参与下丘脑-垂体-甲状腺轴负反馈调控的分子元件研究进展
左卫星, 张志飞, 刘志民, 王超群
1.中国人民解放军第五四六医院, 乌鲁木齐 841700;2.潍坊医学院临床医学院, 山东 潍坊 261053;3.第二军医大学附属长征医院, 上海 200003;4.第二军医大学附属长海医院, 上海 200433
参与下丘脑-垂体-甲状腺轴负反馈调控的分子元件研究进展
左卫星1, 张志飞2, 刘志民3*, 王超群4*
1.中国人民解放军第五四六医院, 乌鲁木齐 841700;2.潍坊医学院临床医学院, 山东 潍坊 261053;3.第二军医大学附属长征医院, 上海 200003;4.第二军医大学附属长海医院, 上海 200433
下丘脑-垂体-甲状腺(hypothalamic-pituitary-thyroid,HPT)轴负反馈调节是维持血清甲状腺激素(thyroid hormone,TH)水平稳定的最重要的机制。目前,普遍认为位于下丘脑室旁核(paraventricular nuclei,PVN)的促垂体区的促甲状腺激素释放激素(thyrotropin-releasing hormone,TRH)神经元是HPT轴的核心调节区域。研究认为在血液循环中,不仅三碘甲状腺原氨酸(T3)参与HPT轴的负反馈调节,甲状腺素(T4)也可通过中枢神经系统伸长细胞的脱碘酶2(Dio2)催化脱碘来影响细胞中T3的可用性,从而参与其中。促垂体区的TRH神经元通过甲状腺激素转运体摄取循环中的TH,而TH进入PVN的TRH神经元或垂体促甲状腺区细胞核与甲状腺激素受体(TRs)(特别是TRβ2)结合后,可招募辅因子,共同参与相应靶基因的调控。此外,中枢神经系统伸长细胞表达的焦谷氨酰肽酶Ⅱ(PPⅡ)可降解释放的TRH,从而影响不同甲状腺功能状态下到达垂体门静脉的TRH水平。综述了参与HPT轴调节的分子元件,以期为甲状腺功能或甲状腺轴异常疾病的科学研究及临床治疗提供参考。
HPT轴;负反馈;甲状腺激素;脱碘酶
反馈调节是生命系统中非常普遍的调节机制,它对于机体维持稳态具有重要意义。在下丘脑-垂体-甲状腺轴中,血液循环中的甲状腺激素水平变化能反馈调节下丘脑释放的TRH和腺垂体分泌的促甲状腺激素(thyroid stimulating hormone,TSH),其涉及的信号通路精密而准确,因此,探究HPT轴调控的分子通路有助于明确下丘脑-垂体-甲状腺轴的中枢调控机制,可为临床上治疗甲状腺功能异常或甲状腺轴异常疾病提供理论基础。虽然HPT轴的反馈调节现象早已被发现,但其分子机制的研究仍在不断推进。故本文将对参与HPT轴中枢负反馈调控的重要信号分子(包括促甲状腺激素释放激素、脱碘酶、甲状腺激素转运体、甲状腺激素受体、辅因子、焦谷氨酰肽酶Ⅱ)进行综述,以期为相关研究提供参考。
1 HPT轴中枢调控模式概述
