significant impact on their biodetection application.The conjugation of biomolecules could endow the UCNPs with both biocompatibility and desired functionality[79-82].The available biomolecules cover from small biomolecules like biotin and folic acid to biomacromolecules like avidin,streptavidin,antibodies,peptides,aptamers and DNA,subjected to the expected target capabilities[83-86].Generally speaking,the commonly used bioconjugation methods for Ln3+-doped UCNPs are primarily based on either electrostatic adsorption or chemical bonding or their combinations(Fig.4).Particularly,the strong coordination capability of naked metal ions such as Ln3+ions on the surface of ligandfree NPs can be exploited for direct bioconjugation.For instance,by virtue of a simple electrostatic attraction approach,we have recently demonstrated the successful avidin-bioconjugation on the surface of ligandfree LiLuF4∶Ln3+UCNPs[58].Meanwhile,we have also synthesized biotinylated NaEuF4and Sr2YF7NPs based on the strong chelation of the exposed Ln3+ions on the surface of their ligand-free counterparts,which can be steadily dispersed in varied buffer solutions for months without any observable aggregates[64,87].Likewise,Lu and co-workers have also developed an exceptionally simple strategy for the direct synthesis of DNA-functionalized NaYF4∶Yb,Er UCNPs from as-prepared hydrophobic ones through electrostatic interaction between the negatively charged phosphates of the DNA and the naked Ln3+ions on the surface of the UCNPs[88].

Fig.4 Schematic illustration of general bioconjugation methods through physical adsorption(a)and chemical bonding(b)
Apart from the electrostatic adsorption,chemical bonding between the functional groups like maleimide,thiol,carboxylic,aldehyde and amine on the surface of Ln3+-doped UCNPs and those of the biomolecules provides another effective strategy for the bioconjugation of Ln3+-doped UCNPs[89].In a typical process of chemical bonding,some typical crosslinking reagents like ethyl(dimethylaminopropyl)carbodiimide(EDC)/N-hydroxysuccinimide(NHS),o-benzotriazole-N,N,N',N'-tetramethyluroniumhexafluoro-phosphate(HBTU)/N,N-diisopropylethy(DIEA)couples are usually utilized to activate the functional groups of biomolecules to react with those of the UCNPs and thus form a tight covalent bond.For instance,in our lab,avidin was readily conjugated to the surface of ultrasmall carboxyl-functionalized Lu6O5F8UCNPs following a standard EDC/NHS bioconjugation protocol[63].Different from this wellestablished EDC/NHS protocol,we have also proposed a new bioconjugation method between aminefunctionalized NPs such as CaF2∶Ln3+and ZrO2∶Ln3+and biomolecules including biotin and amino-terminal fragment(ATF)of urokinase plasminogen activator(uPA)in DMF solution by using HBTU and DIEA as cross-linking reagents,which had never been explored in Ln3+ions doped inorganic NPs before this work[62,90].Note that the bioactivity of the conjugating biomolecules as well as the dispersability of the NPs may decrease during the bioconjugation,therefore the species and the amounts of the cross-linking reagents should be carefully selected.Meanwhile,to avoid unwanted non-specific binding in subsequent bioassays,it is highly desirable to block the residual binding sites on the surface of the UCNPs by using the well-known blocking reagents such as bovine serum albumin(BSA)and human serum albumin(HSA).

Fig.5 Simplified energy level diagrams describing reported UC processes(Reprinted with permission from Ref.[91].Copyright 2013,Royal Society of Chemistry.)
4 Physical Fundamentals for Upconversion Luminescence
Ln3+-doped UCNPs are typically doped with ytterbium(Yb3+)ion as sensitizers,which can absorb infrared radiation and non-radiatively transfer their excitation energy to activator ions such as erbium(Er3+),thulium(Tm3+)or holmium(Ho3+).By virtue of various photophysical UCprocesses such as excited-state absorption(ESA),energy transfer UC(ETU),cooperative sensitization UC(CSU),photon avalanche(PA)and energy migration-mediated UC(EMU),UC luminescence spanning from the ultraviolet to the NIR region can be readily realized in diverse nanomaterials(Fig.5)[13-14].Although recent advances in Ln3+-doped UCNPs have led to accurate control of UCL of Ln3+ions in terms of emission color,lifetime and intensity,many fundamental aspects of the UCL in Ln3+-doped UCNPs are still not fully addressed[92-93].In particular,currently it still lacks a criterion for the quantitative comparison of optical performance among different Ln3+-doped UCNPs.Besides,mechanistic investigations to understand the origins of surface or concentration quenching of UCL and ways to overcome them in Ln3+-doped UCNPs are still under debate.In this section,the recent advances in these two aspects will be reviewed as follows.
4.1 Development of Standard UC Quantum Yield Measurement System
Evaluation on the optical performance of Ln3+-doped UCNPs is of vital significance for optimizing their optical performance and then promoting their commercial applications.UC quantum yield(QY),defined as the ratio of the number of emitted UC photons to the number of absorbed photons,is considered to be the most reliable indicator to evaluate the optical performance of diverse Ln3+-doped UCNPs.However,presently it still lacks a standard and commercially available UC QY measurement system to quantitatively compare the UC luminescent efficiency of different Ln3+-doped UCNPs.Furthermore,unlike the conventional downshifting luminescence(DSL),the absolute UC QY for UCNPs is closely related to the excitation power density rather than constant in view of the nonlinear nature of UC processes,which makes it particularly difficult to directly evaluate their UCperformance measured under different conditions in the literature.To aim at providing such a standard for quantitative comparison of UC efficiency of various UCNPs,we have recently developed an absolute UC QY measurement system by using a simplified patented design strategy(namely,single grating+single detector configuration)(Fig.6(a)).This system allows for the measurement in a broad emission wavelength range from 300 nm to 1 700 nm and enables fast and sensitive measurement of UC QY with a detection limit down to 0.01% .In particular,this system covers a wavelength range of 410-2 400 nm in the UC excitation and UC luminescence lifetime ranging from 1μs to 100 ms.

Fig.6 (a)Schematic design of the absolute UC QY measurement system we recently proposed.(b)Panorama of the optical measurement platform for lanthanide photophysics and photochemistry of UCNPs.(Reprinted with permission from Ref.[14].Copyright 2015,Royal Society of Chemistry.)
Based on the above instrument developments,we further constructed a state-of-the-art optical measurement platform for lanthanide photophysics(Fig.6(b)).The platform shares various excitation sources(including femtosecond,picosecond and nanosecond pulse lasers,CW lasers,and xenon lamps)and integrates diverse modules of the spectrometer systems(including UV to NIR steady-state and transient fluorescence,high-resolution fluorescence,UV to mid-infrared(MIR)steady-state and phosphorescence lifetime,picosecond fluorescence lifetime,femtosecond-to-submillisecond transient absorption(TA),and high-throughput UCL microplate reader)and the cryogenic systems(4 K and 10 K),thus facilitating comprehensive study on lanthanide photophysics and photochemistry properties of UCNPs in the time,frequency,and/or space domains with high resolution.
4.2 Upconversion Luminescence Enhancement
When compared with the commonly used DSL probes like organic fluorophores,lanthanide chelates and QDs,the major bottleneck for Ln3+-doped UCNPs used as a promising bioprobes is their limited brightness and low UC QYs.For the UCNPs doped with a large amount of activators and sensitizers,surface and concentration quenching of UCL is inevitable,thereby leading to an UC efficiency that is much smaller than that using their bulk counterparts.Such notorious surface and concentration quenching of UCL for a given UCNP system is primarily due to the deleterious cross-relaxation between dopant ions or enhanced energy migration via resonant energy transfer to the internal lattice and surface defects in Ln3+-doped UCNPs.
Recent experimental investigations[25]suggest that the most straightforward and effective approach to circumvent the surface and/or concentration quenching is to implement a core-shell design strategy,by which the dominant luminescence quenching through energy migration to surface defects can be largely minimized[62].For instance,we have recently fabricated multi-shell LiLuF4∶Ln3+@LiLuF4core/shell UCNPs by using a novel successive layer-by-layer(SLL)epitaxial shell growth strategy of thermal decomposition[58].It was observed that the overall UC luminescence intensity can be remarkably enhanced upon homogeneous shell passivation,achieving a record-high absolute UC QY of 7.6%for Tm3+in core-shell NPs that was~12 times higher than that of core-only counterparts.Besides such homogeneous epitaxial shell growth,surfaceprotection strategy has been extensively applied to heterogeneous core-shell structures such as NaYF4@In this approach,the epitaxial growth of highquality shell layers with less defective sites at the interface of the core-shell region is of critical importance in improving the UCL efficiency of UCNPs.In particular,Almutairi and co-workers recently demonstrated that,after an inert heterogeneous epitaxial shell growth,the surface and concentration quenching effect in Er3+-heavily-doped(up to 100% mole fraction)NaErF4@NaLuF4coreshell UCNPs can be completely eliminated(Fig.7(a))[92].More importantly,through the use of an additional dopant of Tm3+serving as transient energy trapping,the UCL quenching caused by the internal lattice defects in NaErF4core-only NPs can also be minimized,endowing the resultant NaErF4∶Tm@NaYF4UCNPs with bright red(about 700-fold enhancement)and near-infrared luminescence that was achievable under multiple excitation wavelengths(Fig.7(b))[93].

