异构多羧酸配体的两种Znギ配位聚合物的水热合成和表征
杨诗吟 才 华 张 琦 周玉萍 任晓晨 谢璐璐
(中国民航大学理学院,天津 300300)
异构多羧酸配体的两种Znギ配位聚合物的水热合成和表征
杨诗吟 才 华*张 琦 周玉萍 任晓晨 谢璐璐
(中国民航大学理学院,天津 300300)
利用异构的半刚性多羧酸配体3,5-二(3-羧基苯氧基)苯甲酸(3-H3BCP),3,5-二(4-羧基苯氧基)苯甲酸(4-H3BCP)和刚性双三唑配体4-(4-(4H-1,2,4-三唑-4-基)苯基)-4H-1,2,4-三唑(L)在相似的水热条件下与金属Znギ离子反应,制得混合配体的配位聚合物{[Zn(3-HBCP)(L)]·0.5H2O}n(1)和[Zn(4-HBCP)(L)0.5]n(2)。配合物1为含有双核簇的(4,4)连接的二维网络结构,而配合物2为三重互穿的2D+2D→2D平行网络结构。通过X射线单晶衍射、元素分析、红外光谱、紫外光谱以及荧光光谱其进行了表征。
异构体;半刚性多羧酸;发光性能
0 Introduction
As a class of novel solid materials,functional coordination polymers(CPs)have received great attention because of their interesting structures as well as potentialapplications in storage,gasseparation,magnetism catalysis,microelectronics sensing,and optical properties[1-6].Recently,semi-rigid multi-carboxylateligandswith twoormore aromatic rings separated by O atoms have been employed to buildinteresting coordination frameworks,especially some flexible networks with breathing[7-11].Besides,when the auxiliary ligands were introduced to build these networks,the final packing architectures have greater tenability[12-15].Recent study shows that auxiliary bridging triazole linkers holding different lengths and flexibilities have great effects on the final packing supramolecular and topology as well as coordination modes and molecular conformations of aromatic multicarboxylate acids[16-17].As we all known,the mixed ligand strategy have added the scope of the functional CPs,giving diverse polymeric structures with interesting structures and unusual properties.Therefore,it is worth trying to prepare novel functional metal-organic hybrid complexes by using such kind of isomeric semi-rigid multi-carboxylate and auxiliary bridging triazole linkers.The aforementioned points inspired us to assembly novel coordination frameworks with semirigid 3,5-bi(3-carboxyphenoxy)benzoic acid(3-H3BCP),3,5-bi(4-carboxyphenoxy)benzoic acid(4-H3BCP)and auxiliary bridging triazole linkers(L).Herein,under similar solvothermal conditions,two novel mixedligand Znギcoordination polymers,namely,{[Zn(3-HBCP)(L)]·0.5H2O}n(1)and[Zn(4-HBCP)(L)0.5]n(2)have been isolated.Complexes 1 and 2 are prepared and have been investigated by elemental analysis,FTIR thermal analysis and fluorescence characterization.
1 Experimental
With the exception of the ligand L,which was prepared according to the reported method[18],all reagents and solvents for synthesis and analysis were commercially available and used as received.Fourier transform(FT)IR spectra(KBr pellets)were taken on an AVATAR-330(Nicolet)spectrometer.Microanalyses of C,H,and N were carried out on a CE-440(Leemanlabs)analyzer.Thermogravimetric analysis(TGA)was carried out on a Dupont thermal analyzer from room temperature to 600℃N2atmosphere at a heating rate of 10℃·min-1.Solid-state UV-Vis diffuse reflectance spectra was performed at room temperature using Shimadzu UV-3600 double monochromator spectrophotometer,BaSO4wasused asa100%reflectance standard for all material.Fluorescence spectra of the polycrystalline powder samples were performed on a HITACHI spectrofluorimeter(F7000)equipped with a xenon lamp and quartz carrier at room temperature.