下丘脑室旁核(PVN)促垂体区的TRH神经元是分泌TRH的主要部位(图1)。先前的研究发现人的TRH基因的启动子区有3个负向甲状腺激素反应元件(TREs),而甲状腺激素受体(TRs)能够以单体、同二聚体、异二聚体的形式与TREs结合,进而介导甲状腺激素对TRH靶基因的负反馈调控作用[1]。但HPT轴的调节不仅与循环中的TH水平相关,还需要多种分子的共同参与才能使循环中的TH进入中枢神经系统发挥调控作用。首先,甲状腺激素是由核受体介导发挥生物学效应的激素,甲状腺激素必须通过甲状腺激素转运体穿过血-脑和血-脑脊液屏障最终进入TRH神经元细胞核内才能发挥其调控作用。进入细胞后,其不同活性形式的三碘甲状腺原氨酸(T3)、甲状腺素(T4)相互转化受细胞(如伸长细胞)内脱碘酶(Dio)的调控。进入TRH神经元细胞核后,与大多数核受体一样,甲状腺激素对基因的转录调控还依赖于甲状腺激素受体及辅因子的参与。另外,伸长细胞表达的焦谷氨酰肽酶Ⅱ(PPⅡ)能降解释放的TRH,从而影响不同甲状腺功能状态下到达垂体门静脉的TRH水平[2]。
2 参与HPT轴反馈调控的分子元件
参与HPT轴反馈调控的分子元件包括促甲状腺激素释放激素、脱碘酶、甲状腺激素转运体、甲状腺激素受体、辅因子、焦谷氨酰肽酶Ⅱ(表1)。
2.1 促甲状腺激素释放激素
下丘脑室旁核(PVN)的TRH神经元被认为是HPT轴的核心调节区域[3~5]。早有证据表明这些神经元的缺失会严重损害TSH分泌的调节[6],即TRH在决定TSH生物活性中起着至关重要的作用[7~9]。成熟TRH是一种源自促甲状腺激素释放激素前体(pro-TRH)经激素原转化酶加工而来的三肽,通过正中隆起分泌到达垂体,刺激TSH的合成和释放。TRH的转录[10,11]和翻译后处理[12]均能被T3所抑制。对下丘脑促垂体区TRH神经元的负反馈调节是保证循环中甲状腺激素水平稳定的重要调节机制[11]。当循环中甲状腺激素水平升高时,TRH基因表达下降;而甲状腺功能减退时,TRH基因表达增加[13]。研究表明甲状腺激素对TRH转录的调节相对迅速,在外源性甲状腺激素给药后5 h内即可抑制PVN区TRH基因的转录[10]。Nikrodhanond等[8]的研究发现,TRβ敲除小鼠的TH和TSH水平显著升高,而双敲除(TRβ、TRH均被敲除)小鼠比TRβ敲除小鼠显示出更低的TH和TSH水平,并且只有双敲除小鼠经丙硫氧嘧啶(PTU)处理35 d后,其TSH不能反馈性升高。该研究表明TRH对TSH和TH的合成均很重要,在HPT轴的调节中占据主导地位。但在甲状腺功能严重减退、TRH较低时,垂体依然能够促进TSH的合成[8,14],说明TH除了在TRH神经元水平上发生负反馈作用,还会在TSH的水平上进行调节。
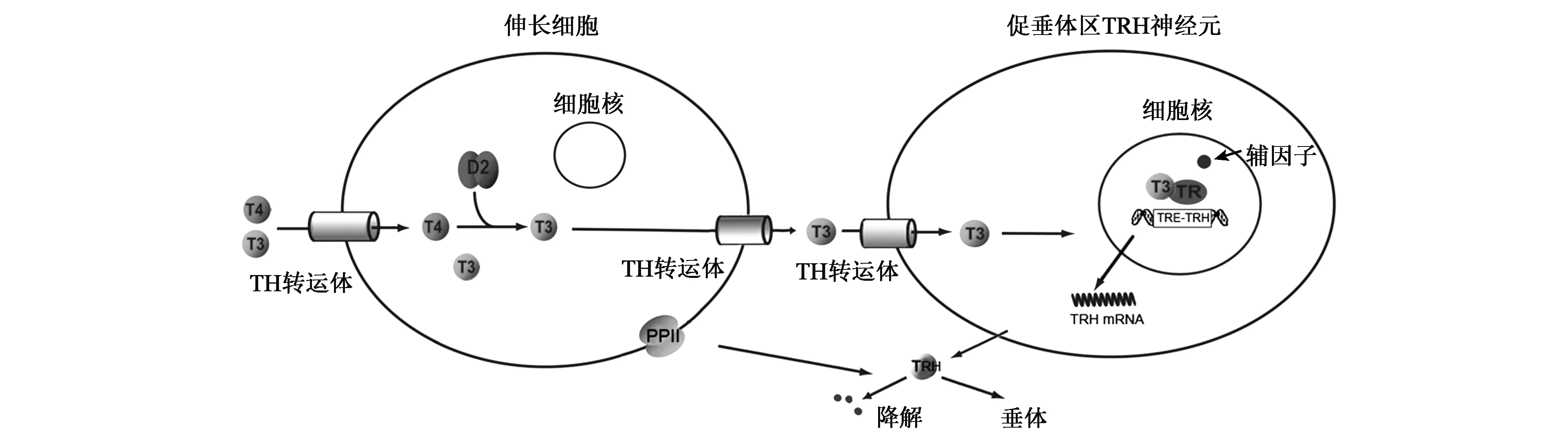
图1 HPT轴中枢调控分子模式图Fig.1 Molecular pattern of central modulation in HPT axis.
