纤维二糖水解酶的研究进展
袁茂翼,叶发银,雷琳,赵国华,2*
1(西南大学 食品科学学院,重庆,400715)2(重庆市特色食品工程技术研究中心, 重庆, 400175)
纤维二糖水解酶的研究进展
袁茂翼1,叶发银1,雷琳1,赵国华1,2*
1(西南大学 食品科学学院,重庆,400715)2(重庆市特色食品工程技术研究中心, 重庆, 400175)
纤维素是世界上最丰富的可再生资源,将其降解为小分子糖并转化为燃料或精细化学品一直是研究的难点和热点。纤维二糖水解酶是生物降解纤维素的关键酶之一,它属于外切酶,作用于结晶纤维素的链末端依次切开相隔的β-1,4-糖苷键,释放纤维二糖。论文对纤维二糖水解酶的来源、分类、结构,对纤维素的作用机理、酶学性质、分子进化、商业酶制剂生产情况及其应用特性进行了总结,同时对该研究进行了展望。
纤维二糖水解酶;酶学性质;结构;催化机理;应用
纤维素酶是能将纤维素水解的酶的统称,是一类复杂的多酶体系,主要包括内切葡聚糖酶(EC3.2.1.4)、外切葡聚糖酶(EC3.2.1.91和EC 3.2.1.176)和β-葡萄糖苷酶(EC3.2.1.21),它们协同作用于纤维素使其彻底降解为葡萄糖。其中,内切葡聚糖酶主要作用于纤维素的非结晶区,将纤维素长链降解为小分子纤维素或寡糖链;外切葡聚糖酶又称作纤维二糖水解酶(cellobiohydrolase,CBH),与内切葡聚糖酶协同作用并负责降解纤维素的结晶区,将纤维素链剥离并水解β-1,4-糖苷键释放纤维二糖[1]。β-葡萄糖苷酶不直接作用于纤维素,主要是将内切葡聚糖酶和外切葡聚糖酶作用产生的寡糖链和纤维二糖水解为葡萄糖。鉴于CBH在食品物料改性以及生物质能源生产上巨大的应用潜能,本文在主要查阅国内外近10年文献的基础上,对CBH生物来源、理化特征及潜在应用进行了综述,并对CBH研究中存在的问题和今后的研究方向进行了探讨,以期推动CBH的深入研究及产业化应用。
1 纤维二糖水解酶的分类及来源
目前,对CBH的命名法主要包括EC法、CAZy法和mycoCLAP法等。在酶的国际系统分类编号(EC)中,纤维二糖水解酶占两个编号:EC3.2.1.91(GH5、GH 6、GH 9)和EC 3.2.1.176(GH7、GH 9、GH 48),其中CBHⅠ(EC 3.2.1.176)作用于纤维素链的还原端,CBHⅡ(EC 3.2.1.91)作用于纤维素链的非还原端[2-3]。真菌是纤维二糖水解酶的主要来源,主要是木霉、青霉和曲霉,且菌株多为野生型;另外也有产生CBH的细菌,但其产量比较低,因此相关研究不多(表1)。

表1 纤维二糖水解酶的来源Table 1 The source of cellobiohydrolasees
CAZy数据库(http://www.cazy.org/)是专门针对碳水化合物活性酶的基因组、结构和生物化学信息的开放获取网络。CAZy数据库根据酶催化结构域氨基酸序列的相似性[4-5],将已报道的糖苷水解酶划归为144个家族,其中真菌CBH归属到GH6和GH7两个糖苷水解酶家族,对于细菌来源的CBH属于GH48、GH9、GH6、GH5。此外,mycoCLAP数据库(http://mycoclap.fungalgenomics.ca)提供木质纤维素相关酶和活性蛋白质的信息,该数据库提供的命名法能直接反映出酶的功能、所属糖苷水解酶家族以及来源微生物的种属信息。如名称为CBH6A_COPCI的酶指来自Coprinopsiscinerea,属于GH6家族的纤维二糖水解酶。
2 纤维二糖水解酶的结构
