SnS2/GCP(石墨烯复合粉末)微米复合材料作为锂离子电池负极材料的电化学性能
林 健 崔永福 崔金龙 文钟晟 孙俊才
(大连海事大学,大连 116026)
SnS2/GCP(石墨烯复合粉末)微米复合材料作为锂离子电池负极材料的电化学性能
林 健 崔永福 崔金龙*文钟晟 孙俊才*
(大连海事大学,大连 116026)
以石墨烯复合粉末为添加剂,采用一步水热法制备了一种SnS2/GCP微米复合材料。在所得到的复合材料中,SnS2纳米片相互缠绕组成多孔球状SnS2颗粒,石墨烯复合粉末均匀的包裹在球状SnS2颗粒表面。将所制备的SnS2/GCP微米复合材料用作锂离子电池负极材料测其电化学性能。结果显示,在0.1 A·g-1的电流密度下可逆比容量为795.6 mAh·g-1,循环100次后比容量损失不到1%。相比于SnS2其比容量和循环稳定性得到了明显改善,主要是由于石墨烯复合粉末的加入,不仅缓解了SnS2颗粒在充放电过程中的团聚和体积膨胀,而且还提高了SnS2颗粒的电导率。
锂离子电池;二硫化锡;负极材料;石墨烯复合粉末
0 Introduction
Lithium-ion batteries (LIBs)have been widely utilized in portable electronic devices,electric vehicles and plug-in hybrid electric vehicles owing to their high energy density,light weight and remarkable cycling performance[1-2].For anode materials,current commercial graphite anodes for LIBs have atheoretical capacity of 372 mAh·g-1,which cannot meet the increasing requirement for LIBs with better electrochemical properties[3-4]. Therefore,significant efforts have been devoted to explore advanced anode materials with higher capacity,super cycle stability and better rate capability,as well as lower cost[5].Among variousanode materials,tin-based anode materials have aroused widespread concern because of their high theoretical reversible capacity (987 mAh·g-1)[4-6].Among Sn-based materials,Sn metal and SnO2have been extensively studied[7-10].In addition,transition metal sulfides,such ashave been broadly investigated as promising anode materials for LIBs,owing to their high specific capacity,safety,low cost and effortless synthesis[11-14].SnS2has a typicallayered CdI2-type crystalline structure in which Sn atoms are sandwiched between two layers of hexagonally compactly arranged S atoms,and the adjacent S layers are connected with weak van der Waals forces[15].This specialized crystalline structure is conducive to intercalation of lithium-ions(Li-ions)and hence many researchers have made great efforts to synthesize SnS2with different structures as alternative anode materials[16-17].
Nevertheless,the practical usage of stannum sulfide for LIBs is severely hampered by remarkable volume change that occurs during Li-ions insertion and extraction reactions.It usually causes the aggregation and pulverization of active material particles with fast capacity fading[18].Consequently,various efforts have been devoted to solving this problem by using SnS2nanostructured particles,such as nanoplates[19-20]and nanosheets[21],hollow spheres[22],and hierarchical structures[17],as well as 3D-hierarchical structures[15-16].These structures could reduce absolute local volume changes and shorten charge transfer pathway,both of which can undoubtedly contribute to improving electrochemical Li-ions storage performance of SnS2electrodes[23].Another specifically effective approachis to fabricate composites of metal sulfides with carbonaceous materials,such as C[24-25],graphene[26-30]or carbon nanotube[31],which not only improves the conductivity,but also buffers the mechanical stress induced by volume change[32].Among these carbonaceous materials,graphene has become the spotlight in LIBs because it owns several desirable features,including superior electrical conductivity,excellent mechanical flexibility,large surface area,and high thermal and chemical stability[33-34].Forming a composite with graphene has been proven to be a useful strategy to improve the cycling stability of SnS2electrodes[26-30].The flexible graphene nanosheets act not only as a buffer to accommodate the volume changes during conversion reactions,but also as a separator to prevent the particles from aggregating upon long life cycling[35].Even so,the high price of graphene increases the production cost of SnS2/Graphene electrodes[36].