Fig.7 Proposed mechanisms involving the use of an inert heterogeneous epitaxial shell growth(a) and a Tm3+-mediated trapping center in Er3+-heavilydoped core-shell UCNPs(b)to effectively avoid the notorious concentration and surface quenching effects for highly enhanced UCL(Reprinted with permission from Refs.[92 - 93].Copyright 2017,American Chemical Society and Wiley-VCH Verlag GmbH & Co.KGaA.)
Another effective approach to circumvent the surface and/or concentration UCL quenching effect is to manipulate the deleterious cross-relaxation type energy transfer(CR-ETs)in Ln3+-heavily-doped UCNPs.It is well known that the unwanted CR-ETs among adjacent Ln3+ions may quench the excitation energy and dissipate it nonradiatively as heat in the lattices of the UCNPs,thereby leading to weak UCL.Note that the probability of CR-ETs for two neighboring Ln3+ions is strongly dependent on their spatial distance[25],therefore,the most straightforward and feasible way to manipulate the CR-ETs is to control the distance among Ln3+ions that are embedded in one identical nanoparticle.Attempts to solve this problem include lowering the doping concentration of Ln3+ion or confining doped Ln3+ions in different layers of core-shell structured UCNPs[97-99].However,only very limited distance between Ln3+emitters(typically <5 nm)can be tuned for these two approaches due to their intrinsic limitations such as elemental migration across the core-shell interfaces and inhomogeneous growth of different shell layers for core-shell structured UCNPs[50,97,100], which thereby cannot completely minimize the negative effect of the deleterious CRETs.To this end,very recently,we proposed a rational design strategy to fundamentally circumvent concentration quenching effect in Ln3+-doped UCNPs.This design strategy was primarily based on manipulating the CR-ETs at an extremely large length scale(>20 nm)through fine-tuning the distances among Ln3+dopants that are spatially confined into different layers by inserting thicknesstunable pure host interlayers into one single nanorod with specially designed multilayer structures(Fig.8).The successful inhibition of the CR-ETs led to significantly enhanced UCL signal with an intensity~70 times higher than co-doped conventional UCNPs.The absolute UC QY for these multilayerstructured nanorods was determined to be 3.9%,which was found to be even higher than that of the well-established Yb/Er doped UC NaYF4bulks or nanomaterials measured at the same laser excitation power density[101].

Fig.8 Schematic design of multilayer-structured KSc2 F7 nanorod comprising different Yb/X pairs separately incorporated layers and thickness-tunable pure KSc2 F7 interlayers to manipulate CR-ETs in one single nanorod for highly enhanced UCL(Reprinted with permission from Ref.[65].Copyright 2017,Royal Society of Chemistry.)
5 In vitro UCL Bioassay of Tumor Markers
Sensitive detection and accurate analysis of tumor markers in fluid samples of cancer patients such as serum,saliva and urine at the earliest stage possible are of crucial importance for early cancer diagnosis and therapy and consequently increase their survival rates.For this purpose,some luminescent bioassay methods including heterogeneous ELISA,TR-FIA and DELFIA have been well developed in the past decades by using organic dyes,QDs and lanthanide chelates as luminescent bioprobes[2-4,102-107].However,the use of these luminescent bioprobes somewhat suffers from a series of disadvantages like the large background noise,poor photochemical stability and high cost-to-use[12].As a new type of luminescent bioprobes,the UCNPs can offer a remarkable advantage over the traditional ones in that the distinct NIR excitation for UC induces only zero or very weak background fluorescence,thereby leading to an improved detection signal-to-noise and sensitivity[89,98,108-120]. As such,many novel and efficient bioassay techniques based on Ln3+-doped UCNPs have recently been developed.According to their signal detection formats,the presently well-developed UCL bioassays based on Ln3+-doped nano-bioprobes can be roughly divided into two broad classes,namely,heterogeneous and homogeneous UCL bioassays(Fig.9).In this section,we will provide a brief survey on the most recent advances in the development of Ln3+-doped UCNPs as nano-bioprobes as well as their homogeneous and heterogeneous in-vitro UCL detection of diverse tumor markers.
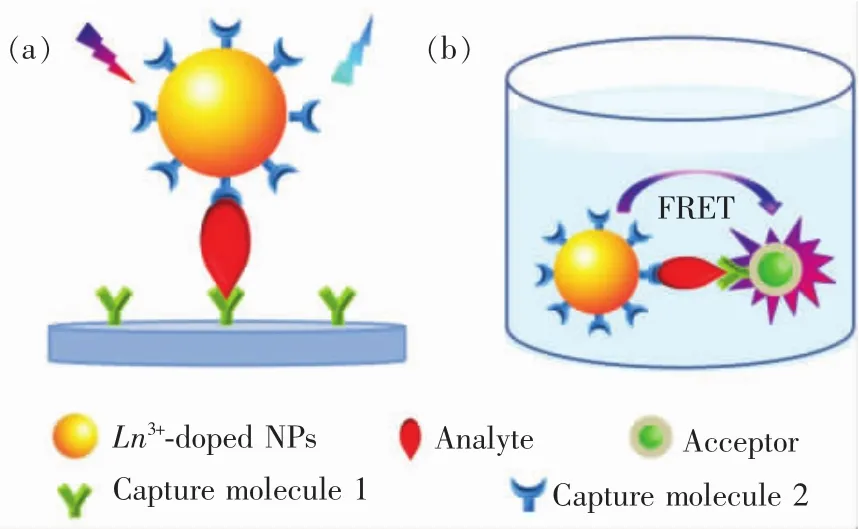
Fig.9 Schematic illustration of typical(a)heterogeneous and(b)homogeneous UCL bioassays of tumor marker based on Ln3+-doped nano-bioprobes
5.1 Heterogeneous UCL Bioassay
Heterogeneous UCL bioassay takes advantage of the specific recognition and high binding affinity between target analyte and capture molecule anchored on a solid substrate to realize the detection of trace amount of analyte(Fig.9(a)).In a typical procedure for heterogeneous UCL bioassay,the capture molecules are first immobilized on a solid substrate like 96-well microplate via incubation,and then conjugated with the analyte solution.Subsequently,washing is performed in order to eliminate other biological species present in the sample,which might interfere in the last detection step.A solution of capture molecule labelled with UCNPs is further added in order to mark the analyte.As such,the analyte is captured between the capture molecule immobilized on the solid phase and the UCNP-labelled capture molecule.After washing off excess unbound UCNP-labelled capture molecules thoroughly,the microplate is finally subjected to a UCL measurement(Fig.10(a)).As a consequence,the concentration of the analyte can be quantified by directly measuring the optical signal of the UCNP label.
One of the earliest examples of the heterogeneous UCL bioassay based on Ln3+-doped UCNPs was demonstrated by Zijlmans and co-workers in 1999,who employed Y2O2S∶Yb,Er(or Yb,Tm)submicrons as UCL reporter for the detection of PSA in human prostate tissue[121].Since then,considerable efforts have been devoted to the use of UCNPs as sensitive bioprobes for diverse heterogeneous UCL bioassay of diverse tumor markers[122-125].Particularly,very recently,we designed a novel UCL biodetection system for high-throughput bioassay based on a commercial microplate reader(Synergy 4,BioTek)integrated with a 980 nm NIR diode laser as the excitation source,where the concentration of tumor markers can be quantified by measuring the optical signals of UC nanoprobes bound to a 96-well microplate upon 980 nm NIR excitation(Fig.10(a)).By employing the surface-functionalized Ln3+-doped UCNPs as nano-bioprobes,a number of important tumor markers like CEA,AFP,PSA,β-HCG and suPAR were successfully detected.For instance,by using LiLuF4∶Yb,Er@LiLuF4core/shell UCNPs as sensitive UCL nano-bioprobes,we achieved the successful heterogeneous UCL biodetection of trace amount ofβ-HCG through specific antibody-antigen recognition(Fig.10(b))[58].The content of β-HCG in standard solution was quantified by measuring the integrated UCL intensity of the UCNPs that were conjugated to the biotinylated β-HCG antibody.It was observed that the UCL intensity of the nanoprobes gradually increased with the increased amount ofβ-HCG antigen and exhibited a linear dependence with the concentration ofβ-HCG at 0 -310 ng·mL-1(Fig.10(c) - (d)).The limit of detection(LOD)ofβ-HCGwas determined to be ~3.8 ng·mL-1,which is comparable to the β-HCG level in the serum of normal humans.
