Synthesis of Highly Efficient Lanthanide Upconversion Nanoparticles
WANG Rui,ZHANG Fan
Synthesis of Highly Efficient Lanthanide Upconversion Nanoparticles
WANG Rui,ZHANG Fan*
(State Key Laboratory of Molecular Engineering of Polymers,Department of Chemistry,Fudan University,Shanghai200433,China)*Corresponding Author,E-mail:zhang_fan@fudan.edu.cn
Upconversion nanoparticles can emit ultraviolet/visible/near-infrared light under near-infrared excitation,also known as anti-Stokes emission.This unique optical property precludes background fluorescence and light scattering from biological materials,which opens up new avenues for optical bio-applications,such as bioimaging,photodynamic treatment,drug delivery,etc.In order to achieve better application performance,highly efficient lanthanide upconversion nanoparticles is desired in many cases.Generally,the quality of the upconversion nanoparticles is always related to their synthetic methods.In this review,we will focus primarily on the various synthetic procedures for the upconversion nanoparticles.Moreover,several effective ways to obtain high luminescence efficiency are summarized.
upconversion nanoparticles;synthetic methods;core-shell structure
1 Introduction
Upconversion materials,which emit high-energy photons under excitation by the near-infrared(NIR)light(anti-Stokes shift)were first discovered in the 1960s[1],but have primarily been exploitedfor the development of some remarkably effective optical devices such as infrared quantum counter detectors[2-3],temperature sensors[4-5]and compact solidstate lasers[6-9].Thus,for more than 30 years the use of the upconversion effect has been limited to bulk glass or crystalline materials[10-13].With the rapid developments of nanoscience and nanotechnology over the past few decades,nano-sized upconversion materials,also known as upconversion nanoparticles(UCNPs),have been generated and reported for many promising bioapplications[14-15].Because of their suitable size(small enough to go in and out through many biological host materials,such as the cytoplasm or nucleus of a cell)and unique properties,such as high chemical stability,low cytotoxicity,and high signal-to-noise ratio,the biological applications of UCNPs for analytical assays and bioimaging were readily recognized[14-17].
In order to achieve better performance for the further application,highly efficient lanthanide-based UCNPs are desired.Generally,efficient UCNPs are composed of three components:a host matrix,a sensitizer,and an activator.An ideal host matrix needs to be optically transparent and has low lattice phonon energy,in order to minimize non-radiative losses and maximize radiative emission.Until now,oxides like Y2O3[18]and fluorides like NaYF4[19-21],NaGdF4[22-23],LiLuF4[24],NaLuF4[25],LaF3[26],CaF2[27],etc have been proven to be the ideal candidates.On the other hand,the rational choice of different lanthanide ions as sensitizer and activator is also very important.Typically,Yb3+is always chosen as sensitizer because of its large absorption cross-section of around 980 nm.Er3+,Tm3+and Ho3+feature ladder-like arranged energy levels which favor multiphoton process,and thus are frequently used as activators[28].Besides these three components,rational design of core-shell structure to minimize surface quenching effects and improve the luminescence efficiency of UCNPs is also very crucial[29-31].
With the development of nanotechnology,several methods have been used to fabricate uniform monodispersed UCNPs with controlled crystalline phases and sizes,including co-precipitation,thermal decomposition,hydro(solvo)-thermal synthesis,seed-mediated heat-up method,etc.Furthermore,many efforts have been also devoted to the structural design of the core-shell UCNPs with higher luminescence efficiency,such as one-pot heating-up method,successive layer-by-layer strategy(SLBL),Ostwald ripening strategy and cation exchange strategy.It is the aim of this review to present a systematic discussion on the basic principles and recent progress achieved on synthetic method optimization,morphology controlling,and luminescence enhancing of the UCNPs,and to gain insights into their future development.
2 Typical Synthetic Methods of UCNPs
Many classical wet chemical(solution-based)synthetic methods,including co-precipitation,thermal decomposition,hydrothermal/solvothermal synthesis,seed-mediated heat-up methods have been explored to synthesize high-quality lanthanide-based UCNPs.In addition,in some specific cases,such as nanocrystals for superlattice assembly,when very narrow particle size distribution is required,special methods(such as a salt bath)or a combination of several methods are also developed by researchers recently.Moreover,no matter what kind synthetic method is used,optimization of experimental conditions is extremely important for the synthesis of nanocrystals with controlled particle size,chemical composition,morphology and optical properties.Typically,the particle size and morphology can be controlled by reaction temperature,ligand and reactant concentration,while the chemical composition and optical properties are related to dopant species.In this section,we will introduce the history and principle of each synthetic method in UCNPs synthesis briefly;then some important factors like ligand concentration,sodium concentration,reaction temperature,etc will be discussed in detail;finally,we will make a comparison among these synthetic methods.
2.1 Co-precipitation
The co-precipitation method may be the most convenient method for synthesizing UCNPs and holds great potential for large-scale production.Compared with other methods,the co-precipitation method is a simple,inexpensive,and quick way to synthesize UCNPs with mild reaction conditions(low temperature and pressure).In some cases,the crystallized nanoparticles can be obtained directly from co-precipitation without calcination or post-annealing processes.In 2002,LaF3nanocrystals doped with Ln3+(Ln refer to lanthanide)were prepared by van Veggel et al.[32].Later in 2005,Chow et al.improved and optimized the water-soluble inorganic precursors to synthesize upconverted LaF3nanocrystals with smaller particle size and narrower particle size distribution(Fig.1(a) - (b))[33].In their experiments,Ammonium di-n-octadecyldithiophosphate was used as a surfactant to control the growth of the particles and to prevent agglomeration of the particles.These 10 nm or lower UCNPs can be redispersed in solution and are expected to act as fluorescent probes for biomolecules of several nanometers to tens of nanometers in size.In addition to LaF3nanocrystals,Haase,Gudel,Chen and Li et al.also synthesized NaYF4∶Yb/Er(Fig.1(c) -(f))[34],LuPO4∶Yb/Tm and YbPO4∶Er[35]and other nanocrystals by co-precipitation and post-annealing.Commercial ligands such as PVP(polyvinylpyrrolidone)and PEI(polyethyleneimine)have been widely used to control particle growth and to help achieve nanocrystal solubility and surface functionalization[36-37].In particular,PEI-coated nanoparticles provide a platform for the direct realization of functionalization of biomolecules.

Fig.1 (a,b)TEM images of well-dispersed LaF3∶12%Yb,3%Er UCNPs at a magnification of 50 000(a)and higher magnification(b).The inset in(b)is the HRTEM showing the high crystallinity of the particles[33].(Reproduced with permission from Ref.[33])(c) - (f)TEM images of NaYF4∶Yb/Er prepared in the acetic acid(c)and ethanol(d)in the presence of CTAB,and in acetic acid(e)and ethanol(f)using EDTA[34].(Reproduced with permission from Ref.[34])
Although so much progress has been made so far,there are still some inevitable drawbacks in the UCNPs synthesized by co-precipitation method,including low efficiency,poor monodispersity and broad size distribution.The low luminescence efficiency mostly results from the poor crystallinity caused by the low reaction temperature(<100℃).High-temperature calcination can enhance the luminescence efficiency at the cost of serious agglomeration because of the demolishing of their surface ligands.Moreover,despite the protection of surfactants,fast reaction rates often lead to explosive nucleation-growth,making the reaction out of control,eventually leading to poor morphology,monodispersity and broad size distribution.In order to synthesize highly crystallized UCNPs at moderate reaction rate,the researchers have made a lot of attempts to reduce the reaction rate,one of the most effective is the hydrothermal/solvothermal method.
2.2 Hydrothermal/Solvothermal Method
Hydrothermal/Solvothermal technology is becoming one of the most important tools for advanced material processing,especially due to its wide range of technical applications in the processing of nanostructured materials,such as electronics,optoelectronics,catalysis,ceramics,magnetic data storage of biomedical and biological photonics.The hydrothermal/solvothermal process uses solvents above the critical temperature and pressure to increase the solubility of the reactants to accelerate the chemical reaction process.Compared with other synthetic methods,this method has the advantage of being able to increase the crystallinity at a lower temperature.