里氏木霉(Trichodermareesei)CBH是真菌CBH的典型代表,对其结构的研究也最为完善。研究CBH结构发现包含了具有降解活性的催化结构域(catalytic domain,CD)和吸附纤维素的纤维素结合结构域(cellulose binding domain,CBD),这两个结构域由一段O-糖肽链的连接桥(linker)相连接。里氏木霉CBH的CBD为“楔型”结构,一面亲水,另一面疏水,且亲水面上的3个酪氨酸残基组成纤维素的吸附位点[6]。这些结构域位于肽链的羧基端(C-端)或氨基端(N-端)。在此基础上也有人研究其他菌,比如来自NeocallimastixpatriciarumJ11的CBH的CBD位于N-端,包含Asn、Ala、Gly和Gla等残基,而CD位于C-端,其连接桥包含Asn(28.8%)、Ala(13%)、Gly(13.7%)和Gla(10.1%)[7]。CBH的糖基化包括CD的N-糖基化和连接桥的O-糖基化,前者发生在天冬酰胺残基上,后者发生在丝氨酸和苏氨酸残基上[8]。有报道认为高度糖基化会降低重组里氏木霉CBHⅠ对结晶纤维素的水解活性[9]。
2.1 催化结构域(CD)
里氏木霉CBHⅠ的CD的结构在1994年被解析,为反平行β-折叠形成的三明治主体结构,包含有4个短α-螺旋和β-束形成的线圈结构,并有10个二硫键[10],CBHⅠ通道至少有10个结合位点(-7到+3)[11],4个色氨酸残基(Trp)促进纤维素糖单元的疏水堆积相互作用,这4个Trp分别是进口处的Trp-40,中心处的Trp-38,围绕催化位点的Trp-367和Trp-376[12]。催化活性位点的3个氨基酸在催化机制中起着关键作用,Glu-217是催化酸和催化基础,Glu-212是形成酶-糖基中间产物的亲核试剂,Asp-214通过侧链氢键与亲核试剂形成稳态相互作用[11]。研究Phanerochaetechrysosporium的Cel7A发现,N端氨基酸谷氨酰胺循环产生焦谷氨酸残基,有Asn188和Asn286两个潜在的糖基化位点。Asn 286结合N-乙酰氨基葡萄糖[4]。
2.2 纤维素结合结构域(CBD)
目前已确定约有130个不同的CBD的结构。根据其氨基酸序列的相似性划分为13个不同的CBD家族。同一家族中的CBD的结构相似,而不同家族的CBD结构具有拓扑性。真菌CBH的CBD大约由35个氨基酸残基构成[13]。脱离了母体CBH的CBD没有催化活性,不能水解纤维素。王禄山等[14]提出CBD在CBHⅠ降解纤维素过程中担负两个角色:1)通过其吸附于纤维素表面而增加底物与酶的接触;2)裂解纤维素晶体表面的纤维素分子间的氢键。敲除CBD编码序列后获得的CBH对不溶性纤维素的水解活性明显下降,而对可溶性纤维素的降解作用没有明显影响。Cel1和CelD是来自Aspergillusniveus的属于GH7家族的两种CBH,它们有相似的CD,但前者有CBD后者没有,发现Cel1对结晶纤维素降解活性明显大于CelD[15]。
2.3 连接桥
连接桥是一段高度糖基化的多肽,它的长短控制着CBH的CBD和CD间的距离,并对酶的构象和活性有显著影响。连接桥的有效长度及柔性是CBH发挥催化活性所必需的。丝状真菌CBH的连接桥一般包含30~40个氨基酸残基,而细菌CBH则由约100个氨基酸残基组成[6]。
3 纤维二糖水解酶的作用机理
CBH对游离纤维素链的水解机理首先是纤维素链结合到CBD上,并推动其进入CD的催化通道,CD沿着纤维素链滑动,进而从还原端或非还原端以纤维二糖为切割单元被降解(图1)。CBD与酶底物结合包括氢键结合和疏水作用[16]。但截至目前,CBH对结晶纤维素的降解机理仍然不清楚,其过程推测如下[14-15]: 1)CBH通过其CBD的介导吸附到结晶纤维素表面(锚定)[4];2)锚定的CBH链在结晶纤维素表面扩散移动的过程中,CD可识别到裸露于结晶纤维素表面的纤维素链末端(还原端或非还原端)并与之结合,使糖苷键水解并释放纤维二糖。