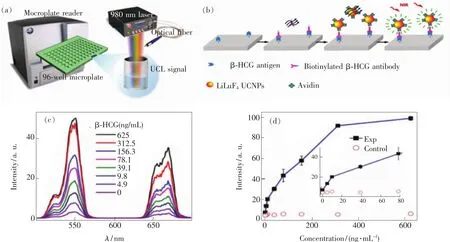
Fig.10 (a)Experimental setup for heterogeneous UCL bioassay of diverse tumor markers based on Ln3+-doped UCNPs.(b)The process and principle of heterogeneous UCL detection ofβ-HCG by using LiLuF4∶Yb,Er@LiLuF4 core-shell UCNPs as sensitive nano-bioprobes.(c)UCL spectra of the bioassays as a function ofβ-HCGconcentration.(d)Calibration curve of UCL detection for the integrated UCL intensity vs.the concentration ofβ-HCG.The control experiment was conducted by employing BSA instead ofβ-HCG antigen as analyte under otherwise identical conditions.(Reprinted with permission from Refs.[58]and[126].Copyright 2013 and 2014,Royal Society of Chemistry and Wiley-VCH Verlag GmbH & Co.KGaA.)
Meanwhile,we also developed a rational coreshell-shell(CSS) design strategy to construct Eu3+-activated NaGdF4∶Yb/Tm@NaGdF4∶Eu@NaEuF4CSS NPs functionalized with efficient UCL and intense dissolution-enhanced DSL of Eu3+for the ultrasensitive detection of AFP(Fig.11(a))[127].By utilizing the CSS NPs as red luminescent nanoprobes,we demonstrated the successful UCL(Fig.11(b))and DSL(Fig.11(c))bioassays of a typical hepatic carcinoma biomarker of AFPin human serum samples.The UCL bioassay showed a LOD of AFP down to 20 pg/mL(290 fmol/L)that was about 30-fold improvement relative to that of commercial dissociation-enhanced lanthanide fluoroimmunoassay kit,while the DSL bioassay by employing the identical CSS NPs can serve as a self-referential validation for the reliability and accuracy of the UCL bioassay for AFP detection.The AFP levels in 20 human serum samples determined independently by UCL assay,DSL assay,and the commercial DELFIA kit were essentially consistent(Fig.11(d) - (e)),thus verifying the high reliability of the assays.The CVs for the assays of human sera by the addition of distinct amount of AFP standard solutions were below 5%and the analytical recoveries were in the range of 102% -109%,reconfirming the high accuracy and precision of the red luminescent CSS nanoprobes for AFP detection.More encouraging results in our lab have also been obtained for the assays of uPAR,CEA and PSA with LODs lower than 20 pmol/L,which are much lower than those in conventional fluorescence immunoassays like ELISA.Further work is underway on the optimization of the nanoprobes and the assay protocols involved in the ultrasensitive UCL biodetection system for the analysis of human fluid samples such as serum,saliva and urine.
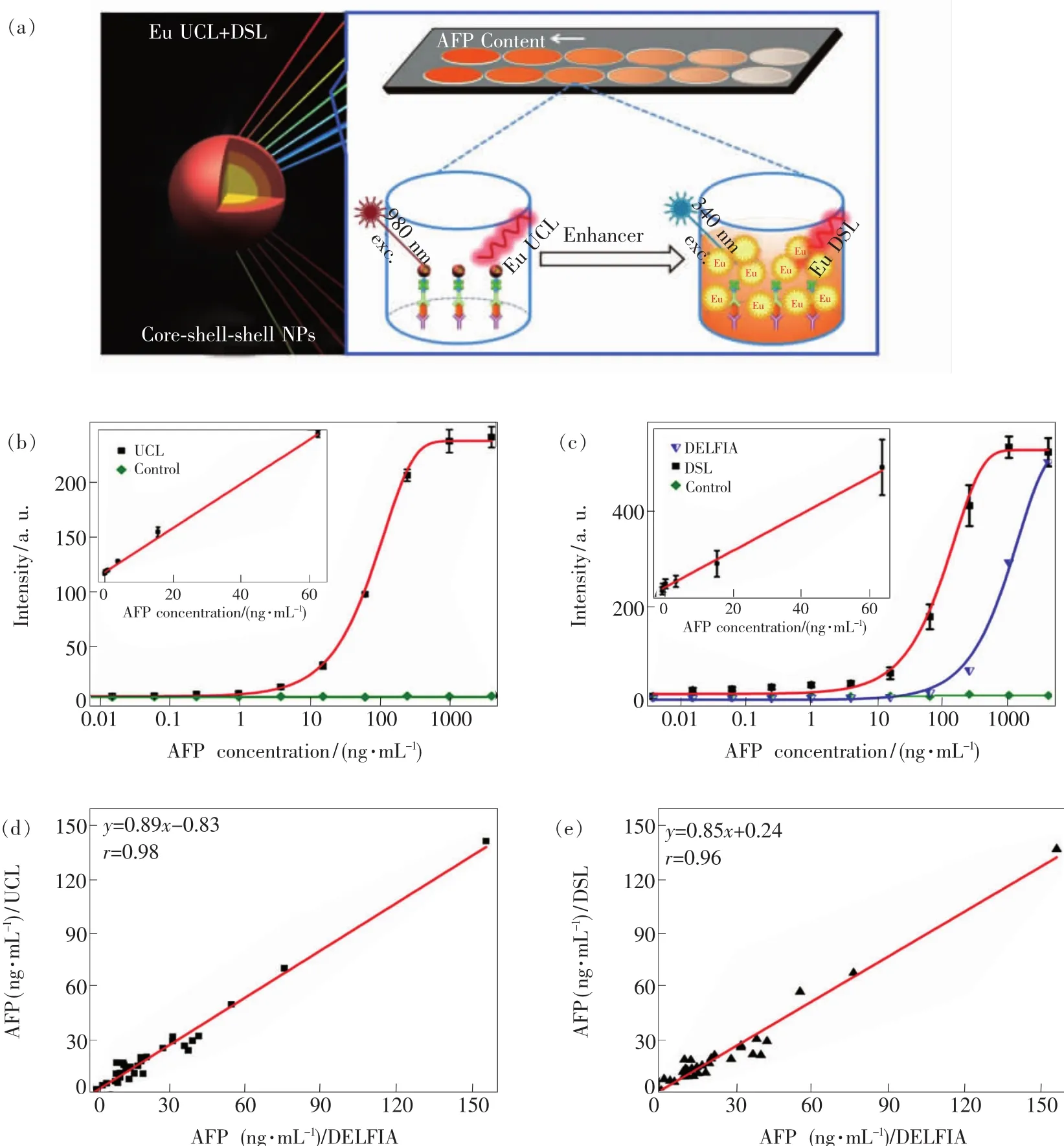
Fig.11 (a)Schematic illustration for the CSSnanoparticle comprising NaGdF4∶Yb/Tm core,NaGdF4∶Eu and NaEuF4 shells to produce intense UCL and DSL of Eu3+(Left)and the proposed UCL and DSL bioassays of AFP(right)based on the biotinylated CSSNPs.(b)Calibration curves for AFP detection via UCL bioassay based on the biotinylated CSSNPs.(c)Calibration curves for AFPdetection via DSL bioassay based on the biotinylated CSSNPs and commercial DELFIA kit based on Eu3+-DTTA complex,respectively.The control experiments in(b)and(c)were conducted by using BSA instead of the AFP antigen as analyte under otherwise identical conditions.By contrast,the UCL and DSL signals were hardly detectable due to the lack of specific binding between BSA and anti-AFP antibody.The insets of(b)and(c)show the corresponding linear range of the calibration curve for AFPdetection via UCL and DSL bioassays.(d,e)Correlations between UCL bioassay,DSL bioassay and commercial DELFIA kit for the AFP detection in 20 human serum samples,where the ordinate and abscissa denote the AFPlevel determined in UCL bioassay,DSL bioassay and DELFIA kit,respectively.(Reproduced with permission from Ref.[127].Copyright 2016,Royal Society of Chemistry.)