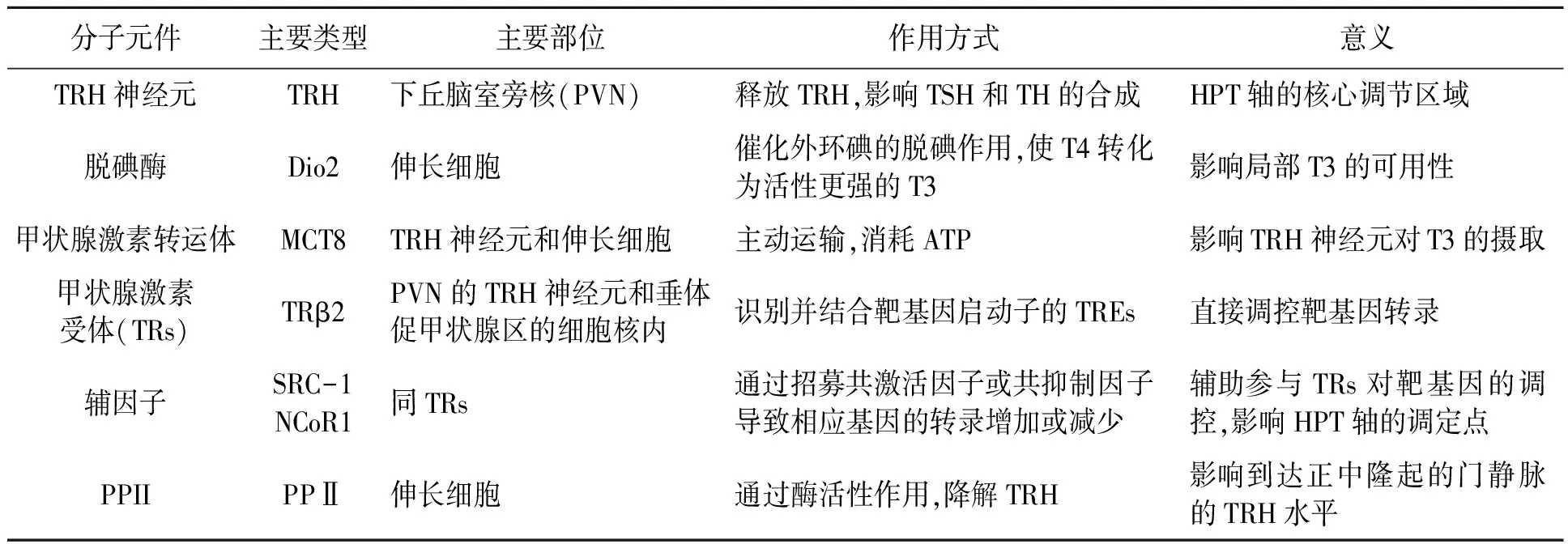
表1 参与HPT轴反馈调控的分子元件的比较Table 1 Comparison of related molecular components involved in HPT axis feedback regulation.
2.2 脱碘酶
早期研究认为在循环中只有T3水平可影响HPT轴的负反馈调节,但Kakucska等[15]发现甲减大鼠循环中的T3水平恢复正常而不干预T4时,下丘脑PVN的TRH mRNA并未恢复正常。只有循环中的T3水平严重高于正常值时,TRH mRNA的表达才降到正常范围内[16]。由此,研究者认为循环中T4在中枢神经系统中转化为T3是参与反馈调节机制的一个重要环节。甲状腺激素(TH)的激活或灭活是通过脱碘酶脱碘实现的。脱碘酶(Dio)包括3型,其中Dio1、Dio2主要催化外环碘的脱碘作用,使T4转化为活性更强的T3;而Dio3只能对内环碘脱碘,使T3、T4失活为T2和rT3。中枢神经系统主要表达Dio2和Dio3,其中,Dio2主要在漏斗核和正中隆突的神经胶质细胞及第三脑室细胞中表达,而Dio3主要存在于室旁核、视上核和漏斗核。
脱碘酶能影响脑组织细胞内T3的可用性[17],脑组织中的T4在Dio2催化下转换成活性更强的T3。Dio2缺乏时,尽管循环中T3水平正常,但下丘脑中T3含量会减少[17],因此在下丘脑中发挥作用的T3至少部分是从局部T4通过脱碘酶转换而来的。而下丘脑脱碘酶主要在伸长细胞(tanycytes)中表达[18, 19]。伸长细胞是位于第三脑室底部腹侧壁和正中隆起处室管膜上的特殊胶质细胞,是血和脑脊液之间的选择性双向转运通路。目前认为伸长细胞中的Dio2对HPT轴的动态平衡起着重要作用。尽管Dio2也存在于正中隆起和弓状核的星形胶质细胞(astrocytes)中[18],但选择性敲除小鼠星形胶质细胞中的Dio2对TRH的反馈无明显影响[20],说明星形胶质细胞在HPT调控中作用较弱。在中枢神经系统内,Dio3表达分布广泛,其表达能被T3所促进,而伸长细胞也可表达Dio3[21],但是否在影响促垂体的TRH神经元的T3水平中起着重要作用尚不清楚。
若循环中的T4水平和T3水平下降,Dio2的主要作用是保持脑区局部T3浓度稳定[22]。如在大脑皮质,甲状腺功能减退时会上调Dio2活性从而产生更多的T3,而甲状腺功能亢进时会下调Dio2活性[23]。因此,即使循环中的T4浓度在一个较宽的范围内变化,大脑皮层的局部T3浓度仍可保持不变[22]。伸长细胞中Dio2转录水平也受细胞内甲状腺激素的调控[24],但是研究发现伸长细胞中Dio2基因表达的增加并不伴随着Dio2活性的增加[25]。虽然甲状腺功能减退导致大脑皮质Dio2活性以超过4倍的速度增加[26],但对下丘脑内侧基底部(MBH)的Dio2活性没有影响[25]。另外,在碘缺乏的条件下,大脑大部分区域的Dio2活性增加而MBH的Dio2活性无改变[27]。由此可见,在MBH中甲状腺激素对Dio2转录后活性减弱,表明Dio2在这一区域的主要作用并不是为了是维持局部T3水平的稳定,而是有助于下丘脑接收外周甲状腺激素水平变化的信号。这一作用极为重要,因为稳定的下丘脑T3浓度反而会降低PVN的TRH神经元反馈调节的敏感性。