图1 纤维二糖水解酶的作用机理[46]Fig.1 The action mechanism of cellobiohydroalses[46]
4 纤维二糖水解酶的酶学特性
表2给出了常见CBH的来源菌、分类及酶学特性。由表2可知,不同CBH的最适pH范围为3.0~9.0,大多数位于4.5~6.0的范围内;最适催化温度为35~70 ℃,分子质量约40~70 kDa,CBH的活性主要受pH、温度、金属离子、产物反馈抑制以及其他成分的影响。COLUSSI等[17]对来自Trichodermaharzianum的CBH(ThCel7A)研究发现,在pH3~5范围内,酶活性随着pH值增大而提升;在pH5~7范围内,酶活性随着pH值增大而降低。pH=5时,在20~50 ℃范围内酶活性随温度上升而增加;当温度超过50 ℃,酶分子发生变性其活性显著降低。WANG等[7]发现,Hg2+和Ag+对来自NeocallimastixpatriciarumJ11的CBH有强烈抑制作用,该菌的酶在大肠杆菌表达后,加入10 mmol Hg2+可使酶完全失活,而10 mmol的Ag+使酶仅保留9%的活性。而对来自产紫青霉(Penicilliumpurperogenum)HBZ003的CBH,Fe2+与Mn2+表现出较强的激活作用,Ca2+、Co2+、Cu2+有弱的激活作用,而Mg2+、Zn2+及Al3+则呈现抑制作用[18]。
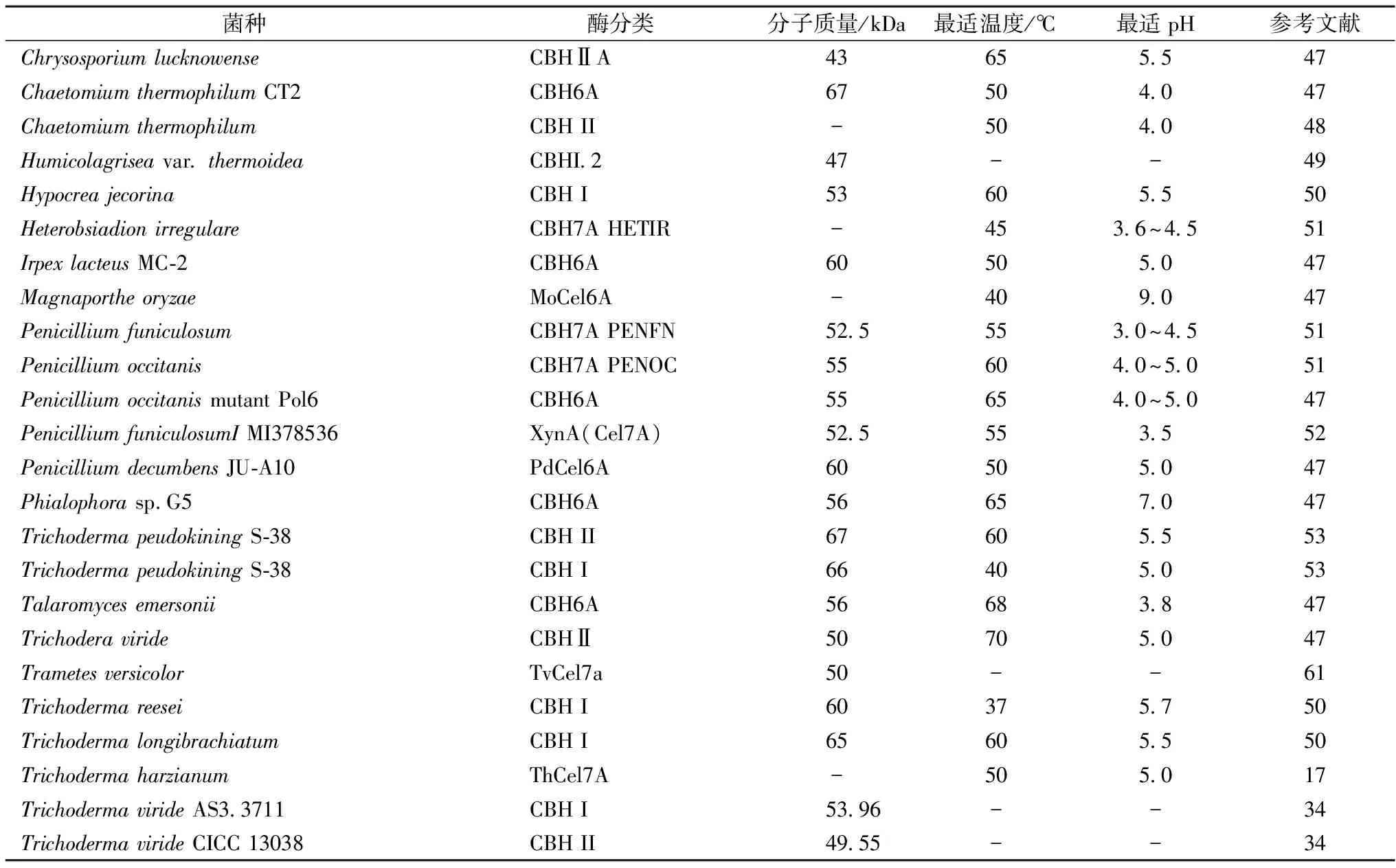
表2 常见纤维二糖水解酶的来源菌、分类及酶学特性Table 2 The origin, classification and enzymatic properties of common cellobiohydroalses
酶解产物纤维二糖对CBH有反馈抑制作用,尤其对CBHⅠ的影响更明显[8,19-20]。KARI等[21]发现,β-型纤维二糖比α-型的抑制作用更强,抑制常数可达12.5 μmol/L。BARAMEE等[22]发现,低于100 mmol/L的纤维二糖对来自Cellulomonasfimi的CBH无抑制作用,而在255 mmol/L时其抑制作用可达50%。向反应液中添加β-葡萄糖苷酶、纤维二糖磷酸化酶[23]、纤维二糖脱氢酶[24]可有效消除反馈抑制。半胱氨酸可通过与CBH酶蛋白分子发生巯基-二硫键交换反应而引起酶失活[25]。可溶性木聚糖能与CBH的CBD结合,从而竞争性地影响酶与纤维素链的结合而降低催化效率[26]。多酚类物质以及漆酶氧化形成的酚低聚物对CBH也有明显的抑制作用[27]。
5 纤维二糖水解酶的分子进化
来自于天然真菌的CBH虽然种类多,但往往其酶产量不高,适宜pH范围窄,易发生热变性以及底物反馈抑制明显,这些缺陷严重限制了CBH的工业化应用。人们试图通过构建基因工程菌株来克服这些缺点。表3给出目前CBH基因工程菌株构建情况及对酶特性的改善情况。

表3 CBH基因工程菌株构建宿主及酶特性改善情况Table 3 Construction of CBH gene engineering strains and improvement of enzyme characteristics
由表3可以看出,目前构建CBH基因工程菌株选择的表达宿主主要有大肠杆菌、酵母(毕赤酵母、酿酒酵母、解脂耶氏酵母等)、丝状真菌(木霉、曲霉和青霉等)[8]。通过基因工程可有效提升CBH酶的热稳定性,拓宽其适宜工作pH范围,降低产物的反馈抑制作用以及提升酶的产量。但总的来看,对酶热稳定性的改善效果较好,但在提升酶产量等方面效果还不十分理想。