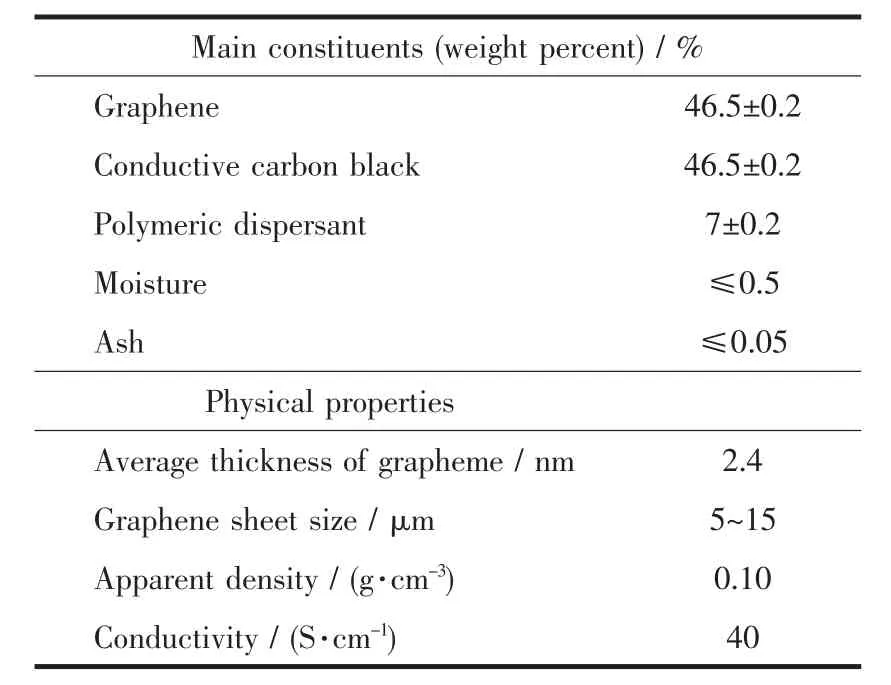
Table1 Characteristics of graphene composite powder(GCP)
In order to reduce the production cost of SnS2/graphene electrodes,graphene composite powder(GCP)was chosen to substitute graphene as an additive in the SnS2synthesis process.GCP is mainly composed of graphene (46.5%,weight percent)and conductive carbon black (46.5%)(Table 1),so it is much cheaper than graphene.In this work,SnS2/GCP microcomposites were synthesized via a facile one-pot hydrothermal approach using stannic chloride(SnCl4·5H2O)and thiourea (CH4N2S)as starting materials in the presence of GCP.The resulting SnS2/GCP microcomposites were composed of SnS2particles intertwined with the interconnected GCP and GCP was uniformly coated on the surfaces of SnS2particles.This unique morphology gave rise to the synergistic effect between SnS2and GCP,which was contributed to an improved specific capacity,high rate capability,as well as outstanding cycle stability up to 400 cycles.
1 Experimental
1.1 Materials
All reagents used in the synthetic process were analytical grade without further purification.Graphene composite powder (GCP) (GC1-Powder 4lib)was supplied by Ningbo Mexi Technology Co.Ltd.The characteristics of GCP are listed in Table 1.Distilled water was employed in all synthesis process.
1.2 Preparation of SnS2/GCP
For preparation of SnS2/GCP microcomposite,0.3 g GCP was dispersed in 40 mL deionized water with ultrasonic to form a homogeneous GCP dispersion.3.5 g SnCl4·5H2O and 2.25 g CH4N2S were dissolved into 20 mL deionized water with ultrasonic to obtain 0.5 mol·L-1SnCl4and 1.5 mol·L-1CH4N2S solution,respectively.Then,these two solutions were added dropwise to the above GCP dispersion with buret under continuously stirring orderly.After vigorously stirring for another 0.5 h,the mixed dispersion was transferred to a 150 mL Teflon-lined stainless steel autoclave and heated in an electric oven at 200℃for 24 h.Finally,the autoclave was allowed to cool down to room temperature naturally.The resulting precipitate was collected by vacuum filtration and washed several times with deionized water and ethanol,and finally dried at 80℃in vacuum overnight.As a comparative experiment,SnS2wasprepared underthe same condition without adding GCP.
