手性化合物[Co(L/D-thr)3]·4.5H2O的合成、结构以及圆二色性
田菊梅 张景萍
(1厦门医学院口腔医学系,厦门 361023)
(2东北师范大学化学学院,长春 130024)
手性化合物[Co(L/D-thr)3]·4.5H2O的合成、结构以及圆二色性
田菊梅*,1张景萍2
(1厦门医学院口腔医学系,厦门 361023)
(2东北师范大学化学学院,长春 130024)
合成了2个基于手性配体L-和D-苏氨酸(L/D-thr)的Coバ配合物的对应异构体[Co(L-thr)3]·4.5H2O(L-1)和[Co(L-thr)3]·4.5H2O(D-1),并对2个化合物进行了单晶X射线衍射、红外、热重、紫外可见光谱以及CD谱性质研究。晶体结构分析表明,2个化合物分别结晶在四方晶系P43212和P41212手性空间群。固体CD谱测试进一步证实2个化合物具有手性。
圆二色;手性;氨基酸
0 Introduction
The homochirality of life,one ofthe great mysteries in science,has become a pressing problem in the field of biochemistry.Current life forms could exist in a world where chirality of monomers such as natural amino acids and sugars forming the biological building blocks of proteins and polysaccharides[1-3].Chirality is present in biological machinery extensively,but only one enantiomer of chiral amino acids is included in proteins[2,4-6].Therefore,a tremendous amount of work and interest focus on finding out the reason of this bias for one enantiomer[4].This further relates to the origin of life beings[7-11].
It is known that circular dichroism (CD)is the most powerful implement for probing the chirality of matter[7,12-13].Natural circular dichroism(NCD)related to natural optical activity is characterized as differentabsorption when chiral media are crossed by left(LCP)and right(RCP)circularly polarized lights.This effect is a consequence of lack of mirror symmetry[14-15].Another effectofresemblant phenomenology is magnetic circular dichroism (MCD),where LCP and RCP are differentially absorbed when chiral objects subject to a magnetic field[16-20].The chiral systems present different optical properties for light propagating parallel or antiparallel to the magnetic field direction[21-23].For a given relative direction of magnetic field,the enantiomers exhibit the opposite signs.This effect results from the breaking of the time-inversion symmetry of the medium[14,16-18,21,24-26].Magnetic induction of chiroptical response of chiral matter may be a plausible candidate for elucidating the homochirality of life[1,27-32].Consequently,chemical,biological,and physical scientists pay considerable attention on synthesis and characterization on the chiropticalactivity ofchiralcompounds.Atthe meantime,we focus our attention on exploring the change of chiroptical features under the condition of the magnetic field and without magnetic field.
Amino acids are known to play a vital role in biochemistry and life sciences[3,33-34].Threonine,which is one of twenty kinds of Amino acids,has a potential application in medicine,chemical reagents and food enhancer.L-threonine is found to be a part of several proteins of human being such as hemoglobin,insulin,β-lactoglobulin and γ-globulin[35].Furthermore,metalcontainingmetalloproteinsbonded to amino acid residues play pivotal roles in biological systems,especially cobalt-containing metalloproteins[36-40].Cobalt,instead of other metal ions,has been employed to gather information about the change of metal sites in proteins during protein function[39].Moreover,cobalt atom,which is mostly six-coordinated,can be easy to coordinate with the rich O and N atoms of threonine to form the mononuclear chiral compound.This simple system can eliminate many effects of complicated factorand predigestourinvestigation the chiral response to magnetic field.
With the consideration above in mind,we choose cobalt bonded to amino acid threonine(L/D-threonine)to successfully get a simple chiral Co-containing system,which is helpfulto understanding the correlation ofstructure-property and figuring out whether the chirality could be alternated under the induction of the magnetic field.In the present report,oxidation of Coギ to Coバ in the presence of L/D-threonine and [Mn3O(O2CMe)6(pyr)3](ClO4)produced chiral mononuclear Coバ enantiomer,[Co(L-thr)3]·4.5H2O(L-1)and[Co(D-thr)3]·4.5H2O(D-1).To the best of our knowledge,although early papers have focused on mononuclear Cobaltバ L-threonine complexes about Λ and Α-β isomers for the basic research,which have been isolated using different methods[41-42],void information exists about MCD in the enantiomer.It is surprising that we observe the change of the compared NCD and MCD in the diamagnetic Coバsystem.