In a typical hydrothermal/solvothermal synthesis procedure,suitable reaction precursors,solvents and surfactants with appropriate chelating groups are mixed and then heated in an autoclave.Surfactants such as PEI,ethylenediaminetetraacetic acid(EDTA),cetyltrimethylammonium bromide(CTAB)and oleic acid(OA)provide chelating ability with cationic ions to regulate their reaction concentration,which is critical for controlling the crystalline phase,size and morphology,as well as the surface functional groups of the resulting UCNPs[38-41].The most representative example of hydrothermal/solvothermal synthesis is provided by the work of Li and his coworkers who reported on a general‘liquid-solid-solution(LSS)’strategy for the synthesis of monodisperse inorganic nanoparticles[42-43].This strategy is based on the phase transfer and separation mechanism occurring at the interface of the liquid,solid and solution phases.After the phase transfer of the metal ions from the aqueous solution to the solid phase,the metal ions may react with other anions such as F-ions to produce Ln F3nanoparticles.Alternatively,the metal ions may be dehydrated by coprecipitation into oxides and/or complex oxides.The lanthanide-doped NaYF4single-crystal nanorods,hexagonal nanosheets and nanoparticles were also synthesized using the LSSmethod[44].The effects of reaction temperature,duration,the molar ratio of NaF to Ln(NO3)3,and reactant content on the size,morphology and phase purity of the product have been studied in detail.The results showed that high NaF content,low Ln(NO3)3content,suitable reaction temperature and long reaction time favor the growth of the nanoparticles.The authors also prepared lanthanide elements doped Na(Y1.5Na0.5)F6single-crystal nanorods and controlled the aspect ratio of nanorods by tuning the reaction temperature[45].The high temperature and versatile ligand species properties in the hydrothermal process make it possible to synthesize highly anisotropic UCNPs.Zhang et al.reported the synthesis of NaYF4nanorods,nanotubes,and flower-patterned nanodisks by a solvothermal method using oleic acid as solvent[46].In 2007,a combination method of thermal decomposition and solvothermal was used to synthesize LaF3∶Yb,Er(Tm,Ho)nanoplates with multicolor upconverted emission[47].
In 2010,Liu et al.developed a novel Gd3+doping method to simultaneously control the crystal phase,size and optical properties of NaYF4UCNPs during hydrothermal/solvothermal synthesis[21].It was found that Gd3+doping not only resulted in cube to hexagonal phase change rapidly within 2 h,but also resulted in a decrease in size,resulting in ultra-small hexagonal NaYF4UCNPs at a significantly low reaction temperature(Fig.2).Later,some other groups further validated that impurity doping can induce phase transformation,size controlling and morphology optimization.For example,Zhao et al.[48]and Hao et al.[49]found that doping Mn2+in the conventional UCNPs synthesized by hydrothermal method can greatly change its crystal phase,size and luminescence properties(red/green ratio).It is believed that the size of the substitutional dopant ions plays a key role in stabilizing a specific crystalline phase in the NaYF4based hybrid materials.The substitution ions with larger ionic radius favor the hexagonal structures(Fig.2(f) - (h))[21],whereas the smaller substitution ions tend to produce the cubic phase of the final products.Therefore,introducing the Mn2+ions(r=0.081 nm)with a smaller size than Y3+(r=0.089 nm)into NaYF4host should dominate the formation of pure cubic-phase NaYF4UCNPs.Meanwhile,substitution of Y3+by Mn2+in NaYF4could cause an extra F-ion on the grain surface and then induce transient electric dipoles with their negative poles pointing outward,which substantially hinder the diffusion of the F-ions needed for crystal growth from the solution to the grain surface due to the charge repulsion,consequently resulting in retardation of the NaYF4nanocrystal growth[48].
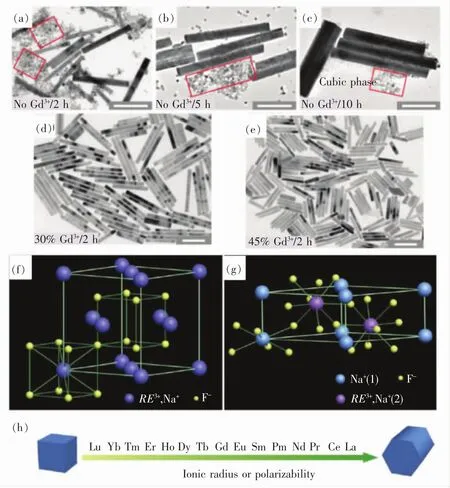
Fig.2 (a) - (c)TEM images of NaYF4∶Yb/Er(18%/2%)products obtained by hydrothermal method after heating for 2,5 and 10 h in the absence of Gd3+dopant ions.Formation of the cubic phase products is partially marked by red squares.(d) -(e)TEM images of the NaYF4∶Yb/Er(18%/2%)products obtained after heating for 2 h in the presence of 30%and 45%Gd3+dopant ions,respectively.(f) - (g)Schematic presentation of cubic-and hexagonal-phase Na RE F4(RE is short for rare-earth)structures,respectively.In the cubic phase,equal numbers of F- cubes contain cations and vacancies.In the hexagonal phase,an ordered array of F- ions offers two types of cation sites:one occupied by Na+and the other occupied randomly by RE3+and Na+.(h)General trend of phase transition from cubic to hexagonal as a function of ionic radius(or polarizability)of the lanthanide dopant ions[21].(Reproduced with permission from Ref.[21])
A prominent advantage of hydrothermal/solvothermal processes is the generation of highly crystalline phases at lower temperatures while implementing a set of reactions at the same time.However,there are still some serious drawbacks to be solved,including the need for specialized reaction vessels called autoclaves and the impossibility of observing the nanocrystal as it grows.Moreover,relatively large nanoparticles are usually produced due to possible Ostwald ripening and recrystallization processes during the crystal growth.
2.3 Thermal Decomposition
The thermal decomposition synthesis is based on co-thermolysis of Na(CF3COO)and Ln(CF3COO)3in oleic acid/oleyamine/1-octadecene(OA/OM/ODE)at elevated temperatures.It was first developed by Yan et al.for the synthesis of monodisperse LaF3nanocrystals[26].Then,this method was extended to a general method for the synthesis of highquality NaYF4nanocrystals[50-51].Generally,oleic acid and oleylamine with polar capping groups are usually adopted as capping agents to control the size and morphology of UCNPs by selective adsorption on specific facets,while octadecene is the most frequently used high-boiling-point organic solvents.As the temperature elevates,C -F bond breaking and nucleation could happen toward target nanoparticles[41].The synthesis reaction was separated as four stages including nucleation in a delayed time,particle growth by monomer supply,size shrinkage by dissolution,and aggregation(Fig.3(a))[52].It was proved that various sizes and shapes of cubic NaYF4∶Yb3+,Er3+UCNPs can be obtained by varying the reaction time,concentration of reagents,and reaction temperature.
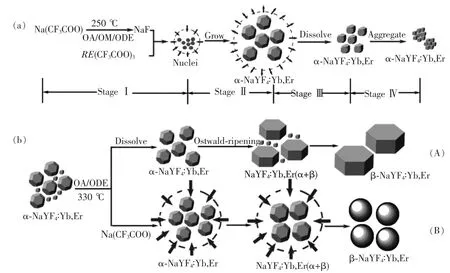
Fig.3 (a)Schematic illustration of the growth stages of cubic NaYF4∶Yb,Er UCNPs via a delayed nucleation pathway.(b)Schematic illustration of the growth process of hexagonal NaYF4:Yb,Er nanocrystals from cubic NaYF4∶Yb,Er monomers via a delayed cubic to hexagonal phase transition[52].(Reproduced with permission from Ref.[52])
As is well-known,hexagonal NaYF4∶Yb,Er is one of the most efficient UC materials for performing infrared-to-visible photon conversion with green emission 10 times stronger and overall emissions 4.4 times higher than those for cubic NaYF4∶Yb,Er[53].Different from cubic NaYF4∶Yb,Er which is a metastable high-temperature phase,hexagonal NaYF4∶Yb,Er is more thermodynamically stable,making the transformation from cubic to hexagonal easily to conduct[50].Mai et al.found this phase transformation is an Ostwald-ripening promoted process,wherein the dissolution and recrystallization are dominant.With cubic NaYF4∶Yb,Er(obtained at 310 ℃for 30 min)as the precursor for a reaction in OA/ODE at 330℃,the cubic to hexagonal transition adopts the Ostwald-ripening process(Fig.3(b),path A).When enough Na(CF3COO)is added in this system,the Ostwald-ripening process is restricted due to the increase of the monomers,and then the cubic to hexagonal transition adopts an Ostwaldripening restricted process,wherein the size of the uniform nanocrystals gradually increases(Fig.3(b),path B).The different pathway will not only lead to different morphology,but also remarkable differences in the UC intensity and fg/r(the intensity ratio of green to red emission).For the pathway A,the variation of UC intensity is mild before the phase transition(at about 8 min).Then it increases abruptly after 8 min,indicative of the beginning of the phase transition,while it increases tardily again at 10 min as the phase transition almost finishes.In contrast,the variation tendency of the fg/ris different.fg/rincreased nearly linearly at the initial stage of the whole process(up to 5 min)with a plateau at the stage of the phase transition.fg/rincreased sharply again after 10 min.As for the pathway B,the variation of UC intensity and fg/ris similar except at the stage before the phase transition.fg/rincreased sharply in the whole process,while the UC intensity shows a sudden increase when the phase transition occurs[53].
Although narrow size distribution and good morphology control can be obtained,the thermal decomposition method still suffers from some drawbacks.It normally requires high reaction temperature(280-330℃),organic solvents,and an oxygen-free with inert gas protection.In addition,most of the synthesized nanomaterials are stabilized by surfactant,which brings difficulties in the biological applications.