此外,垂体促甲状腺区也可表达Dio2,其可直接调控TSH的合成[28]。目前已经证明可通过抑制Dio2来提高TSH水平,而无需依赖TRH水平表达的改变[29,30]。
2.3 甲状腺激素转运体
甲状腺激素必须通过甲状腺激素转运体进入细胞内才能发挥作用,位于细胞内的脱碘酶必须先将TH摄取入细胞才能发挥脱碘效应。由于甲状腺激素的高脂溶性,以前一直认为其可以直接扩散到细胞内,但近30年甲状腺激素的分子生物学研究显示:甲状腺激素主要通过甲状腺激素转运体进出细胞。甲状腺激素的摄取是消耗ATP的主动转运过程。也就是说,细胞内的T3、T4水平不仅依赖于脱碘酶,还与位于细胞膜上的甲状腺素转运体有关。
目前,已知参与脑组织甲状腺激素运输的两个主要转运蛋白是非钠依赖性有机阴离子转运多肽1C1(OATP1C1)和单羧酸转运蛋白8(MCT8),分别属于有机阴离子转运多肽(OATP)和单羧酸转运(MCT)家族[31]。OATP1C1对T3和T4均具有较强亲和力,高度表达于血脑屏障、脉络丛和伸长细胞[32, 33]。OATP1C1敲除动物模型近年才被构建。研究显示OATP1C1敲除小鼠的HPT轴不受影响[34]。这表明OATP1C1在对TRH神经元的反馈调节中作用不大。相反,转运体MCT8主要表达于神经元(包括促垂体区TRH神经元)[35]和伸长细胞,其对T3亲和力强[35]。研究发现MCT8敲除小鼠或破坏小鼠MCT8表达会导致脑中T3的含量减少而TRH的表达增加[36~38],可见MCT8在TRH神经元对T3的摄取起着重要作用。
2.4 甲状腺激素受体
T3进入TRH神经元后,它通过与甲状腺激素受体(TRs)结合发挥生物学效应。TRs是配体依赖性受体,局部T3水平可影响TRs复合物与TREs的结合和解离,其通过识别并结合靶基因启动子的TREs来调节基因转录。但TRs在调节负向靶基因作用中是依赖于其DNA的结合能力还是其与转录因子的相互作用尚不明确,TRs发挥负向调节的精确机制仍不清楚。甲状腺激素受体(TRs)由两个基因(TRα和TRβ)编码,但不同的剪接和转录起始位点可产生不同的亚型。其中TRα1和TRβ1分布广泛,而TRβ2的表达仅限于在特定类型的细胞如PVN的TRH神经元[39]和垂体的促甲状腺区[40,41],被认为是参与HPT轴负反馈调节的主要受体。而有研究表明伴有TRβ位点基因突变的患者会表现出中枢TH抵抗,即中枢神经系统对TH的敏感性降低[42],进一步论证了这一点。另外,TRβ2表达受损的小鼠经PTU干预后,TH水平下降而TRH和TSH水平上升,均体现了TRβ2在HPT轴负反馈调节中的重要作用[43,44]。
2.5 辅因子
TH结合TRs负向调节TRH和TSH亚基基因还需要辅因子的参与。辅因子(cofactor)(包括转录共激活因子和转录共抑制因子)是指与酶(酵素)结合且在催化反应中必要的非蛋白质化合物。辅因子并不直接与DNA结合,但可通过多种机制促进或抑制靶基因的转录。T3存在时,其与TR结合形成复合物能促进招募共激活因子,正向调节基因的启动子,使相应基因的转录增加;而T3缺失时会有助于TR招募核受体共抑制因子,导致相应基因的转录减少。但TRs负向调控基因(如TRH和TSH亚基基因)表达的机制仍不清楚。体内试验显示,共激活因子SRC-1为T3诱导抑制TSH所必需的,而缺乏该共激活因子的小鼠血中的T4和TSH水平增加[45,46];同样,小鼠表达的TRβ若不能正常招募SRC-1,则也有相同的表现[47]。若破坏TR与核受体共抑制剂NCoR1之间的相互作用,则有相反的效应,这表明NCoR1对HPT的激活是必需的[48,49]。上述研究结果表明共激活因子和共抑制因子对负反馈机制至关重要,共激活因子和共抑制剂与TR的结合可能影响HPT轴的调定点。