1 Experimental
1.1 Reagents and physical measurements
All the reagents and solvents were of reagent grade and used as commercially purchased without further purification.
The elemental analyses (C,H and N)were performed on a Perkin-Elmer Model 240C elemental analyzer.The Co elemental analyses were determined by the Leaman inductively coupled plasma(ICP)spectrometer.Infrared spectra (400~4 000 cm-1)were measured on a Perkin-Elmer Fourier transform infrared (FTIR)spectrophotometer using KBr pellets(Fig.S1~S2,supporting information).Thermogravimetric analyses(TGA)were performed on a Perkin-Elmer TG-7 analyzer in flowing nitrogen atmosphere from room temperature to 800℃ with a heating rate of 10℃·min-1.Powder X-ray diffraction(PXRD)measurements were assessed on a Siemens D5005 diffractometer with Cu Kα (λ=0.154 184 nm)in 2θ range of 5°~50°at room temperature.The XRD accelerating voltage and emission current were 40 kV and 30 mA,respectively.
1.2 Synthesis of complexes L-1 and D-1
A mixture of L-threonine(0.059 g,0.5 mmol),Co(Ac)2·4H2O(0.25 g,1 mmol)and[Mn3O(O2CMe)6(pyr)3](ClO4)(0.087 g,0.1 mmol)in 40 mL of aqueous solution was heated at 85℃for 3 h.After the mixture was stirred at room temperature overnight,it was filtered.The filtrate was allowed to stand for several days.The red,rod-like,diffraction quality single crystals of L-1 were collected,washed with a small volume of water(Yield:65% based on Co).Anal.Calcd.(%):Co 11.92,C 29.16,H 6.73,N 8.50;Found(%):Co 11.38,C 29.71,H 6.47,N 8.45.Complex D-1 was prepared in a procedure similar to that of L-1,except that D-threonine was used instead ofL-threonine(Yield:60%based on Co).Anal.Calcd.(%):Co 11.92,C 29.16,H 6.73,N 8.50;Found(%):Co 11.02,C 29.62,H 6.24,N 8.20.
1.3 Details of X-ray crystallography
Single-crystal X-ray diffraction data for complexes L-1 and D-1 were measured on a Bruker SMART APEX CCD area detector diffractometer using graphite monochromated Mo Kα radiation(λ=0.071 073 nm)at 153 K.Structure solution (direct methods)and the refinement of full-matrix least-squares were carried out using the SHELXTL software package[43-45].All the non-hydrogen atoms were refined with anisotropic thermal parameters.The hydrogen atoms attached to carbon and nitrogen atoms were placed in geometrically calculated positions.For both complexes L-1 and D-1,the hydrogen atoms on one non-coordinated H2O molecule were not located.Crystallographic and refinement details for the two complexes are summarized in Table 1.Selected bond lengths and angles are listed in Table S1~S2(Supporting information).
CCDC:1556561,L-1;1556562,D-1.

Table1 Crystallographic data and structure refinement summary for complexes L-1 and D-1
2 Results and discussion
2.1 Synthesis
Heating aqueous solution mixture of L-threonine,Co(Ac)2·4H2O and[Mn3O(O2CMe)6(pyr)3]ClO4in the molar ratio of 5∶10∶1 afforded red rod-like crystals of[Co(L-thr)3]·4.5H2O(L-1).The complex[Co(D-thr)3]·4.5H2O(D-1)was prepared in a procedure similar to that of L-1,except that D-threonine was used instead of L-threonine.Although the element of[Mn3O(O2CMe)6(pyr)3]ClO4do not appear in the crystal structure of complexes L-1 and D-1,its presence in the reaction mixture is essential to generate the crystal product with a good quality.Single crystal X-ray diffraction studies reveal that both enantiopure complexes L-1 and D-1 are isolated by enantioselective synthesis in the presence of enantiopure chiral species,which crystallize in chiral space groups P43212 and P41212,with the absolute structure parameters of-0.002 0(15)and-0.002 0(16),respectively.In each compound,one asymmetric unit contains[Coバ(L-thr)3]or[Coバ(D-thr)3](Fig.1a~1b).