1.1 Synthesis of{[Zn(3-HBCP)(L)]·0.5H2O}n(1)
A mixture containing Zn(OAc)2·2H2O(22 mg,0.10 mmol),L (21.2 mg,0.10 mmol),3-H3BCP(39.4 mg,0.10 mmol)and H2O (10 mL)was sealed in a Teflon-lined stainless steel vessel(20 mL),which was heated at 160℃for 3 days and then cooled to room temperature at a rate of 5 ℃·h-1.Colorless block crystals of 1 was obtained with 47%yield (18.4 mg,based on 3-H3BCP).Anal.Calcd.for C26H17ZnN3O8.50(%):C,55.08;H,3.21;N,6.06.Found(%):C,55.23;H,3.26;N,6.03.IR (KBr,cm-1):3 637m,3 420b,3 211m,1 624s,1 504m,1 434s,1 328s,1 248m,1 154m,1 095w,1 077m,1 053w,1 016m,960m,932 w,904w,778s,744m,690m,640m.
1.2 Synthesis of[Zn(4-HBCP)(L)0.5]n(2)
The same synthetic method as that for 1 was used except that 3-H3BCP was replaced by 4-H3BCP(39.4 mg,0.10 mmol).Colorless block crystals of 2 were obtained with 45%yield (17.6 mg,based on 4-H3BCP).Anal.Calcd.for C26H16ZnN3O8(%):C,55.39;H,2.86;N,7.45.Found(%):C,55.45;H,2.73;N,7.41.IR(KBr,cm-1):3 216m,1 642m,1 576m,1 411s,1 398 s,1 319m,1 259w,1 211m,1 159w,1 121w,1 085w,999m,870m,842m,809m,797m,778m,708w,643w,543w,498w.
1.3 X-ray crystallography
Single-crystal X-ray diffraction data for complexes 1 (Crystal size:0.36 mm×0.28 mm×0.28 mm)and 2(Crystal size:0.22 mm×0.16 mm×0.10 mm)were collected on a Bruker ApexⅡCCD diffractometer at 296(2)K with Mo Kα radiation(λ=0.071 073 nm).There was no evidence of crystal decay during data collection.In general,a semi-empirical absorption correction(SADABS)was applied and the program SAINT was used for integration of the diffraction profiles[19].The structures were solved by direct methods using the SHELXS program of the SHELXTL package and refined with SHELXL[20].The final refinement was performed by full-matrix least-squares methods on F2with anisotropic thermal parameters for all non-H atoms.Hydrogen atoms attached to carbon were generated geometrically and those of methanol or water were first located in difference Fourier syntheses and then treated as riding.Isotropic displacement parameters of H were derived from their parent atoms.A summary of the crystallographic data are shown in Table 1.Selected bond parameters are listed in Table 2,and hydrogen bonds are listed in Table 3.
CCDC:1542779,1;1542780,2.