2.6 焦谷氨酰肽酶Ⅱ
除了通过影响正中隆起的T3的可用性来调节TRH神经元的反馈,最近发现伸长细胞能表达焦谷氨酰肽酶Ⅱ(PPⅡ),PPⅡ能降解在正中隆起释放的TRH,从而影响到达正中隆起的门静脉的TRH水平[50,51]。PPⅡ是一种膜整合蛋白,由一个较小的N端胞内区和一个含有酶活性区域的细胞外结构域组成,其表达和活性受循环中TH的高度调控。有研究证明甲状腺功能亢进时,伸长细胞PPⅡ的表达量增加,从而减少到达垂体门静脉的TRH水平,参与HPT的反馈调节[50,52]。此外,T3也可负向调节TRH所结合的特定细胞膜受体TRHR1的表达,降低其对TRH的敏感性[53~55]。由此可见,T3不仅可以调控TRH mRNA的表达,还可以影响TRH的产生、降解及其受体表达。
3 展望
目前,对HPT轴负反馈调节的分子机制已有了更加深入、全面的了解,但仍有许多问题有待解决。循环中甲状腺激素水平的稳定是由其对PVN的TRH神经元的经典负反馈机制所调控,而这种调控机制非常复杂,目前认为此调控涉及的元件主要包括:脱碘酶、甲状腺激素受体、甲状腺激素转运体、辅因子、PPⅡ等。但这些引起定点改变的信号分子的通路仍有待进一步阐明,在生理条件下哪些因素会产生影响并如何精密调控HPT轴仍有待探究。HPT轴整合机体内环境和外部信号,其调节机制是多水平而复杂的,进一步的研究HPT调控机制有助于阐明疾病引起HPT轴异常的机理,并为疾病提供可行的治疗方法。
[1] Hollenberg A N, Monden T, Flynn T R,etal.. The human thyrotropin-releasing hormone gene is regulated by thyroid hormone through two distinct classes of negative thyroid hormone response elements[J]. Mol. Endocrinol., 1995, 9(5):540-550.
[2] Costa-e-Sousa R H, Hollenberg A N. Minireview: The neural regulation of the hypothalamic-pituitary-thyroid axis[J]. Endocrinology, 2012, 153(9):4128-4135.
[3] Nillni E A. Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs[J]. Front. Neuroendocrin., 2010, 31(2):134-156.
[4] Hollenberg A N. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor[J]. Thyroid, 2008, 18(2):131-139.