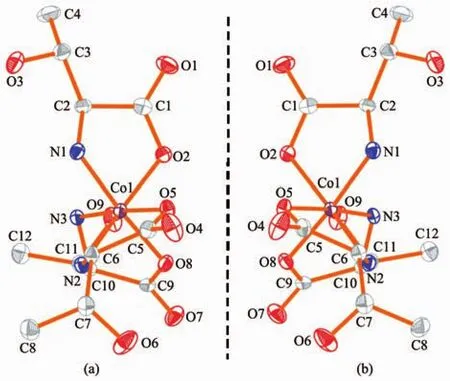
Fig.1 Ellipsoid of[Coバ(L-thr)3]in L-1(a)and[Coバ(D-thr)3]in D-1(b)along a axis,and the probability is 50%

Fig.2 Side view of 1D hydrogen bonding linked chains in L-1(a)and D-1(b)along a axis
2.2 X-ray crystal structures
The cobalt ion displays slightly distorted octahedral coordination geometry by coordination with three N and three O atoms from three L/D-threonine ligands.The Co-O distance is shorter than the Co-N distances in both complexes (Table S1 and S2)(Average Co-O distance:0.190 7 nm;Co-N distance:0.193 2 nm).It is noteworthy that the valence of cobalt has been assigned as+3 oxidation state according to charge balance consideration and crystallographic request as well as confirming by bond valence sum(BVS)calculations[46-48].Intermolecular hydrogen bonds play a dominating contribution on forming supramolecular topology(Fig.2a~2b). The hydrogen bonding linked inter-chains for L-1 and D-1 are showing in Fig.S3.
2.3 Thermal stability and PXRD analysis of L-1 and D-1
We recorded IR spectra of the two complexes compared with that of the free ligands L-thr and D-thr.There are three characteristic absorption bands observed forfreeligandsL/D-thr:thestretching vibration ν(N-H)at 3 027 cm-1,and asymmetric and symmetric stretching vibrations,νas(COO-)at 1 627 cm-1and νs(COO-)at 1 480 cm-1.The enantiomer L-1 and D-1 exhibit similar IR absorptions (Fig.S1~S2).Compared to the ligands L-and D-thr,the two new forming complexes are observed some changes of the characteristic absorption bands(Fig.S1).Therefore,the changes indicate that the coordination to the Coバions are amino nitrogen and carboxylate oxygen of free ligands L-thr/D-thr to form novel complexes.Thermal stabilities measurements indicate that lattice water molecules are easy to release in the temperature range of 25~104 ℃ for the two compounds (Fig.S4).The phase purity is confirmed by powder X-ray diffraction(PXRD)atroom temperature.The experimental patterns of L-1 and D-1 are in fairly good agreement with the simulated ones generated from single-crystal diffraction data(Fig.S5~S6).
2.4 Circular dichroism of L-1 and D-1
Solid-state UV-Vis spectra were recorded for the two complexes L-1 and D-1 at room temperature as shown in Fig.3a.The two complexes show similar absorption bands.There are three peaks at 230,375,521 nm.The broad band at 521nm observed for L-1/D-1 could be attributed to1A1g→1T1gtransition in a distorted octahedral geometry around Coバion[49-51].The two bands with high energy are assigned to ligand to metal transition.In respect that compounds L-1 and D-1 are both insoluble in water and common organic solvents,in order to examine the chiroptical activities,the solid state circular dichroism (CD)spectra of the two compounds were measured by mixing enantiopure crystal pressed in KBr matrices at room temperature.Asshown in Fig.3b,the two compoundsshow pronounced optical activity in the solid state and afford approximately mirror-image CD spectra of each other.It is found that L-1 reveals three negative Cotton effects at 296,369 and 524 nm,respectively.While the D-1 shows opposite Cotton effects at the same wavelengths,indicating that the two compounds are enantiomer.Fig.3c shows the result of MCD for the enantiomer L-1 and D-1.Then,the same disks are put under the 0.7 T magnetic field,which have been employed CD measurement.This feature is observed within the experimental accuracy.It is very interesting to note that the peak at 296 nm has disappeared and new absorption peak has emerged at 420 nm for the enantiomers.To our surprise,when we removed the magnetic field,the CD spectra of the enantiomer have recur as the line shapes of the first CD spectra(Fig.3b and 3d),except that the spectra exhibit slight bathochromic shifts(20 nm)near 316 nm in the UV region.