Table1 Crystallographic data and structural refinement summary for complexes 1~2

Table2 Selected bond distances(nm)and angles(°)for 1~2
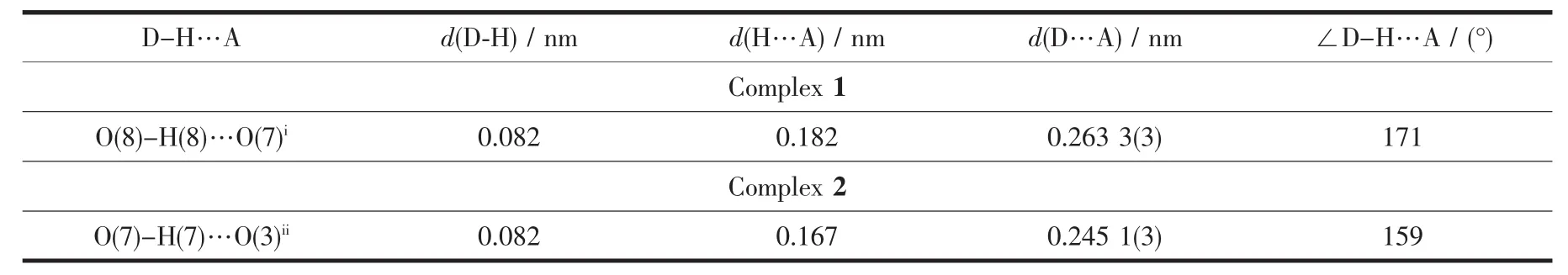
Table3 Hydrogen bond geometries in the crystal structure of 1~2

Fig.1 Crystal structure of 1:(a)Coordination environment of Znギin 1 showing 30%probability displacement ellipsoids;(b)Perspective view of the 2D coordination network;(c)3D supramolecular framework of complex 1 formed through hydrogen-bonding
2 Results and discussion
2.1 Description of the structure
Single X-ray diffraction analysis reveals that complex 1 belongs to space group P1 and possesses a 2D cluster-based Znギcoordination framework.As shown in Fig.1a,the coordination sphere of Zn(1),residing in a tetrahedral coordination environment,is four-coordinated by three oxygen atoms (O1,O2iand O4ii)of three separated 3-HBCP ligands,one nitrogen atom (N1)of L forming ZnN3O donor set.Zn-O bond lengths are in the range of 0.191 97(17)~0.193 50(17)nm and Zn-N bond distances are 0.202 4(2)nm.As shown in Scheme 1a,3-HBCP ligands adopt tridentate η2,η1-bridging coordination mode.Two neighboring Znギcentres are linked by their two carboxyl groups of 3-HBCP ligands to construct dinuclear[Zn(CO2)]2clusters.Such a unit of[Zn(CO2)]2cluster can be viewed as a 4-connecting node to link four other equivalent ones through one pairs of L ligands and two pairs of 3-HBCP ligands,forming a 2D(4,4)layer structure extending along ab plane(Fig.1b).Each grid within this layer has dimensions of 1.054 nm×1.605 nm.One mono-deprotonated 3-HBCP anion is also involved in hydrogen bonding (O8-H8…O7i,Symmetry codes:i-x,3-y,3-z)with another monodeprotonated 3-HBCP anion.These hydrogen bonds connect these neighboring 2D layersinto a 3D supramolecular network(Fig.1c).Therefore complex 1 features a 3D supramolecular framework arising from 2D coordination sheets interlinked through interlayer hydrogen bonds.

Fig.2 Crystal structure of 2:(a)Coordination environment of Znギin 2 showing 30%probability displacement ellipsoids;(b)Perspective view of the 2D layer;(c)Perspective view of the 2D+2D→2D parallel entangled networks;(d)3D supramolecular framework of complex 2 formed through hydrogen-bonding and π…π stacking interactions
Structure analysis reveals complex 2 crystallizes in the monoclinic system,space group P21/c.The asymmetric unit of 2 consists of one Znギion,one 4-HBCP2-ligand,and a half of L ligand (Fig.2a).As shown in Fig.2a,each Znギ centerispentacoordinated by four oxygen atoms from three different 4-HBCP2-linkers,and one nitrogen atom from L ligand and resides in a distorted square-pyramidal coordination environment(τ=0.14)[21].The square base of Zn(1)is nonplanar and occupied by O(1),O(2),O(4)and O(5)from three 4-H3BCP ligands.The axial positions of Zn(1)are coordinated by N(1)from one L ligand.Zn-O bond lengths are in the range of 0.197 3(2)~0.236 0(2)nm and Zn-N bond distances are 0.199 8(2)nm.The 4-H3BCP ligand is partially deprotonated and twisted with the dihedral angle between three phenyl rings are 87.9(1)°,73.9(1)°,and 41.0(9)°,respectively.Three carboxyl groups coordinated with three Znギions with μ1-η1∶η0and μ1-η1∶η1coordination modes(Scheme 1b),leaving a 1D[Zn(4-HBCP)]nladder chain with the Zn…Zn distance is 1.078 4(1)nm.Then L ligand linked the 1D ladder chains,forming an interestingly 2D sheet with the 60-membered large macrocycle(1.353 8(2)nm×1.887(3)nm)(Fig.2b).