[5] Lechan R M, Fekete C. The TRH neuron: A hypothalamic integrator of energy metabolism[J]. Prog. Brain Res., 2006, 153:209-235.
[6] Martin J B, Boshans R, Reichlin S. Feedback regulation of TSH secretion in rats with hypothalamic lesions[J]. Endocrinology, 1970, 87(5):1032-1040.
[7] Beck-Peccoz P, Amr S, Menezes-Ferreira M M,etal.. Decreased receptor binding of biologically inactive thyrotropin in central hypothyroidism: Effect of treatment with thyrotropin-releasing hormone[J]. New Engl. J. Med., 1985, 312(17):1085-1090.
[8] Nikrodhanond A A, Ortiga-Carvalho T M, Shibusawa N,etal.. Dominant role of thyrotropin-releasing hormone in the hypothalamic-pituitary-thyroid axis[J]. J. Biol. Chem., 2006, 281(8):5000-5007.
[9] Taylor T, Gesundheit N, Weintraub B D. Effects ofinvivobolus versus continuous TRH administration on TSH secretion, biosynthesis, and glycosylation in normal and hypothyroid rats[J]. Mol. Cell. Endocrinol., 1986, 46(3):253-261.
[10] Sugrue M L, Vella K R, Morales C,etal.. The thyrotropin-releasing hormone gene is regulated by thyroid hormone at the level of transcriptioninvivo[J]. Endocrinology, 2010, 151(2):793-801.
[11] Segerson T P, Kauer J, Wolfe H C,etal.. Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus[J]. Science, 1987, 238(4823):78-80.
[12] Perello M, Friedman T, Paez-Espinosa V,etal.. Thyroid hormones selectively regulate the posttranslational processing of prothyrotropin-releasing hormone in the paraventricular nucleus of the hypothalamus[J]. Endocrinology, 2006, 147(6):2705-2716.
[13] Fekete C, Lechan R M. Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: Role of neuronal afferents and type 2 deiodinase[J]. Front. Neuroendocrin., 2007, 28(2-3):97-114.
[14] Yamada M, Saga Y, Shibusawa N,etal.. Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene[J]. Proc. Natl. Acad. Sci. USA, 1997, 94(20):10862-10867.
[15] Galton V A, Schneider M J, Clark A S,etal.. Life without thyroxine to 3,5,3′-triiodothyronine conversion: Studies in mice devoid of the 5′-deiodinases[J]. Endocrinology, 2009, 150(6):2957-2963.
[16] Kakucska I, Rand W, Lechan R M. Thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is dependent upon feedback regulation by both triiodothyronine and thyroxine[J]. Endocrinology, 1992, 130(5):2845-2850.
[17] Bianco A C. Minireview: Cracking the metabolic code for thyroid hormone signaling[J]. Endocrinology, 2011, 152(9):3306-3311.
[18] Diano S, Leonard J L, Meli R,etal.. Hypothalamic type II iodothyronine deiodinase: A light and electron microscopic study[J]. Brain Res., 2003, 976(1):130-134.
[19] Fekete C, Mihály E, Herscovici S,etal.. DARPP-32 and CREB are present in type 2 iodothyronine deiodinase-producing tanycytes: Implications for the regulation of type 2 deiodinase activity[J]. Brain Res., 2000, 862(1-2):154-161.
[20] Fonseca T L, Correa-Medina M, Campos M P O,etal.. Coordination of hypothalamic and pituitary T3 production regulates TSH expression[J]. J. Clin. Invest., 2013, 123(4):1492-1500.
[21] Ross A W, Helfer G, Russell L,etal.. Thyroid hormone signalling genes are regulated by photoperiod in the hypothalamus of F344 rats[J]. PLoS ONE, 2011, 6(6):e21351.
[22] Broedel O, Eravci M, Fuxius S,etal.. Effects of hyper- and hypothyroidism on thyroid hormone concentrations in regions of the rat brain[J]. Am. J. Physiol-Endoc. M., 2003, 285(3):E470-E480.
[23] Bianco A C, Salvatore D, Gereben B,etal.. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases[J]. Endocr. Rev., 2013, 23(1):38-89.
[24] Anguiano B, Quintanar A, Luna M,etal.. Neuroendocrine regulation of adrenal gland and hypothalamus 5′deiodinase activity. II. Effects of splanchnicotomy and hypophysectomy[J]. Endocrinology, 1995, 136(8):3346-3352.