1.3 Characterizations
The X-ray diffraction(XRD)(RigakuD/MAX-3A,Rigaku Corporation,Japan)patterns of pure SnS2,GCP and SnS2/GCP microcomposite were obtained using a Co Kα radiation(λ=0.154 06 nm)at operating voltage of 40 kV and a filament current of 40 mA.The diffraction angle was scanned from 10°to 90°with a rate of 4°·min-1.Raman spectra of them were obtained via a T64000 triple Raman system(HORIBA Jobin Yvon,France)with a 532 nm Ar-ion laser.The morphology of pure SnS2and SnS2/GCP microcomposite was observed using a field emission scanning electron microscope(FE-SEM)(SUPRA 55 SAPPHIRE,CARL ZEISS,Germany)and the elements distribution of SnS2/GCP microcomposite was measured via an energy-dispersive X-ray analysis spectrometer(EDS)(X-Max,OXFORD,UK)adjunctto the SEM.Further morphological and structural investigations of SnS2/GCP microcomposite were identified by highresolution transmission electron microscopy(HRTEM)and selected area electron diffraction (SAED)on a JEOL JEM-2100 microscope system operated at 200 kV.The thermogravimetric measurement (TG)was carried out with a Mettler STARe thermal analyzer in a temperature range of 25~900 ℃ under an air atmosphere at a heating rate of 10℃·min-1.
1.4 Electrochemical measurements
The electrochemical properties of the products were tested using CR2025-type coin cells.The working electrodes were prepared by mixing active materials, polyvinylidene fluoride (PVDF) and acetylene black at a mass ratio of 80 ∶10 ∶10 in NMP solvent to form a slurry.The obtained slurry was uniformly pasted on a thin copper foil and then dried at 80℃undervacuumovernight.Mass loading of active material on the collector is 1.8~2.3 mg·cm-2.The electrodes were then assembled into half cells in an Ar-filled glove box using Li foil as a counter electrode,polypropylene microporous sheet(Celgard 2400)as a separator and Ni foam as a filler.1 mol·L-1LiPF6/EC+DMC (1∶1 in volume)solution was applied as the electrolyte. Galvonostatic charge/discharge was measured on a LAND-2100 (Wuhan,China)battery tester in the voltage window of 0.01~3.0 V versus Li/Li+.Cyclic voltammetry (CV)was performed on a CHI660D electrochemical station(CHI Instrument)at a scan rate 0.05 mV·s-1with a voltage range of 0.01~3.0 V.Electrochemical impedance spectroscopy(EIS)measurements were carried out using the CHI660D electrochemical station in a frequency range from 100 kHz to 0.01 Hz at open circuit potential with an amplitude of 10 mV.
2 Results and discussion
2.1 Physicochemical properties
The XRD patterns of GCP,SnS2and SnS2/GCP microcomposite are shown in Fig.1a.For pure SnS2,the sharp diffraction peaks at about 17.47°,32.865°,37.483°,49.048°,58.733°,and 61.749°can be indexed to(001),(100),(101),(102),(110)and(111)planes of orthorhombic phase (PDF,No.23-0677)[28].Theobtained SnS2/GCP microcompositeshowsa similar XRD pattern to pure SnS2,suggesting that the introduction of GCP does not influence the fabrication of SnS2.The only peak for GCP at about 30.732°is observed,which corresponds to the(002)reflection of graphene and obvious peak related to GCP in the SnS2/GCP microcomposite is also observed.In addition,the intensity of the diffraction peak for GCP is weaker,which confirms the higher content of SnS2in thehybrid structure(Fig.1a).As shown in Fig.1b,Raman spectrum of pure SnS2exhibits an peak at about 311 cm-1,which is attributed to the A1gmode according to the group theory analysis conducted by previous studies[25,37].Moreover,a wide peak between 450 and 650 cm-1could also be observed in pure SnS2,which may be attributed to second-order effects[37-38].In the Raman spectrum of GCP,the peak at about 1 586 cm-1(G band),corresponding to an E2gmode of graphitic sheets,is related to the vibration of the sp2-bonded carbon atoms in a two dimensional hexagonal lattice[39].While,the peak at about 1 327 cm-1(D band)can be assigned to the scattering of the A1gmode associated with sp3-bonded disorder carbon,which is related to the defects or disorder in the hexagonal graphitic layers and the existence of disorder conductive carbon black[40].Furthermore,there is another intense peak at about 2 700 cm-1generally named 2D in the Raman spectrum of GCP,which is the second order of zoneboundary phonons[41].All the peaks of both pure SnS2andGCP could be observed in the Raman spectrum of SnS2/GCP microcomposite, confirming that this microcomposite is constituted of SnS2and GCP(Fig.1b).Besides,the intensity ratio of the D band to the G band (ID/IG)for GCP is much smaller than that of SnS2/GCP microcomposite,which can be attributed to the widely separated graphene layers promoted by the intercalation of SnS2nanosheets[42].