2.4 Seed-mediated Heat-up Method
The seed-mediated heat-up method was first developed by Zhang et al.to synthesize highly efficient UCNPs[54-55].Until now,it is one of the most widely used synthetic methods for UCNPs due to its convenience,high yield and environment-friendly properties.The basic principle of this method is to mix the lanthanide source(such as YCl3,Y(CH3COO)3,etc),sodium(such as NaOH)and fluoride source(such as NH4F)in OA/ODE or OM/ODE at room temperature to form amorphous precursor first,then bring to the temperature at which precursor reaction or decomposition occurs sufficiently quickly to result in supersaturation(Fig.4(a),stageⅠ).Supersaturation is again relieved by a“burst nucleation”(Fig.4(a),stage Ⅱ),after which temperature is controlled to avoid additional nucleation events,allowing monomer addition to existing nuclei to occur more rapidly than new monomer formation(Fig.4(a),stage Ⅲ)[56-59].
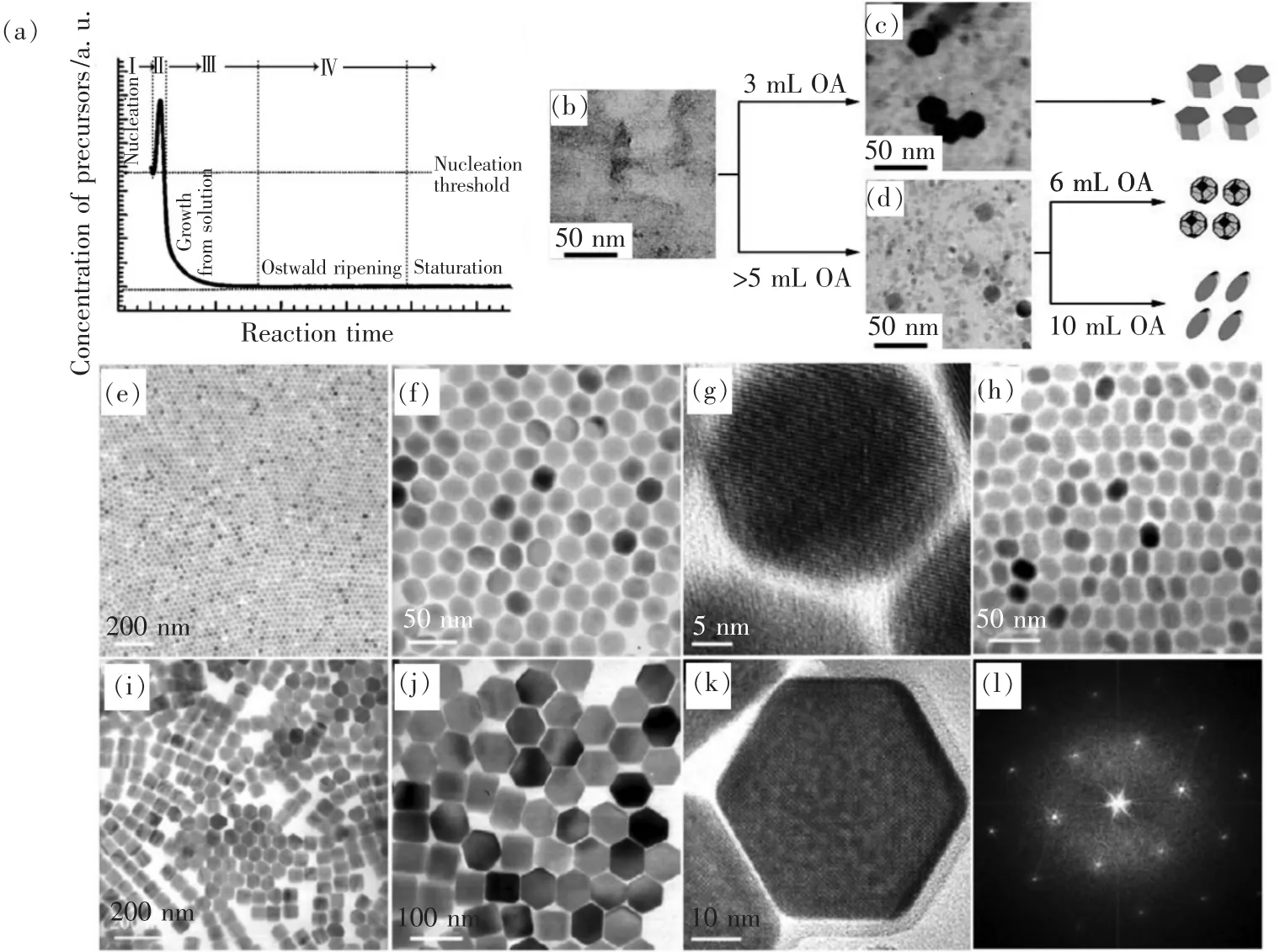
Fig.4 (a)Schematic depicting the four stages of the preparation of monodisperse nanocrystals in the framework of the LaMer model[56].(Reproduced with permission from Ref.[56])(b - d)Illustration of the formation of NaYF4∶Yb,Er,Tm nanoplates,nanospheres and nanoellipses.(e- i)TEM images of NaYF4∶Yb,Er nanospheres((e - g),at different magnifications),nanoellipses(h),and nanoplates((i)-(k),at different magnifications)and Fourier transform of the TEM image in k(i)[54].(Reproduced with permission from Ref.[54])
After a series of attempts to synthesize hexagonal-phase NaYF4∶Yb,Er,Tm UCNPs,Li et al.found that the concentration of the ligand OA plays an important role in the growth rates of UCNPs in different directions(Fig.4(b) - (l))[54].With a low ligand concentration,the nanocrystals grew faster in the[100]direction than the[001]direction and,as such,nanoplates were produced(Fig.4(i) -(l)).With the increase in OA concentration,the growth rates in both directions were comparable and polyhedral nanocrystals were produced(Fig.4(e) -(g)).The growth rate in the[001]direction could be faster than in the[100]direction with an even higher ligand concentration,so elliptical nanocrystals were formed(Fig.4(h)).Besides that,ligand concentration is related inversely to the number of nuclei formed in the nucleation process.It has been suggested that,when a large excess of OA is present,fewer nuclei are formed and these nuclei grow very rapidly and indiscriminately,leading to polydisperse NPs.
The control of appropriate reaction temperature and time is critical when the UCNPs nuclei are prone to aggregate,such as NaGdF4based UCNPs.Because NaGdF4are known to grow faster than NaYF4,the critical step should be adopted to control the nucleation stage and enable enough nuclei formation to suppress the indiscriminate growth[60]. Following this hypothesis,the concentration of OA was systematically reduced to control nucleation with a simultaneous reduction of the reaction temperature to control the growth of those existing nuclei.By altering the reaction time and temperature,van Veggel et al.successfully prepared NaGdF4UCNPs of four sizes,between 2.5 and 8.0 nm,which is very promising for biomedical imaging applications[61].
The sodium concentration is also an important parameter in the seed-mediated heat-up method,because normally the sodium source is NaOH which can create oleate anions(OA-)by reacting with OA.In 2016,Jin et al.experimentally verify the co-existence and different roles of oleate anions(OA-)and OA in the crystal formation[62].They identified that the control over the ratio of OA-to OA can be used to directionally inhibit,promote or etch the crystallographic facets of the UCNPs(Fig.5).This control enables selective grafting of shells with complex morphologies,such as nano-hourglass,nano-flowers, co-axial nano-cylinders, nanoscale spins with double rings,and nano-dumbbells with smooth or sharp ends,thus allowing the fabrication of a diverse library of monodisperse sub-50 nm UCNPs.

Fig.5 (a)NaYF4 core and homogenous NaYF4 nanocrystals after epitaxial growth of NaYF4 in longitudinal direction with 0.5 mmol NaOH and 9.5 mmol OA.(b)Five-section and seven-section‘bamboo-shaped’NaYF4@NaGdF4 nanorods formed by successive heterogeneous growth of periodical shells of NaGdF4-NaYF4 and NaGdF4-NaYF4-NaGdF4 onto NaYF4 core in the longitudinal direction,with 0.5 mmol NaOH and 0.4 mmol KOH and 9.5 mmol OA.Upper part of the panel shows elemental mapping of Y and Gd.(c)NaYF4 core and heterogeneous NaYF4@NaGdF4 nanocrystals after epitaxial growth of NaGdF4 in the transversal direction with 0.15 mmol NaOH and 19 mmol OA[62].
By carefully controlling these parameters in the seed-mediated heat-up method,high-quality UCNPs with a narrow size distribution,good crystallinity,and exceptional optical properties can be readily obtained,moreover,it is more moderate than thermal decomposition method.However,post-modification is still needed before bio-applications.
2.5 Other Methods
The sol-gel process is a typical wet-chemical technique,and the synthesized UCNPs can be used as a film coating and glass material.The sol-gel process is characterized by the hydrolysis and polycondensation of metal alkoxide(or halide)-based precursors.In order to enhance the UCefficiency,it is generally necessary to calcinate the products at high temperature to improve their crystallinity.Prasad et al.developed a modified sol-gel method for the synthesis of Er3+doped ZrO2nanocrystals[63].The method involved a sol-emulsion-gel technique that utilized reverse micelles formed in emulsions as the reactors for growing nanocrystals.The sol-gel method has been developed to prepare a series of metal oxide based UCNPs,including TiO2∶Er,BaTiO3∶Er[64],Lu3Ga5O12∶Er[65]and YVO4∶Yb/Er[66].Despite extensive efforts have been devoted,UCNPs made by sol-gel methods are not suitable for use as probes in biological assays due to their uncontrollable particle size and are serious aggregation when dispersed in aqueous solutions.