6 商业纤维二糖水解酶酶制剂的生产情况
目前用于商业纤维二糖水解酶酶制剂生产的菌株主要是里氏木霉(Trichodermareesei)和长枝木霉(Trichodermalongibrachiatum)。生产商业里氏木霉CBH的公司主要有Sigma-Aldrich、嘉汉生物科技有限公司和杰能科国际股份有限公司。如Sigma-Aldrich公司生产的Celluclast 1.5L以Avicel和pNPC为底物的比酶活分别为0.343 U/mg[28]和0.06 U/mg[29]。而爱尔兰Megazyme、安必奇(Creative Enzymes)和BIOHJ慧嘉生物等都利用长枝木霉生产。如Megazyme的CBHI为无色粉末状,易溶于水,最适pH为6.0,以底物CMC为底物的比酶活为0.1U/mg[30]。除此之外,也有用其他微生物生产商业CBH的案例,如嘉汉生物科技有限公司利用PenicilliumoxalicumJU-A10生产的酶以pNPC为底物的比酶活为0.21 U/mg[29]。研究还发现,来自真菌(酿酒酵母、毕赤酵母和解脂耶氏酵母)的CBH为高糖基化CBH,对可溶性底物或非结晶纤维素活性低[31],但来自酿酒酵母的CBH很适合工业酒精的生产[32]。李亚玲等[33]研究发现,来自嗜热毛壳菌(Chaetomiumthermophilum)CBH的比酶活为1.45 U/mg,回收率为5.25%,半衰期为1h(70 ℃)。目前商业化CBH产品生产存在的主要问题是酶活性、酶热稳定性、pH耐受范围均不理想。OLIVEIRA等[34]发现来自Humicolagriseavar.thermoidea的rCBHI.2有较高的最适温度(60 ℃)、较宽的pH耐受范围(pH4.0~9.0,最适pH为8.0)和良好的热稳定性(70 ℃-240 min可保留88%活性)。
7 纤维二糖水解酶的应用
7.1 在食品工业中的应用
在食品工业中,CBH主要可作为加工助剂提升加工效率或改善产品的品质。利用来自太瑞斯梭孢壳霉(Thielaviaterrestris)的CBH与商业酶CellicCTec2共同对棕榈果进行前处理,可大幅度提升其出油率,其出油率与单独商业酶CellicCTec2作用相比提升了39%[35]。VAILLANT等[36]在百香果汁中加入果胶酶(85 U/L)、CBH(100 U/L)和内切葡聚糖酶(700 U/L),在30 ℃保温处理1 h可使其中的果泥完全液化。向苹果匀浆中添来自黑曲霉(Aspergillusniger)的CBH(29 U/mL)和果胶酶(50 U/mL),在40 ℃处理24 h后获得的苹果混浊汁的稳定性显著改善[5]。在加工脆皮面包和饼干时,向面团中添加羧甲基纤维素酶(9 300 U/g)、CBH(3 800 U/g)和木聚糖酶(2 500 U/g),可降低面包的吸水率并提升其加工性能[5]。在面包生产中使用CBH酶制剂可减少乳化剂的用量[37]。
7.2 在生物工程中的应用
目前CBH的应用基本停留在实验室研究阶段,规模化工业应用尚未实现,核心原因是尚不能提供价廉物美的商业化酶制剂或高产菌株。但就目前的研究情况来看,CBH未来在生物工程领域的潜在应用主要包括纤维二糖的生产、生物燃料的生产、纤维性物料的改性等。纤维二糖是CBH作用于纤维素的主要产物,它是重要的化工原料,可作为生产山梨醇[38]、丙酮[39]、丁醇[40]、丁二醇[41]和乳酸[41-42]的原料。以嗜热芽孢杆菌属(ThermophilicBacilluscoagulans)WCP10-4为例,利用纤维二糖作为唯一的碳源,不用额外加入β-葡萄糖苷酶就可有效地转化纤维二糖为L-乳酸,200 g/L的纤维二糖转化为 196.3 g/L 的L-乳酸,产率达到 97.8%[41]。