5.2 Homogeneous UCL Bioassay
Unlike heterogeneous UCL bioassay,homogeneous UCL bioassay is primarily based on the distance-dependent energy transfer(commonly Frster resonance energy transfer,FRET)between an excited donor and a nearby acceptor via long-range dipole-dipole interactions to detect diverse tumor markers dissolved in the solution,and thus can be performed easily via a simple“mix-and-read”process free of the tedious separation and washing steps typically required in heterogeneous UCL bioassay(Fig.9(b)).In a typical homogeneous UCL bioassay based on FRET(UC-FRET),excitation of the donor by an energy source(e.g.flash lamp or laser)triggers an energy transfer to the acceptor,if they are within a given proximity to each other and spectrally overlapped between the absorption of acceptor and the emission of donor,and the acceptor in turn emits light at its given wavelength.In view of this,the detection sensitivity in a homogeneous UCFRET assay mainly relies on the efficiency of the FRET process,and thereby the donor and acceptor pair used in the UC-FRET bioassay process should be taken into account carefully.The acceptors are commonly those molecules or nanomaterials with large absorption coefficient at the wavelength overlapped with the emission of UCNPs,such as fluorescent proteins,organic dyes,QDs,Au NPs or nanorods,MnO2nanosheets,aromatic polymer nanospheres,carbon NPs or nanotubes,graphenes,and graphene oxides.
The application of UCL phosphors as UC-FRET(or UC-LRET)bioprobes can date back to the work reported by Mitchell and co-workers[130],and subsequently by Kuningas and co-workers[131],who utilized the streptavidin-conjugated UC phosphors as donor and biotinylated phycobiliprotein (biotin-BPE)as acceptor to realize the UC-FRET bioassay.Subsequently,this novel UC-FRET bioassay method was further optimized and extended to other Ln3+-doped nanoprobes to detect biomolecules,temperature,pH and other molecules or ions in solution[132-136].For instance,based on different acceptor entities such as organic dyes,Au NPs,MnO2nanosheets,carbon NPs,graphene and graphene oxide(GO),Liu and co-workers proposed a series of UC-FRET pairs for the detection of diverse biomolecules including glucose,thrombin,DNA,matrix metalloproteinase-2(MMP-2),CEA,and Kanamycin[128,135,137-143].Hao et al.established a sensitive UC-FRET biosensor based on the pair of oligonucleotide-modified BaGdF5∶Yb,Er UCNPs and Au NPs for rapid detection of H7 hemagglutinin genes of avian influenza viruses with an LODdown to7 pmol·L-1[144].

Fig.12 (a)Schematic illustration of UC-FRET inhibition assays using CNPs nanosheets as energy quenchers for the detection of MMP-2.(b)Schematic illustration of the AFP biosensor based on FRET from anti-AFP conjugated UCNPs to gold NRs.(Adapted with permission from Refs.[128-129].Copyright 2012,American Chemical Society.)
Particularly,for the detection of tumor markers,Liu and coworkers designed a sensitive UCFRET biosensor for the detection of matrix metalloproteinase-2(MMP-2,a very important biomarker in blood)by using PEI modified NaYF4∶Yb/Er UCNPs coupled with a polypeptide chain that contains a specific MMP-2 substrate domain andπ-electronrich region as energy donors,and CNPs as energy acceptors(Fig.12(a))[128].Similarly,by using anti-AFP conjugated NaYF4∶Yb,Tm@NaGdF4UCNPs as a donor and ascorbic-acid modified gold nanorods(NRs)as a quencher,Wang et al.constructed a NIR UC-FRET system for the turn-on detection of AFP in human serum samples(Fig.12(b))[129].In their study,the UCNPdonor and gold NR acceptor were initially brought into close proximity to trigger the FRET process through electrostatic attraction,which resulted in an UCL quenching of the donor at 804 nm.Upon addition of AFP,the linkage was cleaved due to the specific binding of AFP with anti-AFP conjugated UCNPs,leading to the recovery of the UCL intensity.It was observed that the UCL intensity of the nanoprobes increased linearly with the increased concentration of AFP in the range from 0.18 to 11.44 ng·mL-1,and the LOD was determined to be 0.16 ng·mL-1.
Another outstanding demonstration was reported by Chu and co-workers[124],who designed another UC-FRET biosensor by using the peptidefunctionalized NaYF4∶Yb,Er UCNPs as energy donors and GO as energy quenchers for the detection of human immunodeficiency virus(HIV)antibody(Fig.13(a)).The UCNPdonors were initially adsorbed on the surface of GO via π-π stacking interactions and hydrophobic interactions between the peptides and GO,which resulted in a complete UCL quenching of the donor through energy transfer or electron transfer processes.Upon addition of anti-HIV-1 gp120 antibody,the adsorption was cleaved through the formation of peptide-antibody complexes,leading to the decrease in quenching efficiency and the recovery of UCL.It was observed that the UCL intensity of the nanoprobes increased linearly with the increased concentration of HIV-1 antibody in the range from 5 to 150 nmol/L,and the LOD was calculated to be 2 nmol/L according to the 3σ rule(Fig.13(b) - (c)).Meanwhile,they also reported a sensitive UC-FRET assay for the ratiometric detection of phospholipase D(PLD,a critical component of intracellular signal transduction and tumor marker)in both cell lysates and living cells by employing phospholipid-modified NaYF4∶Yb,Er UCNPs as an energy donor and the dye rhodamine B as an energy acceptor[145].The PLD level was quantified by the ratio of PL intensity of the UCNP donor at 540 nm and rhodamine B acceptor at 590 nm after the normalization of the Er3+UCL at 655 nm(as an internal reference).The LOD in terms of the rule of 3 times deviation over the blank response was determined to be 4 U·L-1,and the PLD activity in a breast cancer cell line was found at least 7-fold higher than that in normal cells.For point-of-care test,Liu et al.further designed a straightforward paper-based microfluidic device(namely UC-μPAD)for UC-FRET assays[137].Based on UC-μPAD,MMP-2 and CEA in human serum samples were tested with LODs in the order of pg·mL-1.These efforts suggest that Ln3+-doped UCNPs are excellent donor labels in homogeneous UC-FRET assays and may play a critical role in the point-of-care test in future community medical practice[19,146].

Fig.13 (a)Schematic illustration of the UC-FRET bioprobes for the detection of anti-HIV-1 gp120 antibody.(b)UCL spectra of the biosensor with varied concentrations of antibody(0,5,20,40,55,75,95,130,150 nmol/L).(c)Linear relationship between the UCL intensity and the concentration of antibody within the range of 5-150 nmol/L.(Adapted with permission from Ref.[124].Copyright 2014,Royal Society of Chemistry.)
6 Conclusions and Prospects
Ln3+-doped UCNPs are recognized as a new generation of luminescent nanoprobes and as an alternative to traditional molecular probes for cancer theranostics due to their superior physicochemical properties.By utilizing their NIR-triggered anti-Stokes UCL,these nanoprobes can completely eliminate the short-lived background noise from scattered light and autofluorescence from biological samples,thus providing a background-free signal for biodetection and offering a remarkable sensitivity than conventional fluorescent immunoassays.These UCL nano-bioprobes,while being in its infancy,have been rapidly developed and pushed forward to a new horizon for bioassay of diverse tumor markers.However,it remains many technical challenges and economic concerns to be overcome when marching towards commercial assay kit applications.
Firstly,Ln3+-doped UCNPs still suffer from low UCL efficiency when compared to their traditional counterparts like organic dyes,lanthanide chelates and QDs.Therefore,one foremost task is to further increase the UCL efficiency of Ln3+-doped UCL nanoprobes,in order to improve the LODs for diverse tumor markers to fulfill the key requirements for clinical diagnosis.We believe such goal can be achieved by the overall optimization of the UCNPs such as suitable host and dopant selection,coreshell architecture design and antenna effect and so on.Secondly,the non-specific binding that deteriorates the sensitivity,specificity and reliability of the detection is always a plague issue in UCNP-based bioassays.To avoid this undesirable effect,it is highly demanded to develop a general and efficient surface functionalization approach that can precisely control the functional moieties on the surface of the nanoprobes and provide desirable bioaffinity,bioactive and targeted capability.Long-term stability and toxicity of the surface-functionalized nanoprobes are the other key issues that need to be tracked,especially when applied for in vivo detection and imaging of tumors.Thirdly,it is highly desirable to develop economic and convenient bioassay devices such as UCL microplate reader system for Ln3+-doped UCL nanoprobes,in order to facilitate the rapid and ultrasensitive detection of tumor markers and high throughput drug screening for point-of-care test.Last but not the least,in view of its extremely high sensitivity,the UCL bioassay technique based on lanthanide nanoprobes can be exploited for non-invasive analysis of human glandular secretions such as saliva and urine,which may bring new opportunity for early detection or screening of cancer,and would ultimately revolutionize current serum-based bioassays in future community or family medical service.