[25] Diano S, Naftolin F, Goglia F,etal.. Fasting-induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus[J]. Endocrinology, 1998, 139(6):2879-2884.
[26] Leonard J L, Kaplan M M, Visser T J,etal.. Cerebral cortex responds rapidly to thyroid hormones[J]. Science, 1981, 214(4520):571-573.
[27] Serrano-Lozano A, Montiel M, Morell M,etal.. 5′deiodinase activity in brain regions of adult rats: Modifications in different situations of experimental hypothyroidism[J]. Brain Res. Bull., 1993, 30(5-6):611-616.
[28] Christoffolete M A, Ribeiro R, Singru P,etal.. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism[J]. Endocrinology, 2006, 147(4):1735-1743.
[29] Burger A, Dinichert D, Nicod P,etal.. Effect of amiodarone on serum triiodothyronine, reverse triiodothyronine, thyroxin, and thyrotropin: A drug influencing peripheral metabolism of thyroid hormones[J]. J. Clin. Invest., 1976, 58(2):255-259.
[30] Rosene M L, Wittmann G, Arrojo e Drigo R,etal.. Inhibition of the type 2 iodothyronine deiodinase underlies the elevated plasma TSH associated with amiodarone treatment[J]. Endocrinology, 2010, 151(12):5961-5970.
[31] Jansen J, Friesema E C H, Milici C,etal.. Thyroid hormone transporters in health and disease[J]. Thyroid, 2005, 15(8):757-768.
[32] Sugiyama D, Kusuhara H, Taniguchi H,etal.. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: High affinity transporter for thyroxine[J]. J. Biol. Chem., 2003, 278(44):43489-43495.
[33] Kalló I, Mohácsik P, Vida B,etal.. A novel pathway regulates thyroid hormone availability in rat and human hypothalamic neurosecretory neurons[J]. PLoS ONE, 2012, 7(6):e37860.
[34] Mayerl S, Visser T J, Darras V M,etal.. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain[J]. Endocrinology, 2012, 153(3):1528-1537.
[35] Heuer H, Maier M K, Iden S,etal.. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations[J]. Endocrinology, 2005, 146(4):1701-1706.
[36] Trajkovic M, Visser T J, Mittag J,etal.. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8[J]. J. Clin. Invest., 2007, 117(3):627-635.
[37] Heuer H, Visser T. Minireview: Pathophysiological importance of thyroid hormone transporters[J]. Endocrinology, 2009, 150(3):1078-1083.
[38] Dumitrescu A M, Liao X H, Weiss R E,etal.. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (Mct) 8-deficient mice[J]. Endocrinology, 2006, 147(9):4036-4043.
[39] Lechan R M, Qi Y, Jackson I M,etal.. Identification of thyroid hormone receptor isoforms in thyrotropin-releasing hormone neurons of the hypothalamic paraventricular nucleus[J]. Endocrinology, 1994, 135(1):92-100.
[40] Hodin R A, Lazar M A, Wintman B I,etal.. Identification of a thyroid hormone receptor that is pituitary-specific[J]. Science, 1989, 244(4900):76-79.
[41] Wood W M, Ocran K W, Gordon D F,etal.. Isolation and characterization of mouse complementary DNAs encoding α and β thyroid hormone receptors from thyrotrope cells: The mouse pituitary-specific β2 isoform differs at the amino terminus from the corresponding species from rat pituitary[J]. Mol. Endocrinol., 1991, 5(8):1049-1061.
[42] Safer J D, O’Connor M G, Colan S D,etal.. The thyroid hormone receptor-β gene mutation R383H is associated with isolated central resistance to thyroid hormone[J]. J. Clin. Endocr. Metab., 1999, 84(9):3099-3109.
[43] Abel E D, Ahima R S, Boers M E,etal.. Critical role for thyroid hormone receptor β2 in the regulation of paraventricular thyrotropin-releasing hormone neurons[J]. J. Clin. Invest., 2001, 107(8):1017-1023.
[44] Gauthier K, Chassande O, Plateroti M,etal.. Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development[J]. EMBO J., 1999, 18(3):623-631.
[45] Weiss R E, Xu J, Ning G,etal.. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone[J]. EMBO J., 1999, 18(7):1900-1904.
[46] Takeuchi Y, Murata Y, Sadow P,etal.. Steroid receptor coactivator-1 deficiency causes variable alterations in the modulation of T3-regulated transcription of genesinvivo[J]. Endocrinology, 2002, 143(4):1346-1352.