由于能源的短缺,生物燃料的生产备受关注。为不与人争粮,生物燃料生产的最佳原料应为自然界丰富的纤维素,目前常用富含木质纤维素的农业废弃物(秸秆等)和固体垃圾(餐饮垃圾等)为原料生产[43]。其关键是将原料中的木质纤维素转化为可发酵的糖,进而通过生物转化形成诸如乙醇的生物燃料。目前常采用含有内切葡聚糖酶、纤维二糖水解酶、β-葡萄糖苷酶等在内的特定纤维素降解酶组合来实现纤维素向可发酵糖的转化[5,44-45]。如利用可表达里氏木霉内切葡聚糖酶,CBHⅡ和棘孢曲霉β-葡萄糖苷酶酿酒酵母基因工程菌株作用于非结晶纤维素和离子液体溶胀纤维素,60 h后发酵液中乙醇含量可达2.1 g/L[4]。
8 结语
微生物来源的纤维二糖水解酶具有良好的工业应用前景。在食品领域,纤维二糖水解酶可与其他食品酶制剂协同降解水果及油料果实细胞壁多糖,以利于提高出汁率或榨油率,在酿造工业中,可降解啤酒中的多糖,以利于啤酒过滤澄清,在其他工业领域,纤维二糖水解酶可用于纤维二糖的生产,纺织工业中天然纤维物料的改性,以及能源工业中燃料乙醇的生产等等。虽然近年来对于纤维二糖水解酶的研究取得了突出成绩,但仍存在许多值得深入探讨的问题:1)纤维二糖水解酶作为纤维素酶大家族的一类关键酶,其催化水解结晶纤维素的机制有待进一步明确,比如酶与底物相互作用的精确模式、纤维二糖水解酶与其他纤维素酶的协同机制等;2)获得具有商业价值的酶制剂是纤维二糖水解酶走向工业应用的重要途径。一方面,通过高通量筛选技术挖掘新的酶种;另一方面,通过分子进化来改善酶的作用条件和催化性能,对于食品用途酶的生产菌株,还需重视来源菌株的安全性评价;3)要进一步探索纤维二糖水解酶在食品领域的潜在利用方面,如对结晶纤维素的有限可控降解,使获得新型食品配料或添加剂成为可能。
[1] GUSAKOV A V. Alternatives toTrichodermareeseiin biofuel production[J]. Trends in Biotechnology,2011, 29(9): 419-425.
[2] HAMID S B A, ISLAM M M, DAS R. Cellulase biocatalysis: key inuencing factors and mode of action[J]. Cellulose. 2015, 22(4): 2 157-2 182.
[3] FANG Hao, XIA Li-ming. Cellulase production by recombinantTrichodermareeseiand its application in enzymatic hydrolysis of agricultural residues[J]. Fuel,2015, 143: 211-216.
[4] CHUKEATIROTE E, MAHARACHCHIKUMBURA S S N, WONGKHAM S, et al. Cloning and sequence analysis of the cellobiohydrolase I genes from some basidiomycetes[J]. Mycobiology,2012, 40 (2): 107-110.
[5] JUTURU V, WU J C. Microbial cellulases: Engineering, production and applications[J]. Renewable and Sustainable Energy Reviews,2014, 33(6): 188-203.