Each sheet can be defined as a(3,4)-connected(42.63.8)(42.6)2D layer with the Znギion and 4-HBCP2-ligand regarded as four and three nodes,respectively.The large macrocycle within each sheet and the 1D[Zn(4-HBCP)]nladder chain make the neighboring layers possible to interpenetrate with each other.Each sheet simultaneously penetrated with two another adjacent ones,finally formed a rarely reported 3-fold 2D→2D parallel entangled network(Fig.2c).
As illustrated in Fig.2d,there also exist O-H…O hydrogenbonds(O7-H7…O3i,Symmetry codes:ix,1+y,z)and π…π stacking between phenyl ring(C20,C21,C22,C23,C24,C25;x=0.833 12(12),y=0.612 95(6),z=0.258 62(12))and triazol ring(N1,N2,C2,N3,C1;x=0.488 75(10),y=0.375 11(5),z=0.718 76(9))(center-to-center distances,0.380 8(2)nm;dihedral angle,8.61°)interactions,which further stabilize this 2D framework.The 2D arrays are interdigitated with the presence ofinterlayer π-π stacking interactions between the adjacent 4-H3BCP molecules and form a 3D supramolecularstructure.Thecenter-to-center distances of phenyl(C13,C14,C15,C16,C17,C18;x=0.861 45(11),y=0.104 25(6),z=0.294 78(12))-phenyl(C20,C21,C22,C23,C24,C25;x=0.833 12(12),y=0.612 95(6),z=0.258 62(12))rings are 0.377 9(2)nm and dihedral angles are 8.48°.
2.2 Role of metal ion and substituent of ligand on structural assembly
It is noteworthy that dramatic structural differences are observed in the coordination polymers 1~2,based on the selection of semi-rigid tricarboxylate ligands with two different coordination modes as building blocks.These tectons show different binding fashions as illustrated in Scheme 1.For 1,the monodentate and bidentate bridging anions are observed in 1,where the μ3-3-HBCP (Scheme 1a)anions afford the 1D chain array.However,the chelating and monodentate anions are found in 2,and the Zn(Ⅱ)metal centers are extended by μ3-4-HBCP(Scheme 1b)linkers to result in a 1D ladder chains.

Scheme 1 Different coordination modes for two isomeric multi-carboxylate 3-H3BCP and 4-H3BCP

Fig.3 Thermogravimetric analysis(TGA)curves of complexes 1~2
2.3 FT-IR,TGA and fluorescence properties studies
In the FT-IR spectra of the complexes 1~2,there are broad,medium bands around 3 000~3 400 cm-1(3 637,3 420,3 211 cm-1for 1 and 3 216 cm-1for 2),showing the existence of O-H and coordinated water molecules in the coordination framework.For 1 and 2,the asymmetric and symmetric stretching vibrations of carboxylate groups are observed in the ranges of 1 504~1 642 cm-1and 1 315~1 434 cm-1,respectively.The absence of strong absorption bands around 1 700 cm-1indicates complete deprotonation of the carboxylic groups in 1 and 2[22].All the FT-IR results are in agreement with the X-ray crystal result.
There was no evidence of crystal decay during X-ray data collection for 1 and 2,which are stable at ambient conditions.Thus,thermogravimetric analysis(TGA)experimentsofthese Znギ coordination polymershave been performed to explore their thermal stabilities (Fig.3).For 1,the host framework remains stable until the decomposition occurs at 256℃with a slow weight loss ending at 403℃,then following with a sharp weight loss not ending until 600℃.With regard to 2,the complex is thermally stable upon heating to 369℃followed by a sharp weight loss ending at 401℃,then following with a slow weight loss not ending until 600℃.