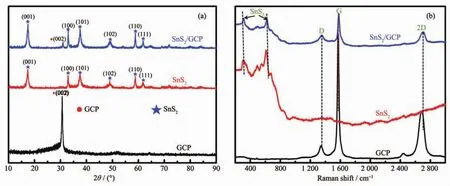
Fig.1 (a)XRD patterns and(b)Raman spectra of GCP,SnS2and SnS2/GCP microcomposite
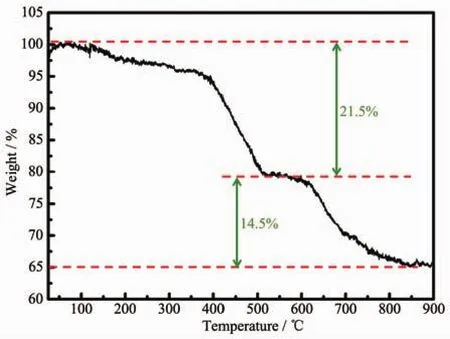
Fig.2 TG curve of SnS2/GCP microcomposite
To further determine the SnS2content in SnS2/GCP microcomposite,the SnS2/GCP microcompo-site was characterized by thermogravimetric analysis(TG)as shown in Fig.2.Two stages of weight loss are seen in the TG curve of SnS2/GCP sample.At first,an apparent weight loss of about 21.5%in the tempera-ture range of 40~500 ℃ is observed,which may be due to the phase conversion from SnS2to.The second weight loss is about 14.5%in the temperature range of 500~900 ℃,which can be attributed to the combustion of GCP.In other words,the weight fraction ofGCP in SnS2/GCP microcompositeis calculated to be 14.5%.After attaining 900℃,the content of remaining SnS2is about 64%in the mixture material.

Fig.3 FE-SEM images of(a)SnS2/GCP microcomposite and SnS2(inset);(b)EDS mappings of C,S and Sn elements of SnS2/GCP microcomposite;(c)TEM image and SAED patterns(inset)and(d)HRTEM image of SnS2/GCP microcomposite
2.2 Morphology and structure characterization
As shown in Fig.3a,the SnS2/GCP microcomposites are constituted of SnS2particles with sphere-like morphology homogeneously coated by the interconnected GCP.GCP coatings act as flexible and conductive matrix to supportthe SnS2particles,forming three dimensional conductivity network for transportation of Li-ions and alleviates the volume change of SnS2particles[43].Furthermore,the spherelike SnS2particle is made of several intertwining SnS2nanosheets accompanied by the formation of many pores on the surface of it(the inset in Fig.3a).Such a porous structure could provide the efficient transport pathways to their interior voids,which could reduce Li-ions diffusion path length and lighten the volume change and mechanical strains[44].Furthermore,this porous structure of SnS2particles could also increase the electrode-electrolyte interfacial area,facilitating electrochemical reactions and supplying more available Li-ions storage sites[45].Thereupon,these structure characterizations would assure the excellent electrochemical performance of the SnS2/GCP electrode[46-47].EDS mapping images from SEM show that C,S and Sn elements have a relatively uniform distribution,manifesting the good contact between SnS2particles and GCP coatings(Fig.3b).It is contributed to not only improving electrical conductivity of the SnS2particles,but also effectually preventing the aggregation of them during Li-ions insertion and extraction[3,47].The TEM image of SnS2/GCP microcomposite demonstrates once again that the SnS2particles are coated by the interconnected GCP(Fig.3c).The SAED patterns of SnS2/GCP microcomposite(the inset in Fig.3c)show that the diffraction spots of it could be placed in five concentric rings.The lattice plane spaces calculated from these rings are in line with SnS2(101),(102),(110)and(004),as well as GCP(002)[48].As shown in Fig.3d,the measured dspacing values of this composite are approximately 0.278 nm,0.215 nm,0.182 nm and 0.335 nm,which could be associated with the crystalline planes of SnS2(101),(102),(110)and GCP(002),respectively.These results are completely in conformity with the XRD analyses.