[47] Ortiga-Carvalho T M, Shibusawa N, Nikrodhanond A,etal.. Negative regulation by thyroid hormone receptor requires an intact coactivator-binding surface[J]. J. Clin. Invest., 2005, 115(9):2517-2523.
[48] Fozzatti L, Lu C, Kim D W,etal.. Resistance to thyroid hormone is modulatedinvivoby the nuclear receptor corepressor (NCOR1)[J]. Proc. Natl. Acad. Sci. USA, 2011, 108(42):17462-17467.
[49] Astapova I, Vella K R, Ramadoss P,etal.. The nuclear receptor corepressor (NCoR) controls thyroid hormone sensitivity and the set point of the hypothalamic-pituitary-thyroid axis[J]. Mol. Endocrinol., 2011, 25(2):212-224.
[50] Sánchez E, Vargas M A, Singru P S,etal.. Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence[J]. Endocrinology, 2009, 150(5):2283-2291.
[51] Charli J L, Vargas M A, Cisneros M,etal.. TRH inactivation in the extracellular compartment: Role of pyroglutamyl peptidase II[J]. Neurobiology, 1998, 6(1):45-57.
[52] Marsili A, Sanchez E, Singru P,etal.. Thyroxine-induced expression of pyroglutamyl peptidase II and inhibition of TSH release precedes suppression of TRH mRNA and requires type 2 deiodinase[J]. J. Endocrinol., 2011, 211(1):73-78.
[53] Gershengorn M C. Bihormonal regulation of the thyrotropin-releasing hormone receptor in mouse pituitary thyrotropic tumor cells in culture[J]. J. Clin. Invest., 1978, 62(5):937-943.
[54] Yamada M, Monden T, Satoh T,etal.. Differential regulation of thyrotropin-releasing hormone receptor mRNA levels by thyroid hormoneinvivoandinvitro(GH3 cells)[J]. Biochem. Bioph. Res. Co., 1992, 184(1):367-372.
[55] Hinkle P M, Goh K B C. Regulation of thyrotropin-releasing hormone receptors and responses by L-triiodothyronine in dispersed rat pituitary cell cultures[J]. Endocrinology, 1982, 110(5):1725-1731.
ReviewonMolecularComponentsParticipatingNegativeFeedbackRegulationintheHypothalamus-pituitary-thyroidAxis
ZUO Weixing1, ZHANG Zhifei2, LIU Zhimin3*, WANG Chaoqun4*
1.The546thHospitalofChinesePeople’sLiberationArmy,Urumqi841700,China; 2.ClinicalMedicalCollege,WeifangMedicalUniversity,ShandongWeifang261053,China; 3.ChangzhengHospital,SecondMilitaryMedicalUniversity,Shanghai200003,China; 4.ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
Negative feedback regulation in the hypothalamic-pituitary-thyroid (HPT) axis primarily functions to maintain normal and circulating levels of thyroid hormone (TH). Hypophysiotropic thyrotropin-releasing hormone (TRH) neurons in the paraventricular nucleus (PVN) of the hypothalamus are believed to represent the regulatory core of the HPT axis. Studies have showed that not only circulating triiodothyronine (T3), tetraiodothyronine (T4) could also regulate intracellular T3 availability by type 2 deiodinase (Dio2) in tanycytes and responsible for negative feedback regulation of hypophysiotropic TRH. TH was transported into the hypophysiotropic TRH neurons by TH transporters and bound to thyroid hormone receptors (TRs), especially TRβ2, with the recruitment of coregulators by the TRs, participating in regulation of the corresponding target gene. In addition, tanycytes have been shown to express pyroglutamyl peptidase II (PPII), which degraded TRH releasing from TRH neurons and furthermore affected the TRH concentration in portal blood in different thyroid states. This article summarized molecular components of HPT axis regulation, which was aimed to provide reference for the scientific researches and clinical treatment of thyroid dysfunction or HPT axis abnormalities.
HPT axis; negative feedback; thyroid hormone; deiodinase
2017-05-22;接受日期2017-07-20
左卫星,主治医师,主要从事甲状腺疾病临床及基础研究。E-mail:303.2006@163.com。*通信作者:刘志民,主任医师,主要从事甲状腺疾病临床及基础研究。E-mail:981857201@qq.com;王超群,住院医师,主要从事甲状腺疾病临床及基础研究。E-mail:wangcqvip@163.com
10.19586/j.2095-2341.2017.0048