[1 ]GBD 2015 Mortality and Causes of Death Collaborators.Global,regional,and national life expectancy,all-cause mortality,and cause-specific mortality for 249 causes of death,1980-2015:a systematic analysis for the Global Burden of Disease Study 2015 [J].The Lancet,2016,388:1459-1544.
[2]CHARBONNIERE L J,HILDEBRANDTN,ZIESSEL R F,et al..Lanthanides to quantum dots resonance energy transfer in time-resolved fluoro-immunoassays and luminescence microscopy[J].J.Am.Chem.Soc.,2006,128:12800-12809.
[3]MATSUMOTOK,YUAN JG,WANGGL,et al..Simultaneous determination of alpha-fetoprotein and carcinoembryonic antigen in human serum by time-resolved fluoroimmunoassay[J].Anal.Biochem.,1999,276:81-87.
[4]WU B Y,WANG H F,CHEN J T,et al..Fluorescence resonance energy transfer inhibition assay for alpha-fetoprotein excreted during cancer cell growth using functionalized persistent luminescence nanoparticles[J].J.Am.Chem.Soc.,2011,133:686-688.
[5]BUNZLI J C G.Lanthanide luminescence for biomedical analyses and imaging [J].Chem.Rev.,2010,110:2729-2755.
[6]ZHOU V,HAN S,BRINKER A,et al..A time-resolved fluorescence resonance energy transfer-based HTSassay and a surface plasmon resonance-based binding assay for heat shock protein 90 inhibitors[J].Anal.Biochem.,2004,331:349-357.
[7]LEQUIN R M.Enzyme immunoassay(EIA)/enzyme-linked immunosorbent assay(ELISA) [J].Clin.Chem.2005,51:2415-2418.
[8]RISSIN D M,KAN CW,CAMPBELL T G,et al..Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations[J].Nat.Biotech.,2010,28:595-599.
[9]CLAPPA R,MEDINTZ I L,MAURO J M,et al..Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors[J].J.Am.Chem.Soc.,2003,126:301-310.
[10]MEDINTZI L,CLAPPA R,BRUNEL FM,et al..Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates[J].Nat.Mater.,2006,5:581-589.
[11]GEILER D,STUFLER S,LHMANNSRBEN H G,et al..Six-color time-resolved Frster resonance energy transfer for ultrasensitive multiplexed biosensing[J].J.Am.Chem.Soc.,2012,135:1102-1109.
[12]CHEN Z,ZHENGW,HUANGP,et al..Lanthanide-doped luminescent nano-bioprobes for the detection of tumor markers[J].Nanoscale,2015,7:4274-4290.
[13]LIU Y S,TU D T,ZHU H M,et al..Lanthanide-doped luminescent nanoprobes:controlled synthesis,optical spectroscopy,and bioapplications[J].Chem.Soc.Rev.,2013,42:6924-6958.
[14]ZHENG W,HUANG P,TU D T,et al..Lanthanide-doped upconversion nano-bioprobes:electronic structures,optical properties,and biodetection[J].Chem.Soc.Rev.,2015,44:1379-1415.
[15]ZHANG P,ROGELJS,NGUYEN K,et al..Design of a highly sensitive and specific nucleotide sensor based on photon upconverting particles[J].J.Am.Chem.Soc.,2006,128:12410-12411.
[16]ACHATZ D E,ALI R,WOLFBEISO S.Luminescent chemical sensing,biosensing,and screening using upconverting nanoparticles[J].Top.Curr.Chem.,2011,300:29-50.
[17]LIU J L,CHENG JT,ZHANG Y.Upconversion nanoparticle based LRET system for sensitive detection of MRSA DNA sequence[J].Biosens.Bioelectron.,2013,43:252-256.
[18]ZHAO P,WU Y Y,ZHU Y H,et al..Upconversion fluorescent strip sensor for rapid determination of vibrio anguillarum[J].Nanoscale,2014,6:3804-3809.
[19]YUAN F,CHEN H Q,XU J,et al..Aptamer-based luminescence energy transfer from near-infrared-to-near-infrared upconverting nanoparticles to gold nanorods and its application for the detection of thrombin[J].Chemistry,2014,20:2888-2894.
[20]DACOSTA M V,DOUGHAN S,HAN Y,et al..Lanthanide upconversion nanoparticles and applications in bioassays and bioimaging:a review[J].Anal.Chim.Acta,2014,832:1-33.
[21]LU Y Q,ZHAO JB,ZHANG R,et al..Tunable lifetime multiplexing using luminescent nanocrystals[J].Nat.Photon.,2014,8:33-37.
[22]TSANGM K,BAI GX,HAOJH.Stimuli responsive upconversion luminescence nanomaterials and films for various applications[J].Chem.Soc.Rev.,2015,44:1585-1607.
[23]YANG PP,GAI SL,LIN J.Functionalized mesoporous silica materials for controlled drug delivery[J].Chem.Soc.Rev.,2012,41:3679-3698.
[24]CHENG L,YANG K,LI Y G,et al..Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy[J].Angew.Chem.Int.Ed.,2011,50:7385-7390.
[25]ZHOU B,SHI B Y,JIN D Y,et al..Controlling upconversion nanocrystals for emerging applications[J].Nat.Nanotechnol.,2015,10:924-936.
[26]ZHOU J,LIU Q,FENG W,et al..Upconversion luminescent materials:advances and applications[J].Chem.Rev.,2015,115:395-465.
[27]HUANG P,TU D T,ZHENG W,et al..Inorganic lanthanide nanoprobes for background-free luminescent bioassays[J].Sci.China Mater.,2015,58:156-177.
[28]BAST R C,BADGWELL D,LU Z,et al..New tumor markers:CA125 and beyond [J].Int.J.Gynecol.Cancer,2005,15:274-281.
[29]FOUNDATION M SC.Understanding tumor markers-grades/prognosis[R/OL].2015-11-04.http://www.cancer.gov/cancertopics/factsheet/detection/tumor-markers.
[30]DUFFY M J.Tumor markers in clinical practice:a review focusing on common solid cancers[J].Med.Princip.Practice:Int.J.Kuwait Univ.,Health Sci.Centre,2013,22:4-11.
[31]STURGEON CM,DUFFY M J,HOFMANN B R,et al..National academy of clinical biochemistry laboratory medicine practice guidelines for use of tumor markers in liver,bladder,cervical,and gastric cancers[J].Clin.Chem.,2010,56:e1-48.
[32]NCInstitute.Tumor marker tests[R/OL].2014-02-27.http://www.cancer.net/all-about-cancer/cancernet-feature-articles/treatments-tests-and-procedures/understanding-tumor-markers.
[33]NGUYEN M H,KEEFFE EB.Screening for hepatocellular carcinoma[J].J.Clin.Gastroenterol.,2002,35:S86-S91.
[34]PONSKY L E,SHARMA S,PANDRANGI L,et al..Screening and monitoring for bladder cancer:refining the use of NMP22 [J].J.Urol.,2001,166:75-78.
[35]DIAMANDISE.Tumor Markers:Past,Present,and Future.in Tumor Markers:Physiology,Pathobiology,Technology,and Clinical Applications[M].Washington,DC:AACC Press,2002:3-8.
[36]LUDWIG JA,WEINSTEIN JN.Biomarkers in cancer staging,prognosis and treatment selection[J].Nat.Rev.Cancer,2005,5:845-856.
[37]ZHANG B H,YANG B H,TANG Z Y.Randomized controlled trial of screening for hepatocellular carcinoma[J].J.Cancer Res.Clin.Oncol.,2004,130:417-422.
[38]GEBO K A,CHANDER G,JENCKESM W,et al..Screening tests for hepatocellular carcinoma in patients with chronic hepatitis C:a systematic review[J].Hepatology,2002,36:S84-S92.
[39]MIZEJEWSKI G J.Alpha-fetoprotein structure and function:relevance to isoforms,epitopes,and conformational variants[J].Experiment.Bio.Med.,2001,226:377-408.