Inorganic-organic hybrid coordination polymers have been investigated for fluorescence properties and for potential applications as luminescent materials,such as light-emitting diodes(LEDs).Owing to the abilityofaffectingtheemission wavelength and strength of organic materials,syntheses of inorganicorganic coordination polymers by the judicious choice of conjugated organic spacers and transition metal centers can be an efficient method for obtaining new types of electroluminescent materials,especially for d10or d10-d10systems[23].In the present work,we have explored the luminescent properties of free ligands L,3-H3BCP and 4-H3BCP and organic/inorganic coordination polymers 1~2 based on the ligands in the solid state.
As is shown in Fig.4,at ambient temperature,the free ligands L,3-H3BCP and 4-H3BCP in the solid state are luminescent and show the broad emission maximum at 318,354 and 352 nm,respectively(λex=279 nm).For these ligands the chromospheres are the aromatic rings and the observed emission is due to the π-π*transition.Solid-state fluorescence spectra of 1~2 at room temperature have been determined.In comparison with those of free ligand L and 3-H3BCP,1 shows strong emission bands centered at 334 nm(λex=279 nm),which should be ascribed to ligand-tometal charge-transfer (LMCT)bands.Complex 2 shows strong emission bands centered at 353 nm(λex=279 nm),which is somewhat similar with that of 4-H3BCP,which should be ascribed to intraligand fluorescent emissions based on 4-H3BCP.

Fig.4 Solid-state fluorescent emissions of complexes 1(a)and 2(b)at room temperature
2.4 UV-Vis absorption spectra
The UV-Vis absorption spectra of complexes 1~2 show intense wide absorption peaks in the range of 220~350 nm for 1 and 2,which can be assigned as ligand-to-metal charge-transfer(LMCT)transitions[24-25],While lower energy bands(270~300 nm for 1,250~300 nm for 2)are assigned as the π →π*electron transition between ligands(Fig.5).

Fig.5 UV-Vis absorption spectra of complexes 1~2
3 Conclusions
In summary,one rigid bis(triazole)ligand 4-(4-(4H-1,2,4-triazol-4-yl)phenyl)-4H-1,2,4-triazole(L)and two isomeric semi-rigid 3,5-bi(3-carboxyphenoxy)benzoic acid(3-H3BCP),3,5-bi(4-carboxyphenoxy)benzoic acid(4-H3BCP)have been employed to prepare two distinct mixed-ligand luminescent coordination polymers{[Zn(3-HBCP)(L)]·0.5H2O}n(1)and[Zn(4-HBCP)(L)0.5]n(2).Both 1 and 2 exhibits network topology as well as high thermal stabilities and strong fluorescent emissions.This work clearly demonstrates that 4-(4-(4H-1,2,4-triazol-4-yl)phenyl)-4H-1,2,4-triazole ligand and various isomeric semi-rigid aromatic poly-carboxylate ligands can be applied as versatile building blocks to constructthese coordination polymerswith interestingnetwork structuresand unique functional properties.