In stark contrast to sol-gel and hydrothermal methods that require a heating time up to several days,UCNPs synthesized by combustion method can be obtained within minutes.Once the heat source is initiated,highly exothermic reactions with temperatures ranging typically from 500 to 3 000℃occur in the form of a combustion wave that propagates through the reaction materials in a self-sustained manner without requiring additional heat.Zhang[67],Luo[68]and Capobianco[69]et al.successfully applied this low power consumption method for the synthesis of various oxysulfides and oxides(Y2O3,La2O2S,Gd2O3)based UCNPs.However,morphology controlling and monodispersity are still critical issues for this method.
3 Synthetic Methods of Core@Shell Structured UCNPs
In order to achieve better performance in the field of optical bioapplications,it is essential to obtain UCNPs with high luminescence efficiency.As discussed before,the choice of suitable luminescent centers,matrix and synthesis methods is critical.But,in order to further optimize the luminescence efficiency,nano-structural design should be done before the synthesis.Moreover,a reasonable nanostructure is always rooted in fully understanding of the reason for the fluorescence quenching of UCNPs.
UCNPs usually have a large proportion of dopant ions(including luminescent centers and sensitizers)on the crystal surface with imperfectly coordinated environments.These surface-doping ions,due to the lack of matrix protection,can easily be quenched by high-energy quenchers,such as external ligands,solvents and impurities.The excited state energy of the internal doping ions can also be consumed by passing the energy to the surface through adjacent dopant ions.In addition,the incomplete coordination environment can result in a decrease in the intensity of the crystal field around the surface dopant ions and further reduces the luminescence efficiency[70-71].Therefore,it is generally believed that the upconversion luminescence efficiency of the nanocrystals is weaker than that of the corresponding bulk materials,and the smaller the size of the nanocrystals,the weaker the luminescence efficiency.In 2010,Veggel et al.measured that the quantum efficiencies of UCNPs with particle sizes of 30 nm and 100 nm were 0.10% ± 0.05%and 0.30% ± 0.10%,respectively,and only 0.005% ±0.005%for 8 -10 nm UCNPs.In comparison,the quantum efficiency of bulk upconversion crystals is as high as3.0% ±0.3%[72].Finding an efficient way to protect the surface ions from quenching is becoming an urgent task for the researchers.
3.1 One-pot Heating-up Method
In order to protect these dopant ions on the crystal surface,an effective method is to coat an outer layer(also known as epitaxial growth)of a certain thickness of the undoped matrix crystal.The shell,commonly referred as an inert shell,has the same matrix material as the internal doped nanocrystals in which the dopant ions are confined in the nanocrystal core.The shell coating can effectively reduce energy loss caused by surface quenching,result in enhancing luminescence efficiency[28,73-74].In 2007,Chow et al.demonstrated that the luminescence intensity of NaYF4∶Yb,Tm nanocrystals with a diameter of 8 nm would increase nearly 30 times after coating NaYF4inert shells with only 1.5 nm thick[70].Yan et al.also demonstrated that the luminescence intensity of NaYF4∶Yb,Er nanocrystals increased by a factor of 2 when the NaYF4inert shell was coated[71].The quantum efficiency of the coreshell structured UCNPs with a particle size of 30 nm is 0.30% ±0.1%reported by Veggel et al.,which is approximate with that of the 100 nm sized UCNPs with none inert shells coating,further proves that the inert shell coating is an effective way to improve the upconversion quantum efficiency[72].In 2017,Johnson et al.provided direct evidence that the major quenching process at high dopant concentrations is surface as opposed to the common misconception of cross-relaxation between dopant ions(Fig.6(a) -(b))[75].They showed that after an inert epitaxial shell growth by the one-pot heating-up method,Er3+concentrations as high as 100%in NaY(Er)F4@NaLuF4core-shell structured UCNPs enhance the emission intensity of both upconversion and downshifted luminescence across different excitation wavelengths(980,800,658 nm),with negligible concentration quenching effects(Fig.6(c) - (f)).Their results highlighted the strong coupling of concentration and surface quenching effects in UCNPs,and that inert epitaxial shell growth can overcome concentration quenching.These fundamental insights into the photophysical processes in heavily doped UCNPs would give rise to enhanced properties not previously thought possible with compositions optimized in bulk.
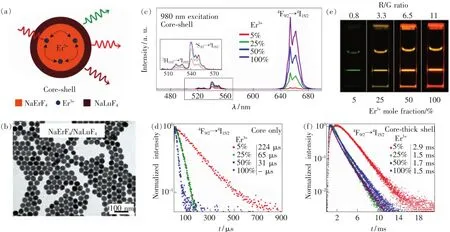
Fig.6 (a)Schematic illustration of the core-shell nanocrystals structural composition.(b)Representative TEM image of the NaErF4-NaLuF4 core-shell structured UCNPs.(c)Upconversion emission spectra of the core-shell nanocrystals with variable Er3+dopant concentrations in the core(NaYF4∶X%Er3+,X=5,25,50,100),Inset shows the green emission bands.(e)Upconversion emission photos of colloidal dispersion of core-shell nanocrystals(λex=980 nm),showing the enhanced emission with increase in dopant concentration.Luminescence decay of the 4 F9/2 level at 650 nm with variable erbium dopant concentration and their corresponding lifetime values of the(d)core nanocrystals and(f)core-shell nanocrystals[75].(Reproduced with permission from Ref.[75])
The whole experiment procedure of one-pot heating-up method for the synthesis of core-shell structured UCNPs is nearly the same as the seed-mediated heat-up method for core only UCNPs synthesis except the presence of the seed nanoparticles.Moreover,the one-pot heating-up method is an apparent case of the separation of nucleation and growth,wherein nucleation is physically separated from growth by using preformed core UCNPs as seed nuclei.This method utilizes heterogeneous nucleation to suppress the formation of additional nuclei by homogeneous nucleation.In this method,the reaction can enter into stageⅢ directly(Fig.4).Preformed nuclei are introduced into the reaction solution,and then the monomers are supplied to precipitate on the surface of the existing nuclei.The lower monomer concentration should be kept during growth to suppress homogeneous nucleation.So,the onepot heating-up method not only can be used in the synthesis of core-only UCNPs but also make a great success in the fabrication of core-shell structured UCNPs.
3.2 Successive Layer-by-layer Strategy
As described in the previous section,inert shell coating can largely increase upconversion by minimizing the surface quenching process.However,recent studies show that the shells of the core-shell structured UCNPs synthesized by conventional onepot heating-up method are very inhomogeneous,resulting in an incomplete inert shell protection[76-77].To solve this problem,Zhang et al.conducted an attempt in 2012:they added the reaction precursors for the inert shells to three or more times to the reaction system and found that compared to conventional inert shell coatings,the UCNPs obtained by this new multiple adding method have stronger luminescence intensity and stability(Fig.7(a))[78].However,this method uses low boiling point NaOH and NH4F methanol solution as a sodium and fluorine source,so in each shell coating process,one should have to wait for the temperature of the reaction solution reducing to room temperature,then adding sodium and fluorine source and repeat this process again.As a result,this method is time-consuming and laborious,especially when it comes to coating a wide variety of active shells.Later,Li et al.modified this process by replacing the precursor with a high boiling point rare earth chloride and sodium trifluoroacetate in the OA/ODE solution[79].By continuously introducing the shell precursor solution under high-temperature conditions,shell thickness of the obtained UCNPs can be finely tuned.This method was named as SLBL strategy(Fig.7(b) - (d)).