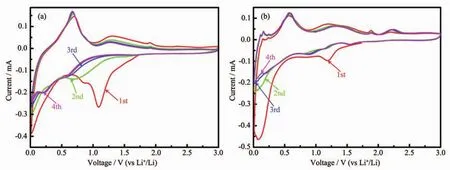
Fig.4 Cyclic voltammograms of(a)SnS2and(b)SnS2/GCP electrodes at a rate of 0.05 mV·s-1in a potential range of 0.01 to 3.0 V
2.3 Electrochemical performance
Cycle voltammetry curves of SnS2and SnS2/GCP electrode were measured between 0.01 and 3.0 V at a scan rate 0.05 mV·s-1,as shown in Fig.4a and b,respectively.For SnS2,the broad peaks at about 1.15 and 0.8 V during the first cathodic scan could be assigned to the conversion of SnS2to Sn(SnS2+4Li++4e-→Sn+2Li2S)[27],and the formation of solid electrolyte interface (SEI),respectively,which should be responsible for the first irreversible capacity loss(Fig.4a).Otherwise,the peak below 0.5 V can be totally due to the formation of LixSn alloy (Sn+xLi++xe-⇌LixSn(1≤x≤4.4))[28].The anodic peak at about 0.7 V could be attributed to the delithiation reaction of LixSn alloy[32].As for the CV curves of SnS2/GCP electrode,the cathodic peak becomes weak and shifts from 1.2 to 1.6 V during the second cycle,which can be attributed to the weak conversion reaction of SnS2caused by the recomination of Sn4+and S2-with Li during the first cycle[26].In addition,the cathodic peak at 0.01~0.5 V could be assigned to the formation of LixSn alloys and the Li-ions insertion into graphene nanosheets[26].The anodic peaks at about 1.8 and 2.15 V might be originated from the oxygenation of generated Sn at higher potential in the charged stage[28].The additional anodic peaks at 0.01~0.25 V would be attributed to the Li-ions extraction from graphene nanosheets[30].By contrast,the cycling stability of the SnS2electrode could be well improved after the addition of GCP.
As shown in Fig.5a,the first discharge and charge capacity of SnS2/GCP microcomposite are 1 834.7 and 874.6 mAh·g-1,so the calculated coulombic efficiency is about 47.6%.This high irreversible capacity could mainly originate from the incomplete conversion reaction[27]and the formation of SEI layer on the electrode surface[49].It is clear that all the charge/discharge plateaus are consistent with the peaks in CV curves.In addition,the curves of the 10th and 50th cycles nearly overlap,confirming that this SnS2/GCP electrode has excellent cycling stability(Fig.5a).In order to evaluate the effect of GCP on the electrochemical properties of SnS2/GCP microcomposite,the discharge specific capacities of SnS2/GCP,SnS2and GCP at a current density of 0.1 A·g-1are displayed in Fig.5b. Obviously, the SnS2/GCP electrode shows an improved cycling stability compared with SnS2electrode.After 5 cycles,a capacity of over 795 mAh·g-1can be maintained till 100 cycles for SnS2/GCP electrode.In contrary,the capacity of SnS2electrode sharply decays from an initial capacity of 1 507.3 to 232.2 mAh·g-1after 70 cycles.Poor cycling stability for SnS2electrode was also observed in the previous report[28].In the SnS2/GCP microcomposite,GCP coatings act as conductive and flexible matrix to support the SnS2particles,forming three dimensional conductivity network for transportation of Li-ions and alleviates the volume change of SnS2particles.Thereby,SnS2/GCP electrode exhibits an improved capacity and superb cycling stability compared with pure SnS2.Although GCP has an excellent cycling stability,its specific capacity is too low to be used as commercial electrode material(332.4 mAh·g-1).In addition to the high reversible capacity and remarkable cycle stability,the SnS2/GCP microcomposite also demonstrates an excellent rate capability (Fig.5c).The charge/discharge rates are programmably modified from 0.1 to 0.2,0.4,0.8,1.0 A·g-1and then back to 0.1 A·g-1for 15 cycles.It can be observed that the discharge capacities varies from 857 to 813,704,615,578,550 mAh·g-1and finally reversibly back to 825 mAh·g-1at current rates of 0.1,0.2,0.4,0.8,1.0 A·g-1and 0.1 A·g-1,respectively.It is noted that the SnS2/GCP electrode can still deliver a capacity of 550 mAh·g-1even at the high current rate of 1.0 A·g-1,which is still higher than the theoretical capacity of traditional graphite.