[1]Dau P V,Cohen S M.Chem.Commun.,2013,49:6128-6130
[2]Férey G,Serre C.Chem.Soc.Rev.,2009,38:1380-1399
[3]Fei H H,Cahill J F,Prather K A,et al.Inorg.Chem.,2013,52:4011-4016
[4]Sun D,Yuan S,Wang H,et al.Chem.Commun.,2013,49:6152-6154
[5]Wang K,Zeng S Y,Wang H L,et al.Inorg.Chem.Front.,2014,1:167-171
[6]Zou J Y,Shi W,Gao H L,et al.Inorg.Chem.Front.,2014,1:242-248
[7]Chen M,Zhao H,Liu C S,et al.Chem.Commun.,2015,51:6014-6017
[8]Cui J H,Yang Q X,Li Y Z,et al.Cryst.Growth Des.,2013,13:1694-1702
[9]Wang Z W,Chen M,Liu C S,et al.Chem.Eur.J.,2015,21:17215-17219
[10]Chaudhari A K,Nagarkar S S,Joarder B,et al.Cryst.Growth Des.,2013,13:3716-3721
[11]Fan L M,Fan W L,Song W K,et al.Dalton Trans.,2014,43:15979-15989
[12]Guo X M,Guo H D,Zou H Y,et al.CrystEngComm,2013,15:9112-9120
[13]Cui J H,Li Y Z,Guo Z J,et al.Chem.Commun.,2013,49:555-557
[14]Cao L H,Li H Y,Zang S Q,et al.Cryst.Growth Des.,2012,12:4299-4301
[15]Hong D H,Suh M P.Chem.Commun.,2012,48:9168-9170
[16]Peng Y F,Li K,Zhao S,et al.Spectrochim.Acta Part A,2015,147:20-25
[17]Ren C,Liu P,Wang Y Y,et al.Eur.J.Inorg.Chem.,2010:5545-5555
[18]Bozena M B,Ewa J W,Ewa T W.Acta Polym.Pharm.,2000,57:199-204
[19]Bruker AXS.SAINT Software Reference Manual,Madison,WI,1998.
[20]Sheldrick G M.SHELXTL NT Ver.5.1,Program for Solution and Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[21]Addison A W,Rao T N,Reedijk J,et al.J.Chem.Soc.Dalton Trans.,1984:1349-1356
[22]Niu D,Yang J,Guo J,et al.Cryst.Growth Des.,2012,12:2397-2410
[23]WANG Xin-Ping(王新萍),LI Ying-Ying(李莹莹),LIU Yong(刘勇),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(5):823-829
[24]CHEN Man-Sheng(陈满生),HUANG Xiu-Yu(黄秀玉),CHEN Xiao-Li(陈小利),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(6):1090-1096
[25]Jeremic'D A,Kaluđerovic'G N,Gómez-Ruiz S,et al.Cryst.Growth Des.,2010,10:559-563
Solvothermal Synthesis and Characterization of Two ZnギCoordination Polymers with Isomeric Multi-carboxylate Ligands
YANG Shi-Ying CAI Hua*ZHANG QiZHOU Yu-Ping REN Xiao-Chen XIE Lu-Lu
(College of Science,Civil Aviation University of China,Tianjin 300300,China)
Two isomeric semi-rigid multi-carboxylate ligands 3,5-bi(3-carboxyphenoxy)benzoic acid(3-H3BCP),3,5-bi(4-carboxyphenoxy)benzoic acid(4-H3BCP)and one rigid bridging bis-triazole 4-(4-(4H-1,2,4-triazol-4-yl)phenyl)-4H-1,2,4-triazole(L)have been employed to react with Znギsalts under similar solvothermal reactions.Two novel zincギ mixed-ligand coordination polymers,namely,{[Zn(3-HBCP)(L)]·0.5H2O}n(1)and[Zn(4-HBCP)(L)0.5]n(2)have been isolated.Complex 1 displays a(4,4)-connected 2D cluster-based network while 2 displays an usual 3D fold 2D+2D→2D parallel entangled network.Solid-state luminescent properties and thermal analyses of 1~2 also have been determined indicating strong fluorescent emissions and good thermal stabilities.Different coordination modes of two semi-rigid multi-carboxylate ligands and L also have been briefly discussed,which also reveal great potential in the construction of these novel mixed-ligand luminescent frameworks with diverse structural motifs and unique functional properties.CCDC:1542779,1;1542780,2.
isomer;semi-rigid multi-carboxylate;photoluminescent properties
O614.24+1
A
1001-4861(2018)01-0179-08
10.11862/CJIC.2018.007
2017-06-12。收修改稿日期:2017-09-19。
中央高校专项研究基金(No.3122017071)、本科生创新创业培训计划(No.20161005959)、天津市分子结构与功能材料重点实验室开放基金(天津师范大学)和国家自然科学基金青年基金(No.21501196)资助项目。
*通信联系人。 E-mail:caihua-1109@163.com