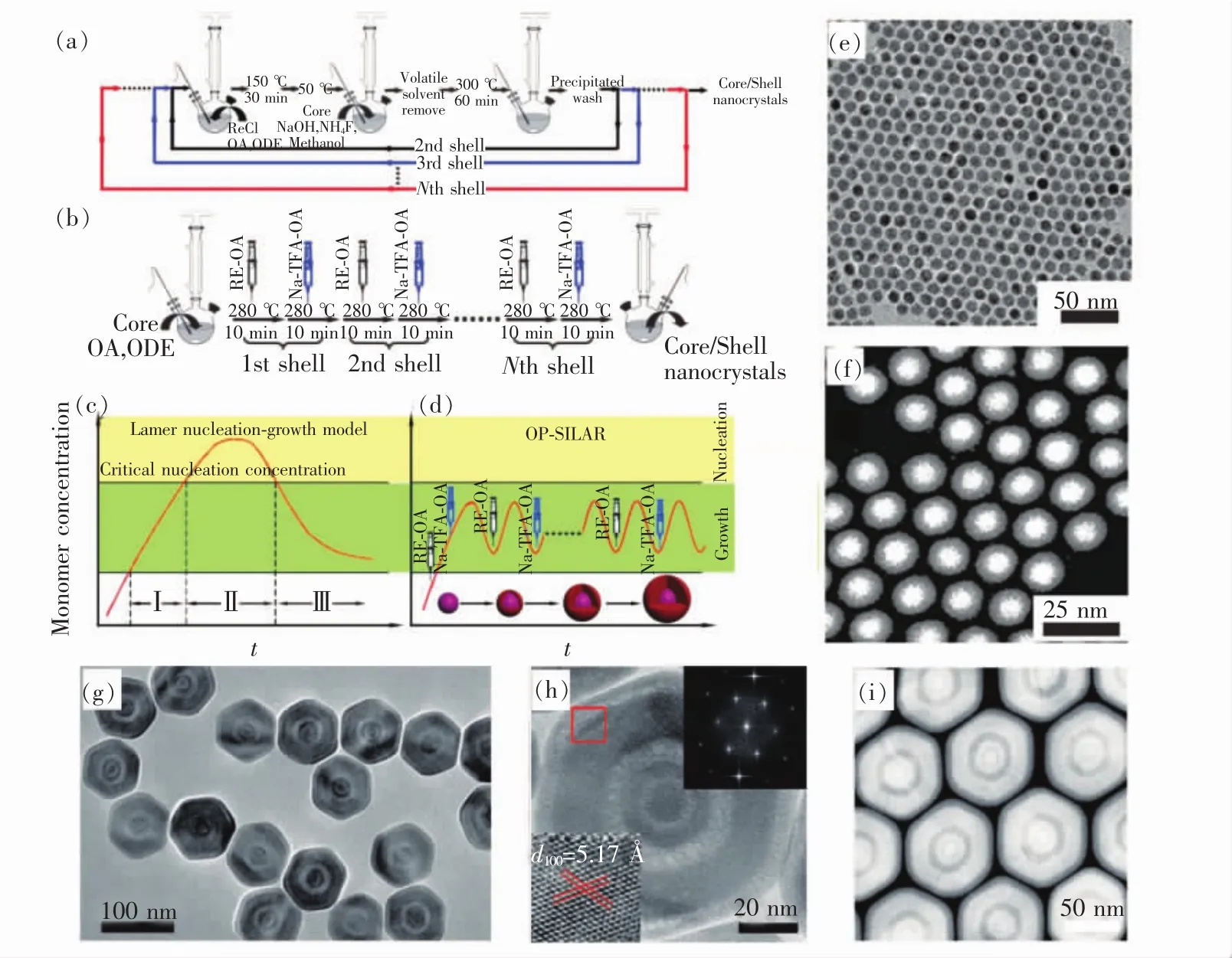
Fig.7 (a)Conventional multi-cycle batch operation for the synthesis of core-shell structured UCNPs[78].(b)SLBL synthetic procedure for the core-shell structured UCNPs.(c,d)Schematic illustration of lamer plot and SLBL method for the controlled synthesis of core-shell structured UCNPs.(e) -(f)Typical TEM(e)and high-angle annular dark field scanning TEM(HAADF-STEM)(f)images of the obtained NaGdF4∶Yb,Er@NaYF4 core-shell structured UCNPs by using the SLBL method[79].(g) - (i)TEM(g),high-resolution TEM(HRTEM)and the corresponding fast Fourier transform(FFT)(h),HAADF-STEM(i)of the multi-shell structured UCNPs with tunable shell thickness in different layers[80].
Their experimental results show that the core of the core-shell structured UCNPs synthesized using SLBL can be completely covered by the shell(Fig.7(e) - (f))[79].The thickness of the shell layer can be continuously adjusted by controlling the amount of shell precursor to be added each time and has a narrower size distribution(Fig.7(g) - (i))[80].The UCNPs synthesized by SLBL have very high quantum efficiency,for example,the quantum efficiency of the synthesized Er-doped UCNPs is as high as 0.51% ±0.08%.In addition,only a single atomic layer thickness of the inert shell coating can be realized by SLBL strategy,further proving its high precision control feature.
3.3 Homogeneous Doping UCNPs
Besides surface quenching effect which always occurs outside of a UCNP,the doping sites of these lanthanide ions will also have a great impact on their fluorescence property[81-83].In a traditional seed-mediated heat-up way,UCNPs grown in single step reactions have been found to have uneven distributions of dopants across the volume of individual UCNPs.For example,van Veggel and co-workers profiled gradients of Gd3+,Y3+,Nd3+,and Tb3+in NaGdF4nanocrystals by probing the particles with X-ray photoelectron spectroscopy(XPS)at a series of excitation energies[84].Later,Li et al.also found the similar phenomenon.With NaGdF4∶Yb3+,Er3+as a model system,they found that the UCNPs synthesized under the regular seed-mediated heat-up synthesis approach are heterogeneous doping during the spontaneous growth process,and the total(Yb3+and Er3+)local relative doping concentration of the dopants in a single particle is gradually decreased as the particle growth radially(Fig.8(a) - (b))[85].They assumed that the heterogeneous doping property should result from different reactivity of lanthanide ions during the reaction,which further leads to varied nucleation and growth rate.
To engineer more homogeneous dopant distributions within the NaYF4sub-lattice,Li et al.used SLBL protocol to grow thin layers of NaGdF4∶Yb3+,Er3+that in theory exhibit dopant distributions with rapid,shallow oscillations rather than a single,large gradient(Fig.8(d) - (i))[85].As shown in Fig.8(j)and(k),due to suppression of concentration quenching,these homogeneous UCNPs grown with such sub-lattice engineering were approximately twice as bright as heterogeneous UCNPs grown using conventional,seed-mediated heat-up reactions.
3.4 Ostwald Ripening Strategy
Like SLBL strategy,Ostwald ripening strategy is also a kind of hot-injection strategy except that small sacrificial nanoparticles are used as shell precursors,rather than lanthanide and sodium trifluoroacetate[86].Driven by Ostwald repining,the small nanoparticles can be quickly dissolved and deposited on the core nanoparticles.It enables the facile synthesis of multishelled nanoparticles through the standard oleate route.Moreover,the shell thickness and multiple layers of such core-shell structured UCNPs can be precisely controlled by manipulating the number of small sacrificial nanoparticles injected and/or repeating the above defocusing and self-focusing cycle.Furthermore,this method is flexible and can be easily extended to prepare other high-quality core-shell structured UCNPs with different compositions.
3.5 Cation Exchange Strategy
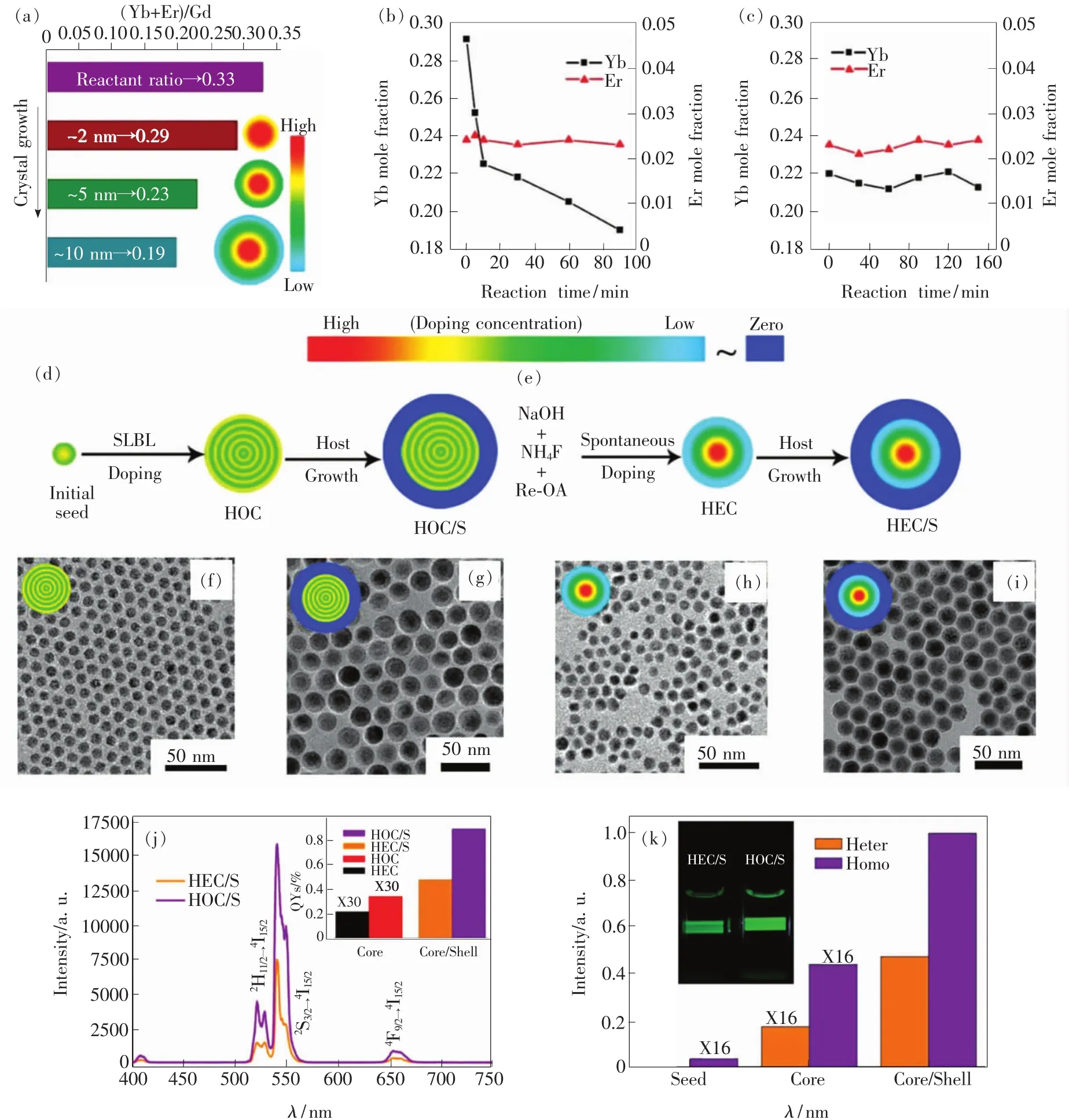
Fig.8 (a)Total doping concentration of Yb3+and Er3+ions determined by the EDX at different growth stage of the spontaneous growth process.The evolutions of Yb3+and Er3+doping concentrations during the spontaneous growth process(b)and SLBL doping process(c)determined by ICP-AES.(d)Synthetic procedures for the homogeneous doping core(HOC)and HOC/S(Sis short for shell)UCNPs based on the SLBL doping strategy.(e)Spontaneous growth process of the UCNPs by using the one pot heating-up method.(f)-(i)TEM images of HOC(f),HOC/S(g),HEC(h)and HEC/S(i)UCNPs.(j)Upconversion emission spectra of NaGdF4∶Yb,Er@NaYF4 HEC/S,and HOC/Snanoparticles under identical experimental conditions.Inset shows the absolute quantum yields(QYs)of the HEC,HOC,HEC/S,and HOC/Snanoparticles.(k)Comparison of the relative emission intensity of the HEC,HOC,HEC/S,and HOC/S nanoparticles[85].