[6] KRAULIS P J, CLORE G M, NILGES M, et al. Determination of the three-dimensional solution structure of the C-terminal domain of cellobiohydrolase I fromTrichodermareesei. A study using nuclear magnetic resonance and hybrid distance geometry-dynamical simulated annealing[J]. Biochemistry,1989, 28(18): 7 241-7 257.
[7] WANG Hui-chang, CHEN Yo-chia, HUANG Ching-tsan, et al. Cloning and characterization of a thermostable and pH-stable cellobiohydrolase fromNeocallimastixpatriciarumJ11[J]. Protein Expression and Purication,2013, 90(2): 153-159.
[8] ZOGLOWEK M, LÜBECK P S, AHRING B K, et al. Heterologous expression of cellobiohydrolases inlamentous fungi-An update on the current challenges, achievements and perspectives[J]. Process Biochemistry,2015, 50(2): 211-220.
[9] GUSAKOV A V, DOTSENKO A S, ROZHKOVA A M, et al. N-Linked glycans are an important component of the processive machinery of cellobiohydrolases[J]. Biochimie,2017, 132: 102-108.
[10] DIVNE C, STAHLBERG J, RENINIKAINEN T, et al. The three-dimensional crystal structure of the catalysis core of cellobiohydrolases I fromTrichodermareesei[J]. Science,1994, 265 (5171): 524-528.
[11] GHATTYVENKATAKRISHNA P K,ALEKOZAI E M,BECKHAM G T,et al. Initial recognition of a cellodextrin chain in the cellulose-binding tunnel may affect cellobiohydrolase directional specificity[J]. Biophysical Journal,2013, 104(4): 904-912.
[12] NAKAMURA A,TSUKADA T,AUER S, et al. The tryptophan residue at the active site tunnel entrance ofTrichodermareeseicellobiohydrolase Cel7A is important for initiation of degradation of crystalline cellulose[J]. Journal of Biological Chemistry,2013, 288(19): 13 503-13 510.
[13] KUHAD R C, DESWAL D, SHARMA S, et al. Revisiting cellulase production and redening current strategies based on major challenges[J]. Renewable & Sustainable Energy Reviews,2016, 55: 249-272.
[14] 王禄山, 张玉忠, 高培基. 纤维二糖水解酶I吸附结构域的新功能[J]. 中国科学C辑:生命科学, 2008,38(7): 678-686.
[15] SEGATO F, DAMASIO A R L, GONCALVES T A, et al. Two structurally discrete GH7-cellobiohydrolases compete for the same cellulosic substrate fiber[J]. Biotechnol Biofuels,2012, 5(1): 21.
[16] 王春卉, 汪天虹, 高培基. 纤维素酶分子的纤维素吸附区的研究进展[J]. 纤维素科学与技术, 1997, 5 (4): 1-10.
[17] COLUSSI F, GARCIA W, ROEEETO F R, et al. Effect of pH and temperature on the global compactness, structure, and activity of cellobiohydrolase Cel7A fromTrichodermaharzianum[J]. European Biophysics Journal. 2012, 41(1): 89-98.
[18] 王一多. 红树林产紫青霉HBZ003纤维二糖水解酶的分离纯化及其基因克隆[D]. 海口:海南大学, 2013.
[19] KUNAMNENI A, PLOU F J, ALCALDE M, et al. Chapter 24-trichoderma enzymes for food industries[J]. Biotechnology and Biology of Trichoderma, 2014: 339-344.
[20] TAKAHASHI M, TAKAHASHI H, NAKANO Y, et al. Characterization of a cellobiohydrolase (MoCel6A) produced byMagnaportheoryzae[J]. Applied & Environmental Microbiology, 2010, 76(19): 6 583-6 590.
[21] KARI J, KONT R, BORCH K, et al. Anomeric selectivity and product profile of a processive cellulase[J]. Biochemistry, 2017, 56(1): 167-178.
[22] BARAMEE S, TEERAVIVATTANAKIT T, PHITSUWAN P, et al. A novel GH6 cellobiohydrolase fromPaenibacilluscurdlanolyticusB-6 and its synergistic action on cellulose degradation[J]. Applied Microbiology and Biotechnology, 2017, 101(3): 1 175-1 188.
[23] WANG Min, LU Xue-feng. Exploring the synergy between cellobiose Dehydrogenase fromPhanerochaetechrysosporiumand cellulase fromTrichodermareesei[J]. Frontiers in Microbiology,2016, 7(113):620.
[24] HILDEBRAND A, BENNETT ADDISON J, KASUGA J, et al. Cellobionic acid inhibition of cellobiohydrolase I and cellobiose dehydrogenase[J]. Biochemical Engineering Journal,2016, 109: 236-242.
[25] WU I, HEEL T, ARNOLD F H. Role of cysteine residues in thermal inactivation of fungal Cel6A cellobiohydrolases[J]. Biochimica et Biophysica Acta. 2013, 1 834(8): 1 539-1 544.