[40]NGAN H Y,CHEUNG A N,LAUDER I J,et al..Prognostic significance of serum tumour markers in carcinoma of the cervix[J].Eur.J.Gynaecolo.Oncol.,1996,17:512-517.
[41]MAI H X,ZHANG Y W,SIR,et al..High-quality sodium rare-earth fluoride nanocrystals:controlled synthesis and optical properties[J].J.Am.Chem.Soc.,2006,128:6426-6436.
[42]BOYER JC,VETRONE F,CUCCIA L A,et al..Synthesis of colloidal upconverting NaYF4nanocrystals doped with Er3+,Yb3+and Tm3+,Yb3+via thermal decomposition of lanthanide trifluoroacetate precursors[J].J.Am.Chem.Soc.,2006,128:7444-7445.
[43]YI G S,CHOWGM.Synthesis of hexagonal-phase NaYF4∶Yb,Er and NaYF4∶Yb,Tm nanocrystals with efficient up-conversion fluorescence[J].Adv.Funct.Mater.,2006,16:2324-2329.
[44]YE X C,COLLINSJE,KANGY J,et al..Morphologically controlled synthesis of colloidal upconversion nanophosphors and their shape-directed self-assembly[J].Proc.Natl.Acad.Sci.USA,2010,107:22430-22435.
[45]LI Z Q,ZHANG Y.An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF4∶Yb,Er/Tm nanocrystals with controllable shape and upconversion fluorescence[J].Nanotechnology,2008,19:345606.
[46]WANGF,DENGR,LIU X.Preparation of core-shell NaGdF4nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes[J].Nat.Protoc.,2014,9:1634-1644.
[47]WANG X,ZHUANGJ,PENGQ,et al..A general strategy for nanocrystal synthesis[J].Nature,2005,437:121-124.
[48]CHEN D Q,YU Y L,HUANG F,et al..Modifying the size and shape of monodisperse bifunctional alkaline-earth fluoride nanocrystals through lanthanide doping[J].J.Am.Chem.Soc.,2010,132:9976-9978.
[49]LI C X,LIN J.Rare earth fluoride nano-/microcrystals:synthesis,surface modification and application[J].J.Mater.Chem.,2010,20:6831-6847.
[50]LI X M,WANG R,ZHANG F,et al..Engineering homogeneous doping in single nanoparticle to enhance upconversion efficiency[J].Nano Lett.,2014,14:3634-3639.
[51]PATRA A,FRIEND C S,KAPOOR R,et al..Fluorescence upconversion properties of Er3+-doped TiO2and BaTiO3nanocrystallites[J].Chem.Mater.,2003,15:3650-3655.
[52]PATRA A,FRIEND CS,KAPOOR R,et al..Upconversion in Er3+∶ZrO2nanocrystals[J].J.Phys.Chem.B,2002,106:1909-1912.
[53]DONG CH,VAN VEGGEL F C JM.Cation exchange in lanthanide fluoride nanoparticles[J].ACSNano,2009,3:123-130.
[54]TU D T,LIU Y S,ZHU H M,et al..Breakdown of crystallographic site symmetry in lanthanide-doped NaYF4crystals[J].Angew.Chem.Int.Ed.,2013,125:1166-1171.
[55]LIU Y S,TU D T,ZHU H M,et al..A strategy to achieve efficient dual-mode luminescence of Eu3+in lanthanides doped multifunctional NaGdF4nanocrystals[J].Adv.Mater.,2010,22:3266-3271.
[56]AI Y,TU D T,ZHENG W,et al..Lanthanide-doped NaScF4nanoprobes:crystal structure,optical spectroscopy and biodetection[J].Nanoscale,2013,5:6430-6438.
[57]WANGM,CHENZ,ZHENGW,et al..Lanthanide-doped upconversion nanoparticles electrostatically coupled with photosensitizers for near-infrared-triggered photodynamic therapy[J].Nanoscale,2014,6:8274-8282.
[58]HUANG P,ZHENG W,ZHOU SY,et al..Lanthanide-doped LiLuF4upconversion nanoprobes for the detection of disease biomarkers[J].Angew.Chem.Int.Ed.,2014,53:1252-1257.
[59]ZOU Q L,HUANG P,ZHENG W,et al..Cooperative and non-cooperative sensitization upconversion in lanthanidedoped LiYbF4nanoparticles[J].Nanoscale,2017,9:6521-6528.
[60]WANG Y,LIU Y,XIAO Q,et al..Eu3+doped KYF4nanocrystals:synthesis,electronic structure,and optical properties[J].Nanoscale,2011,3:3164-3169.
[61]LIU R,TU D T,LIU Y S,et al..Controlled synthesis and optical spectroscopy of lanthanide-doped KLaF4nanocrystals[J].Nanoscale,2012,4:4485-4491.
[62]ZHENG W,ZHOU SY,CHEN Z,et al..Sub-10 nm lanthanide-doped CaF2nanoprobes for time-resolved luminescent biodetection[J].Angew.Chem.Int.Ed.,2013,52:6671-6676.
[63]XU J,ZHOU SY,TU D T,et al..Sub-5 nm lanthanide-doped lutetium oxyfluoride nanoprobes for ultrasensitive detection of prostate specific antigen[J].Chem.Sci.,2016,7:2572-2578.
[64]YANG Y,TU D,ZHENG W,et al..Lanthanide-doped Sr2YF7nanoparticles:controlled synthesis,optical spectroscopy and biodetection[J].Nanoscale,2014,6:11098-11105.
[65]ZHUO Z,LIU Y S,LIU D J,et al..Manipulating energy transfer in lanthanide-doped single nanoparticles for highly enhanced upconverting luminescence[J].Chem.Sci.,2017,8:5050-5056.
[66]ZHANG Y H,ZHANG L X,DENG R R,et al..Multicolor barcoding in a single upconversion crystal[J].J.Am.Chem.Soc.,2014,136:4893-4896.
[67]ZHANG T R,GE J P,HU Y P,et al..A general approach for transferring hydrophobic nanocrystals into water[J].Nano Lett.,2007,7:3203-3207.
[68]DONGA G,YE X C,CHEN J,et al..A Generalized ligand-exchange strategy enabling sequential surface functionalization of colloidal nanocrystals[J].J.Am.Chem.Soc.,2011,133:998-1006.
[69]LIU Y,CHEN T,WU C,et al..Facile surface functionalization of hydrophobic magnetic nanoparticles[J].J.Am.Chem.Soc.,2014,136:12552-12555.
[70]CHEN Z G,CHEN H L,HU H,et al..Versatile synthesis strategy for carboxylic acid-functionalized upconverting nanophosphors as biological labels[J].J.Am.Chem.Soc.,2008,130:3023-3029.
[71]ZHOU H P,XU C H,SUN W,et al..Clean and flexible modification strategy for carboxyl/aldehyde-functionalized upconversion nanoparticles and their optical applications[J].Adv.Funct.Mater.,2009,19:3892-3900.
[72]OZIN GA,BOGDAN N,VETRONE F,et al..Synthesis of ligand-free colloidally stable water dispersible brightly luminescent lanthanide-doped upconverting nanoparticles[J].Nano Lett.,2011,11:835-840.
[73]JIANGG C,PICHAANDI J,JOHNSON N JJ,et al..An effective polymer cross-linking strategy to obtain stable dispersions of upconverting NaYF4nanoparticles in buffers and biological growth media for biolabeling applications[J].Langmuir,2012,28:3239-3247.
[74]DASG K,STARK D T,KENNEDY I M.Potential toxicity of up-converting nanoparticles encapsulated with a bilayer formed by ligand attraction [J].Langmuir,2014,30:8167-8176.
[75]WANG L Y,YAN R X,HAO Z Y,et al..Fluorescence resonant energy transfer biosensor based on upconversion-luminescent nanoparticles[J].Angew.Chem.Int.Ed.,2005,44:6054-6057.
[76]WANG C,CHENG L,LIU Z.Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics[J].Theranostics,2013,3:317-330.
[77]IDRIS N M,GNANASAMMANDHAN M K,ZHANG J,et al..In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers[J].Nat.Med.,2012,18:1580-1585.
[78]LIUJN,BU JW,BUWB,et al..Real-time in vivo quantitative monitoring of drug release by dual-mode magnetic resonance and upconverted luminescence imaging[J].Angew.Chem.Int.Ed.,2014,53:4551-4555.
[79]SEDLMEIER A,GORRISH H.Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications[J].Chem.Soc.Rev.,2015,44:1526-1560.