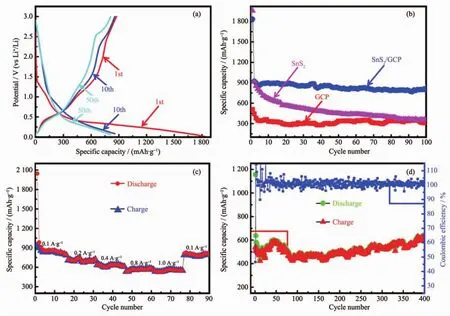
Fig.5 (a)Galvanostatic discharge/charge curves of SnS2/GCP electrode for the 1st,10th,and 50th cycles at 0.1 A·g-1;(b)Discharge specific capacity of SnS2/GCP,SnS2and GCP electrodes at 0.1 A·g-1;(c)Rate capability of SnS2/GCP electrode at 0.1,0.2,0.4,0.8 and 1.0 A·g-1;(d)Cycling performance of SnS2/GCP electrode at 0.5 A·g-1
The cyclic performance of SnS2/GCP electrode was tested at 0.5 A·g-1with voltage range of 0.01~3.0 V,and the result is plotted in Fig.5d.Although the cyclic performance curve is slightly fluctuating,the SnS2/GCP electrode exhibits high reversible capacity,approximately 642.8 A·g-1,which is much higher than SnS2/graphene aerogels (300 mAh·g-1at 0.5 A·g-1)[27]and SnS2/MWCNTs (multiwalled carbon nanotubes)hybrid(510 mAh·g-1at 0.1 A·g-1)[31].Table 2 lists the comparison of specific capacities of SnS2composite synthesized using carbonaceous materials as additive.Notwithstanding the specific capacity of SnS2/GCP microcomposite is not the highest,the lower cost and less usage of GCP would make it widely available for commercial production.Meanwhile,the coulombic efficiency is very close to 100%all over the 400 cycles after the first cycle.The distinct behavior of SnS2/GCP and pure SnS2indicates that the addition of GCP can not only prominently enhance the specific capacities of this microcomposite,but also effectively improve its cycling stabilities.

Table2 Comparison of specific capacities of SnS2composites
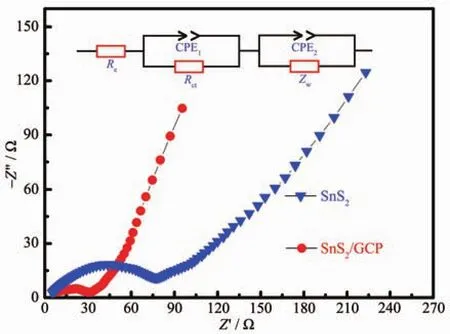
Fig.6 Nyquist plots of SnS2and SnS2/GCP electrodes after 100th cycle
Such outstanding electrochemical properties of this resulting SnS2/GCP microcomposite could be explained by multiple factors:(1)Although the capacity of GCP itself is not very high,the conductive GCP can provide many pathways for electrons and Li-ions and improve the whole conductivity of the composite electrode.(2)C,S and Sn elements have a relatively uniform distribution,which could effectually prevent the aggregation ofSnS2particlesduring Li-ions insertion and extraction.(3)The porous structure and GCP coatings of SnS2/GCP particles could facilitate electrochemical reactions,supply more available Liions storage sites and lighten the volume change and mechanical strains. These characteristics could guarantee high reversible capacity and superb cyclic stability of the resulting SnS2/GCP microcomposite.
To explain the different electrochemical behaviorsbetween SnS2/GCP microcomposite and SnS2,EIS were measured after cycling for 100 cycles as shown in Fig.6.For both electrodes,the Nyquist plots are consisted of a depressed semicircle in the high-to-middle frequency region and a slopping line in the low frequency region[50].These plots are fitted by an equivalent circuit given in the inset of Fig.6.The semicircle in the high frequency region corresponds to the charge transfer resistance (Rct)of the anode and the straight line in the low-frequency domain corresponds to the Li-diffusion process within electrodes.In addition,the intersections of the semicircle with the real axis at high frequency region (denoted as Re)are attributed to the internal resistance,associating with electronic resistance of the anode and the ionic resistance of the liquid electrolyte[3,51].According the fitting results,the values of Reand Rctof SnS2/GCP electrode after 100 cycles are 35.02 and 30.85 Ω,respectively,which are lower than those of SnS2electrode(71.24 and 76.03 Ω).These results indicate that the addition of GCP can significantly increase the electronic conductivity of composite electrode and enhance rapid electron transport during the Li-ions insertion/extraction process,so the electrochemical performance of SnS2could be effectively improved[52].