[26] XIN Dong-lin,GE Xiao-yan, SUN Zong-ping,et al. Competitive inhibition of cellobiohydrolase I by manno-oligosaccharides[J]. Enzyme & Microbial Technology,2015, 68: 62-68.
[27] OLIVA-TARAVILLA A, TOMS-PEJE, DEMUEZ M, et al. Phenols and lignin: Key players in reducing enzymatic hydrolysis yields of steam-pretreated biomass in presence of laccase[J].Journal of Biotechnology,2016, 218: 94-101.
[28] PINHEIRO G L, AZEVEDO-MARTINS A C D, ALBANO R M,et al. Comprehensive analysis of the cellulolytic system reveals its potential for deconstruction of lignocellulosic biomass in a novelStreptomycessp.[J]. Applied Microbiology Biotechnology, 2017, 101 (1) : 301-319.
[29] SONG Wen-xia, HAN Xiao-long, QIAN Yuan-chao, et al. Proteomic analysis of the biomass hydrolytic potentials ofPenicilliumoxalicumlignocellulolytic enzyme system[J]. Biotechnology Biofuels, 2016, 9 (1) : 1-15.
[30] http://www.so.com/link?url=http%3A%2F%2Fwww.labbase.net%2FSupply%2FSupplyItems-2826114.html&q=CBHI%E7%94%9F%E4%BA%A7%E5%85%AC%E5%8F%B8&ts=1497834045&t=b2f4a9980057f78e03be8ce7b0bc7bc&src=haosou.
[31] RIBEIRO O,WIEBE M,ILMÉN M, et al. Expression ofTrichodermareeseicellulases CBHI and EGI inAshbyagossypii[J]. Applied Microbiology and Biotechnology,2010, 87(4): 1 437-1 446.
[32] HAAN R D, KROUKAMP H, ZYL J H D V, et al. Cellobiohydrolase secretion by yeast: Current state and prospects for improvement[J]. Process Biochemistry, 2013, 48(1): 1-12.
[33] 李亚玲, 李多川, 滕芳超. 嗜热毛壳菌外切葡聚糖纤维二糖水解酶的纯化和部分性质研究[J]. 微生物学报, 2006, 46 (1): 143-146.
[34] SONG Jin-zhu,LIU Bei-dong,LIU Zhi-hua, et al. Cloning of two cellobiohydrolase genes fromTrichodermavirideand heterogenous expression in yeastSaccharomycescerevisiae[J]. Molecular Biology Reports,2010, 37(4): 2 135-2 140.
[35] WOON J S, MACKEEN M M, MAHADI N M, et al. Expression and characterization of a cellobiohydrolase (CBH7B) from the thermophilic fungusThielaviaterrestrisinPichiapastoris[J]. Biotechnology & Applied Biochemistry, 2016, 63(5): 690-698.
[36] VAILLANT F, MILLAN P, JARIEL O, et al. Optimization of enzymatic preparation for passion fruit juice liquefaction by fractionation of fungal enzymes through metal chelate affinity chromatography[J]. Food Biotechnology, 1999, 13(1): 33-50.
[37] SHARMA A, TEWARI R, RANA S S, et al. Cellulases: Classification, methods of determination and industrial applications[J]. Applied Microbiol Biotechnology, 2016, 179(8): 1 346-1 380.
[38] ALMEIDA J M A R, VIL D, CARP D, et al. Screening of mono-and bi-functional catalysts for the one-pot conversion of cellobiose into sorbitol[J]. Catalysis Today, 2017, 279: 187-193.
[39] 熊莲, 潘微, 陈新德, 等. 纤维二糖发酵生产丙酮丁醇[J]. 生物加工过程, 2012, 10(2):1-5.
[40] TANIMURA K, TAKASHIMA S, MATSUMOTO T, et al. 2,3-Butanediol production from cellobiose using exogenous beta-glucosidase-expressingBacillussubtilis[J]. Applied Microbiology and Biotechnology, 2016, 100(13): 1-9.
[41] ONG S A, NG Z J, WU J C. Production of high concentration of l-lactic acid from cellobiose by thermophilicBacilluscoagulansWCP10-4 [J]. Applied Microbiol Biotechnology,2016, 100(14): 6 501-6 508 .
[42] TURNER T L,ZHANGGuo-chang,OH E J, et al. Lactic acid production from cellobiose and xylose by engineeredSaccharomycescerevisiae[J].Biotechnology & Bioengineering, 2015, 113(5): 1 075-1 083.