Cation exchange reactions at the nanoscale have recently emerged as a powerful tool for controlling composition and phase in colloidal semiconductor nanocrystals[87-88].These reactions present an alternative solution for modulating emission colors in UCNPs.One of the earliest examples of this technique was demonstrated by van Veggel and Dong,who prepared GdF3@Ln F3core-shell structured nanoparticles by exposing GdF3core nanoparticles to an aqueous solution containing excess Ln3+ions,where the Gd3+ions in GdF3nanoparticles were partially replaced by the Ln3+in the solution[89].By refining this synthetic route,they synthesized multi-functional NaYF4∶Yb,Tm@NaGdF4core-shell structured UCNPs with tunable shell thickness,enhanced upconversion luminescence and outstanding paramagnetic performance via cation exchange of NaYF4∶Yb/Tm nanoparticles with Gd3+ions,making these UCNPs promising for bimodal imaging,namely,optical bio-imaging and magnetic resonance imaging(MRI)[90].
Later in 2016,Liu et al.demonstrated the use of a cation exchange strategy for expeditiously accessing large classes of UCNPs with various luminescence property(Fig.9)[91].By combining the process of cation exchange with energy migration,the luminescence properties of the nanocrystals can be easily tuned while preserving the size,morphology and crystal phase of the initial nanocrystal template(Fig.9(a) - (f)).In particular,they achieved upconversion emission from Ce3+or Mn2+doped in hexagonal-phased UCNPs.This allows them to generate a record long-lived luminescence of~ 600 ms for Mn2+-activated UCNPs(Fig.9(f)).

Fig.9 (a)Schematic representation of a typical cation exchange process,occurring at the particle surface,between lanthanide(Ln3+)and exchange(Xn+)ions.(b)Proposed energy management process for cation exchange-mediated luminescence tuning in the nanocrystals.The energy transfer process mainly comprises energy harvesting,energy migration and energy trapping through different types of lanthanides.DCand UCrepresent downconversion and upconversion processes,respectively.(c)Typical luminescent ions used for cation exchange-mediated luminescence tuning and their main emitting transitions in the ultraviolet and visible part of the electromagnetic spectrum.(d,e)Low-resolution TEM images of the as-prepared NaGdF4∶Yb,Tm@NaGdF4 nanoparticles,obtained before(d)and after(e)incubation with TbCl3.scale bar=50 nm.(f)Typical photoluminescence spectra of the UCNPs treated with TbCl3,EuCl3,MnCl2,DyCl3 and CeCl3,respectively.(g)Typical scanning transmission electron microscopic(STEM)image of the nanoparticles treated with TbCl3.(h)Electron energy loss spectroscopy(EELS)point analysis collected,respectively,from green and red circle marked in g.(i,j)EELSline profile recorded by scanning along the white line shown in g.Note that both elemental analyses in(i,j)reveal that more Tb content is present at particle outer layer,while more Gd content exists at particle inner layer[91].
4 Summary and Perspectives
In the last decade,NIR optical biomedical applications based on UCNPs has played an important role in biotechnology due to their intrinsic advantages against the traditional imaging probes.They benefit from high photostability,non-blinking and low toxicity properties,but they also suffer from shortcomings.One of the most serious among them is low luminescence efficiency.As above mentioned,many efforts have been devoted in a synthetic way to improve the luminescence efficiency of UCNPs,such as inert shell coating,homogeneous doping,etc.Although in some cases(such as co-precipitation and combustion method,etc),the luminescence efficiency is relatively higher than those synthesized by other methods.However,they are generally accepted that at a cost of poor monodispersity and morphology,inhibiting their usage in the bio-applications.Therefore,advanced synthetic methods should be developed to synthesize highly efficient UCNPs.In addition,more reasonable nanostructures should be designed to reduce the crystal defects or modify the crystal field of the host.What's more,in order to obtain the highly efficient upconversion nanoprobes,increasing the doping concentration without dropping down the luminescence efficiency is also a feasible way.
[1]AUZEL F.Upconversion and anti-Stokes processes with f and d ions in solids[J].Chem.Rev.,2004,104(1):139-173.
[2 ]ESTEROWI L,SCHNITZL.A,NOONAN J,et al..Rare earth infrared quantum counter[J].Appl.Opt.,1968,7(10):2053-2070.
[3]LIU ST,MACIOLEK R B.Rare-earth-modified Sr1-xBaxNb2O6ferroelectric-crystals and their applications as infrared detectors[J].J.Electron.Mater.,1974,3(4):864.
[4]MACIEL G S,MENEZESL D,GOMESA S L,et al..Temperature sensor based on frequency upconversion in Er3+-doped fluoroindate glass[J].IEEE Photon.Technol.Lett.,1995,7(12):1474-1476.
[5]BERTHOU H,JORGENSEN C K.Optical-fiber temperature sensor based on upconversion-excited fluorescence[J].Opt.Lett.,1990,15(19):1100-1102.
[6 ]DOWNING E,HESSELINK L,RALSTON J,et al..A three-color,solid-state,three-dimensional display[J].Science,1996,273(5279):1185-1189.
[7 ]JOUBERT M F.Photon avalanche upconversion in rare earth laser materials [J].Opt.Mater.,1999,11(2-3):181-203.
[8 ]STONEMAN R C,ESTEROWITZ L.Efficient resonantly pumped 2.8-μm Er3+GSGG laser[J].Opt.Lett.,1992,17(11):816-818.
[9 ]HUTCHINSON JA,ALLIK T H.Diode array-pumped Er,Yb-phosphate-glass laser[J].Appl.Phys.Lett.,1992,60(12):1424-1426.
[10]PACHECO E M,DEARAUJO C B.Frequency upconversion in a borate glass doped with Pr3+[J].Chem.Phys.Lett.,1988,148(4):334-336.
[11]CHAMARRO M A,CASESR.Energy upconversion in(Yb,Ho)and(Yb,Tm)doped fluorohafnate glasses[J].J.Lumin.,1988,42(5):267-274.
[12]QUIMBY R S,DREXHAGE M G,SUSCAVAGE M J.Efficient frequency upconversion via energy-transfer in fluoride glasses[J].Electron.Lett,.1987,23(1):32-34.
[13]YEH D C,SIBLEY WA,SUSCAVAGE M J.Efficient frequency upconversion of Tm3+ions in Yb3+doped barium thorium fluoride glass[J].J.Appl.Phys.,1988,63(9):4644-4650.
[14]ELISEEVA SV,BUNZLI J C G.Lanthanide luminescence for functional materials and bio-sciences[J].Chem.Soc.Rev.,2010,39(1):189-227.
[15]KOBAYASHI H,OGAWA M,ALFORD R,et al..New strategies for fluorescent probe design in medical diagnostic imaging[J].Chem.Rev.,2010,110(5):2620-2640.
[16]MADER H S,KELE P,SALEH SM,et al..Upconverting luminescent nanoparticles for use in bioconjugation and bioimaging[J].Curr.Opin.Chem.Biol.,2010,14(5):582-596.
[17]CHENG L,YANG K,LI Y G,et al..Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy[J].Angew.Chem.Int.Ed.,2011,50(32):7385-7390.
[18]ZHANG F,SHI Y,SUN X,et al..Formation of hollow upconversion rare-earth fluoride nanospheres:nanoscale kirkendall effect during ion exchange[J].Chem.Mater.,2009,21(21):5237-5243.