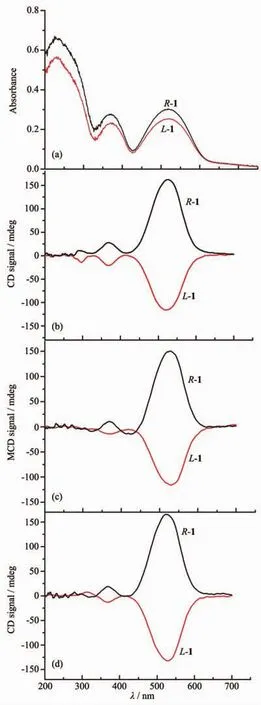
Fig.3 UV-Vis(a),CD(b),MCD(c)and CD(d)spectra of L-1 and D-1
3 Conclusions
In summary,we synthesize a couple of enantiomers L-1 and D-1.In order to examine the chiroptical activities,we measured the solid state CD and MCD spectra,which are both of opposite sign for the enantiomers.Compared to the CD spectra,MCD spectra of the enantiomers show new absorption peaks at 420 nm,while the peaks at 296 nm have disappeared.The essence of the alternate signal of the CD spectra with or without magnetic induction is still an open question for the scientists,which is interesting from the viewpointofpossible contribution for explaining the question above mentioned.The further work,which relates to embed other paramagnetic metal ion coordination with the remaining coordination site to isolate homochiral magnetic complexes,is in progress with an eye on their eventual applications in life sciences.
Supporting information is available at http://www.wjhxxb.cn
[1]Feringa B L.Science,2001,292:2021-2022
[2]Miles A J,Wallace B A.Chem.Soc.Rev.,2016,45:4859-4872
[3]Hou S S,Hsu Y Y,Lin J H,et al.ACS Macro Lett.,2016,5:1201-1205
[4]Foroughi L M,Matzger A J.Nat.Chem.,2011:663-665
[5]Diaz C,Vesga Y,Echevarria L,et al.RSC Adv.,2015,5:17429-17437
[6]Narushima T,Okamoto H.Sci.Rep.,2016,6:35731
[7]Saito M,Ishikawa K,Taniguchi K,et al.Phys.Rev.Lett.,2008,101:117402(4 pages)
[8]Yashima E.Nat.Chem.,2011,3:12-14
[9]Mashhadi Z,Newcomer M E,Brash A R.ChemBioChem,2016,17:2000-2006
[10]Murayama T,Pujals S,Hirose H,et al.Pept.Sci.,2016,106:430-439
[11]Cukras J,Kauczor J,Norman P,et al.Phys.Chem.Chem.Phys.,2016,18:13267-13279
[12]JI Qin(吉沁),CHEN Li-Zhuang(陈立庄).Chinese J.Inorg.Chem.(无机化学学报),2017,33:874-880
[13]WANG Chuan-Zhong(王传忠),ZHU Zhi-Ang(朱志昂),LI Ying(李瑛),et al.Chinese J.Inorg.Chem.(无机化学学报),2001,17:244-248
[14]Rikken G L J A,Raupach E.Nature,1997,390:493-494
[15]Shoji K,Nakayama M,Koseki T,et al.Polymer,2016,97:20-30
[16]Train C,Gruselle M,Verdaguer M.Chem.Soc.Rev.,2011,40:3297-3312
[17]Train C,Gheorghe R,Krstic V,et al.Nat.Mater.,2008,7:729-734
[18]Vallet M,Ghosh R,Le Floch A,et al.Phys.Rev.Lett.,2001,87:183003(4 pages)
[19]RutardottirS,KarnaukhovaE,NantasenamatC,et al.Biochim.Biophys.Acta:Proteins Proteomics,2016,1864:29-41
[20]Baker T M,Nakashige T G,Nolan E M,et al.Chem.Sci.,2017,8:1369-1377
[21]Rikken G L J A,Raupach E.Phys.Rev.E,1998,58:5081-5084
[22]Lee H,Jeon S,Friedman B,et al.Sci.Rep.,2017,7:43804
[23]Liu X L,Tsunega S,Jin R H.Nanoscale Horiz.,2017,2:147-155
[24]Kleindienst P,Wagniere G H.Chem.Phys.Lett.,1998,288:89-97
[25]Zhang H,Xin X,Sun J,et al.J.Colloid Interface Sci.,2016,484:97-106
[26]Zahid M T,Shakoori F R,Zulifqar S,et al.J.Cell.Biochem.,2016,117:1843-1854
[27]Kitagawa Y,Segawa H,Ishii K.Angew.Chem.Int.Ed.,2011,50:9133-9136
[28]Guijarro A,Yus M.Origin of Chirality in the Molecules of Life.Cambridge:RSC Publishing,2009.