3 Conclusions
Through the hydrothermal method,the SnS2/GCP microcompositeswhich could beused asanode materials for lithium-ion batteries were effectively prepared.The resulting SnS2/GCP microcomposites showed an impressive electrochemical performance with a specific capacity of about 642.8 mAh·g-1and excellent cyclic stability without any degradation after 400 charge/discharge cycles at 0.5 A·g-1in the voltage window of 0.01~3.0 V.The addition of GCP was very helpful to provide good electronic conductivity and lighten the volume change of SnS2particles.
[1]Armand M,Tarascon J M.Nature,2008,451:652-657
[2]JI Wen-Xu(纪文旭),WU Di(吴迪),YANG Rong(杨蓉),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(4):659-665
[3]Cui J L,Cui Y F,Li S H,et al.ACS Appl.Mater.Interfaces,2016,8:30239-30247
[4]PENG Peng(彭鹏),WEN Zhao-Yin(温兆银),LIU Yu(刘宇),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30(6):1293-1298
[5]Alias N,Mohamad A A.J.Power Sources,2015,274:237-251
[6]Du Z J,Zhang S C,Xing Y L,et al.J.Power Sources,2011,196:9780-9785
[7]Cheng Y Y,Huang J F,Li J Y,et al.J.Power Sources,2016,324:447-454
[8]Liu R P,Su W M,He P,et al.J.Alloys Compd.,2016,688:908-913
[9]Guo C,Yang Q Q,Liang J W,et al.Mater.Lett.,2016,184:332-335
[10]Wang F,Jiao H X,He E K,et al.J.Power Sources,2016,326:78-83
[11]Shu H B,Li F,Hu C L,et al.Nanoscale,2016,8:2918-2926
[12]Wang Q H,Jiao L F,Han Y,et al.J.Phys.Chem.C,2011,115:8300-8304
[13]Han Y,Wang Y P,Gao W H,et al.Powder Technol.,2011,212:64-68
[14]Chen Q N,Chen W X,Ye J B,et al.J.Power Sources,2015,294:51-58
[15]Guan D S,Li J Y,Gao X F,et al.J.Alloys Compd.,2016,658:190-197
[16]Zai J T,Wang K X,Su Y Z,et al.J.Power Sources,2011,196:3650-3654
[17]Zhu W B,Yang Y W,Ma D M,et al.Ionics,2015,21:19-26
[18]Duan B C,Wang W K,Zhao H L,et al.Rare Metal Mater.Eng.,2012,41(3):93-100
[19]Wang L Y,Zhuo L H,Yu Y C,et al.Electrochim.Acta,2013,112:439-447
[20]Seo J W,Jang J T,Park S W,et al.Adv.Mater.,2008,20:4269-4273
[21]Kim T J,Kim C,Son D,et al.J.Power Sources,2007,167:529-535
[22]Xia J,Li G C,Mao Y C,et al.CrystEngComm,2012,14:4279-4283
[23]Ara M,Wadumesthrige K,Meng T J,et al.RSC Adv.,2014,4:20540-20547
[24]Du Y P,Yin Z Y,Rui X H,et al.Nanoscale,2012,5:1456-1459
[25]Kim H S,Chung Y H,Kang S H,et al.Electrochim.Acta,2009,54:3606-3610
[26]Zhang Q Q,Li R,Zhang M M,et al.Electrochim.Acta,2014,115:425-433
[27]Jiang X,Yang X L,Zhu Y H,et al.J.Power Sources,2013,237:178-186
[28]Tang H L,Qi X,Han W J,et al.Appl.Surf.Sci.,2015,355:7-13
[29]Chang K,Wang Z,Huang G C,et al.J.Power Sources,2012,201:259-266
[30]Ren Y D,Lv W M,Wen F S,et al.Mater.Lett.,2016,174:24-27
[31]Sun H Y,Ahmad M,Luo J,et al.Mater.Res.Bull.,2014,49:319-324
[32]Wei W,Jia F F,Wang K F,et al.Chin.Chem.Lett.,2017,28:324-328
[33]Geim A K.Science,2009,324:1530-1534
[34]Wu Z S,Zhou G M,Yin L C,et al.Nano Energy,2012,1:107-131