[19]BOYER J C,MANSEAU M P,MURRAY J I,et al..Surface modification of upconverting NaYF4nanoparticles with PEG-phosphate ligands for NIR(800 nm)biolabeling within the biological window[J].Langmuir,2010,26(2):1157-1164.
[20]HEBBINK GA,STOUWDAM JW,REINHOUDT DN,et al..Lanthanide(Ⅲ-doped nanoparticles that emit in the nearinfrared [J].Adv.Mater.,2002,14(16):1147-1150.
[21]WANG F,HAN Y,LIM CS,et al..Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping[J].Nature.2010,463(7284):1061-1065.
[22]WANG F,DENG R R,WANGJ,et al..Tuning upconversion through energy migration in core-shell nanoparticles[J].Nat.Mater.,2011,10(12):968-973.
[23]WANG F,WANG J,LIU X.Direct evidence of a surface quenching effect on size-dependent luminescence of upconversion nanoparticles[J].Angew.Chem.Int.Ed.,2010,49(41):7456-7460.
[24]HUANG P,ZHENG W,ZHOU S,et al..Lanthanide-doped LiLuF4upconversion nanoprobes for the detection of disease biomarkers[J].Angew.Chem.Int.Ed.,2014,53(5):1252-1257.
[25]LIU Q,SUN Y,YANGT,et al..Sub-10 nm hexagonal lanthanide-doped NaLuF4upconversion nanocrystals for sensitive bioimaging in vivo[J].J.Am.Chem.Soc.,2011,133(43):17122-17125.
[26]ZHANG Y W,SUN X,SI R,et al..Single-crystalline and monodisperse LaF3triangular nanoplates from a single-source precursor[J].J.Am.Chem.Soc.,2005,127(10):3260-3261.
[27]ZHENG W,ZHOU S,CHEN Z,et al..Sub-10 nm lanthanide-doped CaF2nanoprobes for time-resolved luminescent biodetection[J].Angew.Chem.Int.Ed.,2013,52(26):6671-6676.
[28]WANG F,LIU X G.Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals[J].Chem.Soc.Rev.,2009,38(4):976-989.
[29]CHENG L,WANG C,LIU Z.Upconversion nanoparticles and their composite nanostructures for biomedical imaging and cancer therapy[J].Nanoscale,2013,5(1):23-37.
[30]WANG F,BANERJEE D,LIU Y,et al..Upconversion nanoparticles in biological labeling,imaging,and therapy[J].Analyst,2010,135(8):1839-1854.
[31]ZHOU J,LIU Z,LI F.Upconversion nanophosphors for small-animal imaging[J].Chem.Soc.Rev.,2012,41(3):1323-1349.
[32]STOUWDAM JW,VAN VEGGEL F C.Near-infrared emission of redispersible Er3+,Nd3+,and Ho3+doped LaF3nanoparticles[J].Nano Lett.,2002,2(7):733-737.
[33]YI G S,CHOW G M.Colloidal LaF3∶Yb,Er,LaF3∶Yb,Ho and LaF3∶Yb,Tm nanocrystals with multicolor upconversion fluorescence[J].J.Mater.Chem.,2005,15(41):4460-4464.
转型升级工程推进以来,在政法、地质、海关等专业出版领域,已经构建出相对完善的大数据平台,确立了数据采集、数据存储、知识体系、知识标引、知识计算、大数据模型、大数据服务的“七步法”原理,探索出一条将大数据应用于新闻出版业的可行路径。累计出版了AR出版物300多种,基本形成了“3D模型库、AR编辑器、输出展示系统”的AR出版产业链环节;虚拟现实(VR)技术不断应用于在线教育和教育装备领域;人工智能的深度学习、语音识别、机器人新闻、智能教育机器人、智能科普机器人、图书馆智能盘点机器人等创新型数字产品层出不穷、不断涌现。
[34]ZENG JH,SU J,LI Z H,et al..Synthesis and upconversion luminescence of hexagonal-phase NaYF4∶Yb,Er3+phosphors of controlled size and morphology[J].Adv.Mater.,2005,17(17):2119-2123.
[35]HEER S,LEHMANN O,HAASE M,et al..Blue,green,and red upconversion emission from lanthanide-doped LuPO4and YbPO4nanocrystals in a transparent colloidal solution[J].Angew.Chem.Int.Ed.,2003,42(27):3179-3182.
[36]WANG F,CHATTERJEE D K,LI Z,et al..Synthesis of polyethylenimine/NaYF4nanoparticles with upconversion fluorescence[J].Nanotechnology,2006,17(23):5786-5791.
[37]LI Z,ZHANG Y.Monodisperse silica-coated polyvinylpyrrolidone/NaYF4nanocrystals with multicolor upconversion fluorescence emission[J].Angew.Chem.Int.Ed.,2006,45(46):7732-7735.
[38]QIU P,ZHOU N,CHEN H,et al..Recent advances in lanthanide-doped upconversion nanomaterials:synthesis,nanostructures and surface modification[J].Nanoscale,2013,5(23):11512-11525.
[39]CHEN X,PENG D,JU Q,et al..Photon upconversion in core-shell nanoparticles[J].Chem.Soc.Rev.,2015,44(6):1318-1330.
[40]CHEN G,QIU H,PRASAD P N,et al..Upconversion nanoparticles:design,nanochemistry,and applications in theranostics[J].Chem.Rev.,2014,114(10):5161-5214.
[41]DONG H,DU SR,ZHENG X Y,et al..Lanthanide nanoparticles:from design toward bioimaging and therapy[J].Chem.Rev.,2015,115(19):10725-10815.
[42]WANG X,ZHUANG J,PENG Q,et al..A general strategy for nanocrystal synthesis[J].Nature,2005,437(7055):121-124.
[43]WANGX,ZHUANGJ,PENGQ,et al..Hydrothermal synthesis of rare-earth fluoride nanocrystals[J].Inorg.Chem.,2006,45(17):6661-6665.
[44]WANG L,LI Y.Controlled synthesis and luminescence of lanthanide doped NaYF4nanocrystals[J].Chem.Mater.,2007,19(4):727-734.
[45]WANGL,LI Y.Na(Y1.5Na0.5)F6single-crystal nanorods as multicolor luminescent materials[J].Nano Lett.,2006,6(8):1645-1649.
[46]ZHANG F,WAN Y,YU T,et al..Uniform nanostructured arrays of sodium rare-earth fluorides for highly efficient multicolor upconversion luminescence[J].Angew.Chem.Int.Ed.,2007,46(42):7976-7979.
[47]LIU C,CHEN D.Controlled synthesis of hexagon shaped lanthanide-doped LaF3nanoplates with multicolor upconversion fluorescence[J].J.Mater.Chem.,2007,17(37):3875-3880.
[48]TIAN G,GU Z,ZHOU L,et al..Mn2+dopant-controlled synthesis of NaYF4∶Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery[J].Adv.Mater.,2012,24(9):1226-1231.
[49]ZENGS,YI Z,LU W,et al..Simultaneous realization of phase/size manipulation,upconversion luminescence enhancement,and blood vessel imaging in multifunctional nanoprobes through transition metal Mn2+doping [J].Adv.Funct.Mater.,2014,24(26):4051-4059.
[50]MAI H X,ZHANG Y W,SI R,et al..High-quality sodium rare-earth fluoride nanocrystals:controlled synthesis and optical properties[J].J.Am.Chem.Soc.,2006,128(19):6426-6436.
[51]BOYER JC,CUCCIA L A,CAPOBIANCO J A.Synthesis of colloidal upconverting NaYF4∶Er3+/Yb3+and Tm3+/Yb3+monodisperse nanocrystals[J].Nano Lett.,2007,7(3):847-852.
[52]MAI H X,ZHANGY W,SUN L D,et al.Size-and phase-controlled synthesis of monodisperse NaYF4∶Yb,Er nanocrystals from a unique delayed nucleation pathway monitored with upconversion spectroscopy[J].J.Phys.Chem.C,2007,111(37):13730-13739.
[53]KRMER K W,BINER D,FREI G,et al.Hexagonal sodium yttrium fluoride based green and blue emitting upconversion phosphors[J].Chem.Mater.,2004,16(7):1244-1251.
[54]LI Z Q,ZHANG Y.An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF4∶Yb,Er/Tm nanocrystals with controllable shape and upconversion fluorescence[J].Nanotechnology,2008,19(34):345606.
[55]LIZ Q,ZHANGY,JIANGS.Multicolor core/shell-structured upconversion fluorescent nanoparticles[J].Adv.Mater.,2008,20(24):4765-4769.
[56]LAMER V K,DINEGAR R H.Theory,production and mechanism of formation of monodispersed hydrosols[J].J.Am.Chem.Soc.,1950,72(11):4847-4854.
[57]MURRAY CB,KAGAN C,BAWENDI M.Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies[J].Annu.Rev.Mater.Sci.,2000,30(1):545-610.
[58]PENG Z A,PENG X.Nearly monodisperse and shape-controlled CdSe nanocrystals via alternative routes:nucleation and growth[J].J.Am.Chem.Soc.,2002,124(13):3343-3353.