[29]Ribó J M,Crusats J,Sagués F,et al.Science,2001,292:2063-2066
[30]Tsuda A,Alam M A,Harada T,et al.Angew.Chem.Int.Ed.,2007,46:8198-8202
[31]Escudero C,Crusats J,Díez-Pérez I,et al.Angew.Chem.Int.Ed.,2006,45:8032-8035
[32]Crusats J,Claret J,Díez-Pérez I,et al.Chem.Commun.,2003:1588-1589
[33]Tanaka M,Demizu Y,Doi M,et al.Angew.Chem.Int.Ed.,2004,43:5360-5363
[34]Pilicer S L,Bakhshi P R,Bentley K W,et al.J.Am.Chem.Soc.,2017,139:1758-1761
[35]Jinnah M M A,Umadevi M,Ravikumar B,et al.Spectroc.Acta A,2004,60:2977-2983
[36]Rulíšek L,Vondráek J.J.Inorg.Biochem.,1998,71:115-127
[37]Payne M S,Wu S,Fallon R D,et al.Biochemistry,1997,36:5447-5454
[38]Gavel O Y,Bursakov S A,Calvete J J,et al.Biochemistry,1998,37:16225-16232
[39]Rizzi A C,Brondino C D,Calvo R,et al.Inorg.Chem.,2003,42:4409-4416
[40]Solheim H,Kornobis K,Ruud K,et al.J.Phys.Chem.B,2010,115:737-748
[41]Gillard R D,Laurie S H,Price D C,et al.J.Chem.Soc.Dalton Trans.,1974:1385-1396
[42]Yoshimura Y.Bull.Chem.Soc.Jpn.,2004,77:1861-1867
[43]Sheldrick G M.SHELXS-97 and SHELXL-97,University of Göttingen,Germany,1997.
[44]Huang Y Q,Cheng H D,Chen H Y,et al.CrystEngComm,2015,17:5690-5701
[45]Huang Y Q,Wan Y,Chen H Y,et al.New J.Chem.,2016,40:7587-7595
[46]Tandon S S,Bunge S D,Rakosi R,et al.Dalton Trans.,2009:6536-6551
[47]Alley K G,Bircher R,Waldmann O,et al.Inorg.Chem.,2006,45:8950-8957
[48]Abedin T S M,Thompson L K,Miller D O.Chem.Commun.,2005:5512-5514
[49]Chaudhuri P,Querbach J,Wieghardt K,et al.J.Chem.Soc.Dalton Trans.,1990:271-278
[50]Luo J,Rath N P,Mirica L M.Inorg.Chem.,2011,50:6152-6157
[51]Kember M R,White A J P,Williams C K.Macromolecules,2010,43:2291-2298
Syntheses,Structures and Circular Dichroism Spectroscopy of Chiral Complexes[Co(L/D-thr)3]·4.5H2O
TIAN Ju-Mei*,1ZHANG Jing-Ping2
(1Department of Stomatology,Xiamen Medical College,Xiamen,Fujian 361023,China)
(2Faculty of Chemistry,Northeast Normal University,Changchun 130024,China)
A couple of enantiomer[Co(L-thr)3]·4.5H2O(L-1)and[Co(L-thr)3]·4.5H2O(D-1)consisting the Coバion,based on chiral ligand L/D-threonine(L/D-thr),were prepared and characterized by single-crystal X-ray structure analysis,IR,thermogravimetric analysis,solid-state UV-Vis spectra and circular dichroism analysis.Complexes L-1 and D-1 crystalize in tetragonal system P43212 and P41212,respectively.Moreover,the circular dichroism analysis of complexes L-1 and D-1 was measured with chirality,and the CD spectra of the enantiomer show change under the magnetic field inducing.CCDC:1556561,L-1;1556562,D-1.
circular dichroism;chiral;amino acid
O614.81+2
A
1001-4861(2018)01-0195-06
10.11862/CJIC.2018.010
2017-06-29。收修改稿日期:2017-08-31。
福建省卫生计生青年科研课题(No.2017-2-115)、厦门医学院校内科研平台课题(No.KYPT2015-02)和厦门医学高等专科学校自然科学研究项目(No.K2015-13)资助。
*通信联系人。 E-mail:tjm@xmmc.edu.cn