[80]NAM J,WON N,BANG J,et al..Surface engineering of inorganic nanoparticles for imaging and therapy[J].Adv.Drug Deliver.Rev.,2013,65:622-648.
[81]MEISER F,CORTEZ C,CARUSO F.Biofunctionalization of fluorescent rare-earth-doped lanthanum phosphate colloidal nanoparticles[J].Angew.Chem.Int.Ed.,2004,43:5954-5957.
[82]ERATHODIYIL N,YING JY.Functionalization of inorganic nanoparticles for bioimaging applications[J].Acc.Chem.Res.,2011,44:925-935.
[83]LI L L,ZHANG R,YIN L,et al..Biomimetic surface engineering of lanthanide-doped upconversion nanoparticles as versatile bioprobes[J].Angew.Chem.Int.Ed.,2012,124:6225-6229.
[84]XIA L,KONG X,LIU X,et al..An upconversion nanoparticle-zinc phthalocyanine based nanophotosensitizer for photodynamic therapy[J].Biomaterials,2014,35:4146-4156.
[85]VOLIANI V,GONZLEZ-BJAR M,HERRANZ-PREZ V,et al..Orthogonal functionalisation of upconverting NaYF4nanocrystals[J].Chem.Eur.J.,2013,19:13538-13546.
[86]BEYAZIT S,AMBROSINI S,MARCHYK N,et al..Versatile synthetic strategy for coating upconverting nanoparticles with polymer shells through localized photopolymerization by using the particles as internal light sources[J].Angew.Chem.Int.Ed.,2014,53:8919-8923.
[87]ZHOU S,ZHENG W,CHEN Z,et al..Dissolution-enhanced luminescent bioassay based on inorganic lanthanide nanoparticles[J].Angew.Chem.Int.Ed.,2014,53:12489-12502.
[88]LI L L,WU P,HWANG K,et al..An exceptionally simple strategy for DNA-functionalized up-conversion nanoparticles as biocompatible agents for nanoassembly,DNA delivery,and imaging [J].J.Am.Chem.Soc.,2013,135:2411-2414.
[89]ZHOU J,LIU Z,LI F Y.Upconversion nanophosphors for small-animal imaging[J].Chem.Soc.Rev.,2012,41:1323-1349.
[90]LIU Y S,ZHOU SY,TU D T,et al..Amine-functionalized lanthanide-doped zirconia nanoparticles:optical spectroscopy,time-resolved fluorescence resonance energy transfer biodetection,and targeted imaging[J].J.Am.Chem.Soc.,2012,134:15083-15090.
[91]HUANG X Y,HAN SY,HUANG W,et al..Enhancing solar cell efficiency:the search for luminescent materials as spectral converters[J].Chem.Soc.Rev.,2013,42:173-201.
[92]JOHNSON N JJ,HE S,DIAO S,et al..Direct evidence for coupled surface and concentration quenching dynamics in lanthanide-doped nanocrystals[J].J.Am.Chem.Soc.,2017,139:3275-3282.
[93]CHEN Q S,XIE X J,HUANG B L,et al..Confining excitation energy in Er3+-sensitized upconversion nanocrystals through Tm3+-mediated transient energy trapping[J].Angew.Chem.Int.Ed.,2017,56:7605-7609.
[94]ZHANG F,CHE RC,LIX M,et al..Direct imaging the upconversion nanocrystal core/shell structure at the subnanometer level:shell thickness dependence in upconverting optical properties[J].Nano Lett.,2012,12:2852-2858.
[95]WANGY F,SUN L D,XIAOJW,et al..Rare-earth nanoparticles with enhanced upconversion emission and suppressed rare-earth-ion leakage[J].Chem.Eur.J.,2012,18:5558-5564.
[96]ZHOU B,TAO L,TSANG Y H,et al..Core-shell nanoarchitecture:a strategy to significantly enhance white-light upconversion of lanthanide-doped nanoparticles[J].J.Mater.Chem.C,2013,1:4313-4318.
[97]ZHOU B,TAO L,CHAI Y,et al..Constructing interfacial energy transfer for photon up-and down-conversion from lanthanides in a core-shell nanostructure[J].Angew.Chem.Int.Ed.,2016,128:12544-12548.
[98]DENG R,QIN F,CHEN R,et al..Temporal full-colour tuning through non-steady-state upconversion[J].Nat.Nanotech.,2015,10:237-242.
[99]CHAN E M,HAN G,GOLDBERG JD,et al..Combinatorial discovery of lanthanide-doped nanocrystals with spectrally pure upconverted emission [J].Nano Lett.,2012,12:3839-3845.
[100]ZHANGY,HUANG L,LIU X.Unraveling epitaxial habits in the Na Ln F4system for color multiplexing at the single-particle level[J].Angew.Chem.Int.Ed.,2016,55:5718-5722.
[101]BOYER JC,VAN VEGGEL F CJM.Absolute quantum yield measurements of colloidal NaYF4∶Er3+,Yb3+upconverting nanoparticles[J].Nanoscale,2010,2:1417-1419.
[102]HILDEBRANDT N,CHARBONNIERE L J,BECK M,et al..Quantum dots as efficient energy acceptors in a time-resolved fluoroimmunoassay[J].Angew.Chem.Int.Ed.,2005,44:7612-7615.
[103]KUNINGASK,PAKKILA H,UKONAHO T,et al..Upconversion fluorescence enables homogeneous immunoassay in whole blood[J].Clin.Chem.,2007,53:145-146.
[104]YUAN J L,WANG G L,MAJIMA K,et al..Synthesis of a terbium fluorescent chelate and its application to time-resolved fluoroimmunoassay[J].Anal.Chem.,2001,73:1869-1876.
[105]PERFEZOU M,TURNER A,MERKOCI A.Cancer detection using nanoparticle-based sensors[J].Chem.Soc.Rev.,2012,41:2606-2622.
[106]SIITARI H,HEMMILA I,SOINIE,et al..Detection of Hepatitis-Bsurface-antigen using time-resolved fluoroimmunoassay[J].Nature,1983,301:258-260.
[107]HEMMILA I,DAKUBU S,MUKKALA V M,et al..Europium as a label in time-resolved immunofluorometric assays[J].Anal.Biochem.,1984,137:335-343.
[108]BUNZLI J C G,PIGUET C.Taking advantage of luminescent lanthanide ions[J].Chem.Soc.Rev.,2005,34:1048-1077.
[109]WANG J,DENG R,MACDONALD M A,et al..Enhancing multiphoton upconversion through energy clustering at sublattice level[J].Nat.Mater.,2014,13:157-162.
[110]GARGASD J,CHAN E M,OSTROWSKI A D,et al..Engineering bright sub-10 nm upconverting nanocrystals for single-molecule imaging[J].Nat.Nanotech.,2014,9:300-305.
[111]GAI S,LI C,YANG P,et al..Recent progress in rare earth micro/nanocrystals:soft chemical synthesis,luminescent properties,and biomedical applications[J].Chem.Rev.,2013,114:2343-2389.
[112]GORRISH H,ALI R,SALEH SM,et al..Tuning the dual emission of photon-upconverting nanoparticles for ratiometric multiplexed encoding[J].Adv.Mater.,2011,23:1652.
[113]HAASE M,SCHAFER H.Upconverting nanoparticles[J].Angew.Chem.Int.Ed.,2011,50:5808-5829.
[114]WU SW,HAN G,MILLIRON D J,et al..Non-blinking and photostable upconverted luminescence from single lanthanide-doped nanocrystals[J].Proc.Natl.Acad.Sci.USA,2009,106:10917-10921.
[115]CHATTERJEE D K,GNANASAMMANDHAN M K,ZHANG Y.Small upconverting fluorescent nanoparticles for biomedical applications[J].Small,2010,6:2781-2795.
[116]SUN L D,WANG Y F,YAN C H.Paradigms and challenges for bioapplication of rare earth upconversion luminescent nanoparticles:small size and tunable emission/excitation spectra[J].Acc.Chem.Res.,2014,47:1001-1009.
[117]ZHANG F,SHI Q H,ZHANG Y C,et al..Fluorescence upconversion microbarcodes for multiplexed biological detection:nucleic acid encoding[J].Adv.Mater.,2011,23:3775-3779.
[118]ZHAO JB,JIN D Y,SCHARTNER E P,et al..Single-nanocrystal sensitivity achieved by enhanced upconversion luminescence[J].Nat.Nanotechnol.,2013,8:729-734.
[119]NACZYNSKI D J,TAN M C,ZEVON M,et al..Rare-earth-doped biological composites as in vivo shortwave infrared reporters[J].Nat.Commun.,2013,4:1-10.