[59]PENGX,WICKHAM J,ALIVISATOSA.Kinetics ofⅡ-Ⅵ andⅢ-Ⅴ colloidal semiconductor nanocrystal growth:“focusing”of size distributions[J].J.Am.Chem.Soc.,1998,120(21):5343-5344.
[60]DU HNEN S,RINKEL T,HAASE M.Size control of nearly monodisperseβ-NaGdF4particles prepared from smallα-NaG-dF4nanocrystals[J].Chem.Mater.,2015,27(11):4033-4039.
[61]JOHNSON N J,OAKDEN W,STANISZ G J,et al..Size-tunable,ultrasmall NaGdF4nanoparticles:insights into their T1 MRI contrast enhancement[J].Chem.Mater.,2011,23(16):3714-3722.
[62]LIU D,XU X,DU Y,et al..Three-dimensional controlled growth of monodisperse sub-50 nm heterogeneous nanocrystals[J].Nat.Commun.,2016,7:10254.
[63]PATRA A,FRIEND CS,KAPOOR R,et al..Upconversion in Er3+∶ZrO2nanocrystals[J].J.Phys.Chem.B,2002,106(8):1909-1912.
[64]PATRA A,FRIEND C S,KAPOOR R,et al..Fluorescence upconversion properties of Er3+-doped TiO2and BaTiO3nanocrystallites[J].Chem.Mater.,2003,15(19):3650-3655.
[65]VENKATRAMU V,FALCOMER D,SPEGHINI A,et al..Synthesis and luminescence properties of Er3+-doped Lu3Ga5O12nanocrystals[J].J.Lumin.,2008,128(5-6):811-813.
[66]YANGK S,ZHENGF,WU R N,et al..Upconversion luminescent properties of YVO4∶Yb3+,Er3+nano-powder by solgel method[J].J.Rare Earth,2006,24:162-166.
[67]XU L,YU Y,LI X,et al..Synthesis and upconversion properties of monoclinic Gd2O3∶Er3+nanocrystals[J].Opt.Mater.,2008,30(8):1284-1288.
[68]LUO X X,CAOWH.Ethanol-assistant solution combustion method to prepare La2O2S∶Yb,Pr nanometer phosphor[J].J.Alloys Compd.,2008,460(1):529-534.
[69]VETRONE F,BOYER JC,CAPOBIANCO J A,et al..Significance of Yb3+concentration on the upconversion mechanisms in codoped Y2O3∶Er3+,Yb3+nanocrystals[J].J.Appl.Phys.,2004,96(1):661-667.
[70]YI G S,CHOW GM.Water-soluble NaYF4∶Yb,Er(Tm)/NaYF4/polymer core/shell/shell nanoparticles with significant enhancement of upconversion fluorescence[J].Chem.Mater.,2007,19(3):341-343.
[71]MAI H X,ZHANG Y W,SUN L D,et al..Highly efficient multicolor up-conversion emissions and their mechanisms of monodisperse NaYF4∶Yb,Er core and core/shell-structured nanocrystals[J].J.Phys.Chem.C,2007,111(37):13721-13729.
[72]BOYER JC,VAN VEGGEL F.Absolute quantum yield measurements of colloidal NaYF4∶Er3+,Yb3+upconverting nanoparticles[J].Nanoscale,2010,2(8):1417-1419.
[73]LI X,ZHANG F,ZHAO D.Highly efficient lanthanide upconverting nanomaterials:progresses and challenges[J].Nano Today,2013,8(6):643-676.
[74]LI X,ZHANG F,ZHAO D.Lab on upconversion nanoparticles:optical properties and applications engineering via designed nanostructure[J].Chem.Soc.Rev.,2015,44(6):1346-1378.
[75]JOHNSON NJ,HE S,DIAOS,et al..Direct evidence for coupled surface and concentration quenching dynamics in lanthanide-doped nanocrystals[J].J.Am.Chem.Soc.,2017,139(8):3275-3282.
[76]ABEL K A,BOYERJC,ANDREICM,et al..Analysis of the shell thickness distribution on NaYF4/NaGdF4core/shell nanocrystals by EELSand EDS [J].J.Phys.Chem.Lett.,2011,2(3):185-189.
[77]ABEL K A,BOYER J C,VAN VEGGEL F C J M.Hard proof of the NaYF4/NaGdF4nanocrystal core/shell structure[J].J.Am.Chem.Soc.,2009,131(41):14644-14645.
[78]ZHANG F,CHE R,LI X,et al..Direct imaging the upconversion nanocrystal core/shell structure at the subnanometer level:shell thickness dependence in upconverting optical properties[J].Nano Lett.,2012,12(6):2852-2858.
[79]LI X,SHEN D,YANG J,et al..Successive layer-by-layer strategy for multi-shell epitaxial growth:shell thickness and doping position dependence in upconverting optical properties[J].Chem.Mater.,2013,25(1):106-112.
[80]LI X,GUO Z,ZHAO T,et al..Filtration shell mediated power density independent orthogonal excitations-emissions upconversion luminescence[J].Angew.Chem.Int.Ed.,2016,55(7):2464-2469.
[81]LI X,WANG R,ZHANG F,et al..Nd3+sensitized up/down converting dual-mode nanomaterials for efficient in-vitro and in-vivo bioimaging excited at 800 nm[J].Sci.Rep.,2013,3:3536.
[82]WANG R,LI X M,ZHOU L,et al..Epitaxial seeded growth of rare-earth nanocrystals with efficient 800 nm near-infrared to 1 525 nm short-wavelength infrared downconversion photoluminescence for in vivo bioimaging[J].Angew.Chem.Int.Ed.,2014,53(45):12086-12090.
[83]WANG R,ZHANG F.NIR luminescent nanomaterials for biomedical imaging[J].J.Mater.Chem.B,2014,2(17):2422-2443.
[84]ABEL K A,BOYER JC,VEGGEL F CV.Hard proof of the NaYF4/NaGdF4nanocrystal core/shell structure[J].J.Am.Chem.Soc.,2009,131(41):14644-14645.
[85]LI X,WANGR,ZHANGF,et al..Engineering homogeneous doping in single nanoparticle to enhance upconversion effi-ciency[J].Nano Lett.,2014,14(6):3634-3639.
[86]JOHNSON N J,KORINEK A,DONG C,et al..Self-focusing by Ostwald ripening:a strategy for layer-by-layer epitaxial growth on upconverting nanocrystals[J].J.Am.Chem.Soc.,2012,134(27):11068-11071.
[87]SON D H,HUGHES S M,YIN Y,et al..Cation exchange reactions in ionic nanocrystals[J].Science,2004,306(5698):1009-1012.
[88]ROBINSON R D,SADTLER B,DEMCHENKO D O,et al..Spontaneous superlattice formation in nanorods through partial cation exchange[J].Science,2007,317(5836):355-358.
[89]DONG C,VAN VEGGEL F C.Cation exchange in lanthanide fluoride nanoparticles[J].ACSNano,2008,3(1):123-130.
[90]DONG C,KORINEK A,BLASIAK B,et al..Cation exchange:a facile method to make NaYF4∶Yb,Tm-NaGdF4coreshell nanoparticles with a thin,tunable,and uniform shell[J].Chem.Mater.,2012,24(7):1297-1305.
[91]HAN S,QIN X,AN Z,et al..Multicolour synthesis in lanthanide-doped nanocrystals through cation exchange in water[J].Nat.Commun.,2016,7:13059.
镧系元素掺杂的高效率上转换纳米粒子的合成
王 睿,张 凡*
(复旦大学化学系高分子工程国家重点实验室,上海 200433)
上转换纳米粒子在近红外光激发下发射紫外/可见/近红外光,也称为反斯托克斯发射。这一独特的光学性质排除了来自于生物材料的背景荧光和散射光,为光学生物应用,如生物成像、光动力学治疗、药物输送等开辟了新的途径。为了实现更好的应用性能,在许多情况下需要高效率的镧系元素上转换纳米颗粒。一般来说,上转换纳米粒子的质量往往与其合成方法有关。在这篇综述中,我们将主要阐述上转换纳米粒子的各种合成过程,总结了几种获得高发光效率的上转换纳米粒子的有效途径。
上转换纳米粒子;合成方法;核壳结构
2017-10-23;
2017-12-09
国家自然科学基金杰出青年基金(21725502);国家重大研究计划项目(2017YFA0207303);上海市基础研究重大项目(17JC1400100)资助
O482.31 Document code:A
10.3788/fgxb20183901.0050
Supported by National Science Fund for Distinguished Young Scholars(21725502);National Key R&D Program of China(2017YFA0207303);Key Basic Research Program of Science and Technology Commission of Shanghai Municipality(17JC1400100)

王睿(1991-),男,江西上饶人,博士,2017年于复旦大学获得博士学位,主要从事生物活体成像与生物传感方面的研究。
E-mail:rwang938@gmail.com

张凡(1977-),男,内蒙古呼和浩特人,教授,博士生导师,2008年于复旦大学获得博士学位,主要从事光学成像分析方面的研究。
E-mail:zhang_fan@fudan.edu.cn