[35]Du N,Wu X L,Zhai C X,et al.J.Alloys Compd.,2013,580:457-464
[36]http://www.reagent.com.cn:666/ScrcBackGroup/reagent/cpzx/cpss.jsp
[37]Wang C R,Tang K B,Yang Q,et al.Chem.Phys.Lett.,2002,357:371-375
[38]Julien C,Mavi H S,Jain K P,et al.Mater.Sci.Eng.B,1994,23(2):98-104
[39]Li N,Jin S X,Liao Q Y,et al.Nano Energy,2014,5:105-115
[40]Lv P P,Zhao H L,Gao C H,et al.J.Power Sources,2015,274:542-550
[41]Ferrari A C,Meyer J C,Scardaci V,et al.Phys.Rev.Lett.,2006,97:187401
[42]Luo B,Fang Y,Wang B,et al.Energy Environ.Sci.,2012,5:5226-5230
[43]Huang Y G,Lin X L,Pan Q C,et al.Electrochim.Acta,2016,193:253-260
[44]Tao H C,Huang M,Fan L Z,et al.Solid State Ionics,2012,220:1-6
[45]Li M Q,Zeng Y,Ren Y R,et al.J.Power Sources,2015,288:53-61
[46]Lin J,Cui J L,Cheng F P,et al.J.Alloys Compd.,2017,695:2173-2179
[47]Cui J L,Cheng F P,Lin J,et al.Powder Technol.,2017,311:1-8
[48]Wang Y K,Ding J,Liu Y H,et al.Ceram.Int.,2015,41:15145-15152
[49]GENG Kai-Ming(耿凯明),WU Jun-Jie(吴俊杰),GENG Hong-Bo(耿洪波),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(9):1495-1502
[50]Hu Y S,Demir-Cakan R,Titirici M M,et al.Angew.Chem.Int.Ed.,2008,47:1645-1649
[51]Tao H C,Yang X L,Zhang L L,et al.Ionics,2014,20:1547-1552
[52]YANG Rong(杨蓉),LIU Xu-Wang(刘绪望),PENG Lu-Ming(彭路明).Chinese J.Inorg.Chem.(无机化学学报),2017,33(2):210-218
Electrochemical Performance of SnS2/GCP(Graphene Composite Powder)Microcomposite as Anode Material for Lithium-Ion Battery
LIN Jian CUI Yong-Fu CUI Jin-Long*WEN Zhong-Sheng SUN Jun-Cai*
(Institute of Materials and Technology,Dalian Maritime University,Dalian,Liaoning 116026,China)
SnS2/GCP(graphene composite powder)microcomposite was synthesized through a facile one-pot hydrothermal method.Graphene composite powder was used as an additive in the SnS2synthesis process.In the resulting SnS2/GCP microcomposites,the sphere-like SnS2particles were made of several intertwining SnS2nanosheets accompanied by the formation of many pores on the surface of it and GCP was homogeneously anchored on SnS2particles.When tested as anode material for lithium-ion batteries,the SnS2/GCP microcomposites showed a larger specific capacity (795.6 mAh·g-1at a current density of 0.1 A·g-1)and remarkable cycling stability (less than 1%capacity loss after 100 cycles)compared with pure SnS2.This superb energy storage performance could be mainly attributed to the existence of GCP that could not only prevent SnS2particles from aggregating,but also mitigate the volume changes of SnS2particles during charge/discharge cycle.Otherwise,the GCP could also ameliorate the electric conductivity of the SnS2particles.
lithium-ion battery;SnS2;anode material;graphene composite powder
O614.43+2
A
1001-4861(2018)01-0033-10
10.11862/CJIC.2018.019
2017-02-17。收修改稿日期:2017-08-28。
国家自然科学基金(No.51479019,21476035);中央高校基本科研业务费(No.3132016341)资助项目。
*通信联系人。 E-mail:sunjc@dlmu.edu.cn;jinlong_cuiyuan@hotmail.com