[120]LI P,PENG Q,LI Y D.Dual-mode luminescent colloidal spheres from monodisperse rare-earth fluoride nanocrystals[J].Adv.Mater.2009,21:1945-1948.
[121]ZIJLMANSH JM A A,BONNET J,BURTON J,et al..Detection of cell and tissue surface antigens using up-converting phosphors:a new reporter technology[J].Anal.Biochem.,1999,267:30-36.
[122]WU SJ,DUANN,WANGZ P,et al..Aptamer-functionalized magnetic nanoparticle-based bioassay for the detection of ochratoxin a using upconversion nanoparticles as labels[J].Analyst,2011,136:2306-2314.
[123]WANG L Y,LI Y D.Green upconversion nanocrystals for DNA detection[J].Chem.Comm.,2006,24:2557-2859.
[124]WU Y M,CEN Y,HUANG L J,et al..Upconversion fluorescence resonance energy transfer biosensor for sensitive detection of human immunodeficiency virus antibodies in human serum[J].Chem.Commun.,2014,50:4759-4762.
[125]WANG J,WEI T,LI X Y,et al..Near-infrared-light-mediated imaging of latent fingerprints based on molecular recognition[J].Angew.Chem.Int.Ed.,2014,53:1616-1620.
[126]LIU Y S,TU D T,ZHU H M,et al..Lanthanide-doped luminescent nano-bioprobes:from fundamentals to biodetection[J].Nanoscale,2013,5:1369-1384.
[127]LIU Y S,ZHOU SY,ZHUOZ,et al..In vitro upconverting/downshifting luminescent detection of tumor markers based on Eu3+-activated core-shell-shell lanthanide nanoprobes[J].Chem.Sci.,2016,7:5013-5019.
[128]WANG Y H,SHENP,LICY,et al..Upconversion fluorescence resonance energy transfer based biosensor for ultrasensitive detection of matrix metalloproteinase-2 in blood[J].Anal.Chem.,2012,84:1466-1473.
[129]CHEN H Q,GUAN Y Y,WANGSZ,et al..Turn-on detection of a cancer marker based on near-infrared luminescence energy transfer from NaYF4∶Yb,Tm/NaGdF4core-shell upconverting nanoparticles to gold nanorods [J].Langmuir,2014,30:13085-13091.
[130]MORGAN CG,MITCHELL A C.Prospects for applications of lanthanide-based upconverting surfaces to bioassay and detection[J].Biosens.Bioelectron.,2007,22:1769-1775.
[131]KUNINGASK,RANTANEN T,UKONAHO T,et al..Homogeneous assay technology based on upconverting phosphors[J].Anal.Chem.,2005,77:7348-7355.
[132]WANG X D,WOLFBEISO S,MEIER R J.Luminescent probes and sensors for temperature[J].Chem.Soc.Rev.,2013,42:7834-7869.
[133]TU D T,ZHENG W,LIU Y S,et al..Luminescent biodetection based on lanthanide-doped inorganic nanoprobes[J].Coord.Chem.Rev.,2014,273-274:13-29.
[134]LIU Y,CHEN M,CAO T Y,et al..A cyanine-modified nanosystem for in vivo upconversion luminescence bioimaging of methylmercury[J].J.Am.Chem.Soc.,2013,135:9869-9876.
[135]ZHANG C L,YUAN Y X,ZHANG S M,et al..Biosensing platform based on fluorescence resonance energy transfer from upconverting nanocrystals to graphene oxide[J].Angew.Chem.Int.Ed.,2011,50:6851-6854.
[136]DENG R R,XIE X J,VENDRELL M,et al..Intracellular glutathione detection using MnO2-nanosheet-modified upconversion nanoparticles[J].J.Am.Chem.Soc.,2011,133:20168-20171.
[137]HE M Y,LIU Z H.Paper-based microfluidic device with upconversion fluorescence assay[J].Anal.Chem.,2013,85:11691-11694.
[138]PENG J H,WANGY H,WANGJL,et al..A new biosensor for glucose determination in serum based on up-converting fluorescence resonance energy transfer[J].Biosens.Bioelectron.,2011,28:414-420.
[139]YUAN Y X,WU SF,SHU F,et al..An MnO2nanosheet as a label-free nanoplatform for homogeneous biosensing[J].Chem.Commun.,2014,50:1095-1097.
[140]WANG Y H,BAO L,LIU Z H,et al..Aptamer biosensor based on fluorescence resonance energy transfer from upconverting phosphors to carbon nanoparticles for thrombin detection in human plasma [J].Anal.Chem.,2011,83:8130-8137.
[141]WU Z,LI H,LIU Z.An aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer[J].Sens.Actuators B:Chem.,2015,206:531-537.
[142]LI H,SUN D,LIU Y J,et al..An ultrasensitive homogeneous aptasensor for kanamycin based on upconversion fluorescence resonance energy transfer[J].Biosens.Bioelectron.,2014,55:149-156.
[143]WANG Y H,WU Z J,LIU Z H.Upconversion fluorescence resonance energy transfer biosensor with aromatic polymer nanospheres as the lable-free energy acceptor[J].Anal.Chem.,2013,85:258-264.
[144]YE W W,TSANG M K,LIU X,et al..Upconversion luminescence resonance energy transfer(LRET)-based biosensor for rapid and ultrasensitive detection of avian influenza virus H7 subtype[J].Small,2014,10:2390-2397.
[145]CEN Y,WU Y M,KONG X J,et al..Phospholipid-modified upconversion nanoprobe for ratiometric fluorescence detection and imaging of phospholipase D in cell lysate and in living cells[J].Anal.Chem.,2014,86:7119-7127.
[146]ZHOU F,NOOR M O,KRULL U J.Luminescence resonance energy transfer-based nucleic acid hybridization assay on cellulose paper with upconverting phosphor as donors[J].Anal.Chem.,2014,86:2719-2726.
基于稀土上转换纳米荧光探针的肿瘤标志物体外检测
于莉华1,2,刘永升1*,陈学元1,2*
(1.中国科学院福建物质结构研究所,中国科学院功能纳米结构设计与组装重点实验室福建省纳米材料重点实验室,福建福州 350002;2.福建师范大学材料科学与工程学院,福建福州 350007)
肿瘤标志物的超敏特异性检测对肿瘤患者的早期诊断和治疗以及增加其存活率具有重要的意义。作为新一代的纳米荧光探针,稀土掺杂上转换纳米晶具有独特的近红外激发的反斯托克斯上转换发光以及长荧光寿命等特征,被认为是有机染料、稀土螯合物、量子点等传统荧光探针在肿瘤早期诊疗领域最有应用前景的替代者。本文从上转换纳米荧光探针最基础的物理化学性质如控制合成、表面修饰以及发光物理出发,系统综述了该类材料在肿瘤标志物上转换体外检测方面的最新进展,并对其未来的发展趋势与努力的方向作了进一步的远景展望。
稀土;上转换发光;肿瘤标志物;纳米晶;生物检测
2017-10-26;
2017-11-23
中国科学院战略重点研究计划(XDB20000000,XDA09030102);973国家重大科学研究计划(2014CB845605);国家自然科学基金(21771185,21473205,U1305244,21325104);中国科学院/国家外专局创新研究团队国际合作项目;中国科学院青年创新促进会项目;福建省自然科学基金(2017I0018,2017J01038)资助项目
O482.31;TP394.1 Document code:A
10.3788/fgxb20183901.0027
1000-7032(2018)01-0027-23
Supported by The Strategic Priority Research Program of The CAS(XDB20000000,XDA09030102);973 National Major Scientific Research Program(2014CB845605);National Natural Science Foundation of China(21771185,21473205,U1305244,21325104);CAS/SAFEA International Partnership Program for Creative Research Teams;Youth Innovation Promotion Association of CAS;Natural Science Foundation of Fujian Province(2017I0018,2017J01038)

于莉华(1993-),女,河南漯河人,博士研究生,2016年于河南师范大学获得硕士学位,主要从事稀土纳米材料方面的研究。
E-mail:lhya@fjirsm.ac.cn

陈学元(1970-),男,福建建瓯人,博士,研究员,1998年于中国科学院福建物质结构研究所获得博士学位,主要从事纳米发光材料方面的研究。
E-mail:xchen@fjirsm.ac.cn

刘永升(1977-),男,山东临沂人,博士,研究员,2010年于中国科学院福建物质结构研究所获得博士学位,主要从事无机发光材料方面的研究。
E-mail:liuysh@fjirsm.ac.cn
——李振声