[43] MOMENI M H, UBHAYASEKERA W, SANDGREN M, et al. Structural insights into the inhibition of cellobiohydrolase Cel7A by xylo-oligosaccharides[J].Febs Journal, 2015, 282(11): 2 167-2 177.
[44] CABEZAS L, CALDERON C, MEDINA L M, et al. Characterization of cellulases of fungal endophytes isolated fromEspeletiaspp.[J]. Journal of Microbiology, 2012, 50(6): 1 009-1 013.
[45] KASHYAP D R, VOHRA P K, CHOPRA S, et al. Applications of pectinases in the commercial sector: A review[J]. Bioresource Technology, 2001, 77(3): 215-227 .
[46] THOMPSON A J, HEU T,SHAGHASI T, et al. Structure of the catalytic core module of theChaetomiumthermophilumfamily GH6 cellobiohydrolase Cel6A[J]. Acta Crystallogr D Biol Crystallogr, 2012, 68(8): 875-882.
[47] ZHAO Jun-qi, SHI Peng-jun J, LI Zhong-yuan, et al. Two neutral thermostable cellulases fromPhialophorasp. G5 act synergistically in the hydrolysis of filter paper[J]. Bioresource Technology, 2012, 121(2): 404-410.
[48] WANG Xiu-juan J, PENG Yan-jie J, ZHANG Li-qing, et al. Directed evolution and structural prediction of cellobiohydrolase II from the thermophilic fungusChaetomiumthermophilum[J]. Applied Microbiol Biotechnology, 2012, 95(6): 1 469-1 478 .
[49] OLIVEIRA G S, ULHOA C J, OLIVEIRA M H L, et al. An alkaline thermostable recombinantHumicolagriseavar.thermoideacellobiohydrolase presents bifunctional (endo/exoglucanase) activity on cellulosic substrates[J]. World Journal of Microbiology and Biotechnology,2013, 29(1): 19-26.
[50] GREGOR T, CHRISTOPH O, ROBERT V, et al. Cellobiohydrolases produce different oligosaccharides from chitosan[J]. Biomacromolecules, 2016, 17(6): 2 284-2 292.
[51] http://www.so.com/link?url=http%3A%2F%2Fmycoclap.fungalgenomics.ca%2F&q=mycoCLAP&ts=1492792039&t=2c39cdd3e881e6bc517-e48136-f5-bc-db-&src=haosou.
[52] TEXIER H, DUMON C. Redefining XynA fromPenicilliumfuniculosumIMI 378536 as a GH7 cellobiohydrolase[J]. Journal of Industrial Microbiology & Biotechnology, 2012, 39(11): 1 569-1 576.
[53] 阎伯旭,高培基. 外切葡聚糖纤维二糖水解酶的分离纯化和部分性质研究[J]. 生物化学杂志, 1997, 13 (3): 362-364.
[54] HOBDEY S E, KNOTT B C, MOMENI M H, et al. Biochemical and structural characterizations of twoDictyosteliumcellobiohydrolases from the Amoebozoa kingdom reveal a high level of conservation between distant phylogenetic trees of life[J]. Applied and Environmental Microbiology, 2016, 82(11): 3 395-3 409.
[55] HERRERA-HERRERA A J, PÉREZ-AVALOS O, SALGADO L M, et al. Cyclic AMP regulates the biosynthesis of cellobiohydrolase inCellulomonasflavigenagrowing in sugar cane bagasse[J]. Archives of Microbiology, 2009, 191 (10): 745-750.
[56] GAVLIGHI H A, MEYER A S, DALGAARD M J. Enhanced enzymatic cellulose degradation by cellobiohydrolases via product removal[J]. Biotechnology Letters, 2013, 35(2): 205-212.
[57] TODA H, NAGAHATA N, AMANO Y, et al. Gene cloning of cellobiohydrolase II from the white rot fungusIrpexlacteusMC-2 and its expression inPichiapastoris[J]. Bioscience, Biotechnology, and Biochemistry, 2008, 72(12): 3 142-3 147.
[58] VOUTILAINEN S P, PURANEN T, SIIKA-AHO M, et al. Cloning, expression, and characterization of novel thermostable family 7 cellobiohydrolases[J]. Biotechnology & Bioengineering, 2008, 101(3): 515-528.
[59] TAMURA M, MIYAZAKI T, TANAKA Y, et al. Comparison of the structural changes in two cellobiohydrolases, CcCel6A and CcCel6C, fromCoprinopsiscinerea-A tweezer-like motion in the structure of CcCel6C[J]. FEBS Journal,2012, 279(10): 1 871-1 882.
[60] WU I, ARNOLD F H. Engineered thermostable fungal Cel6A and Cel7A cellobiohydrolases hydrolyze cellulose efficiently at elevated temperatures [J]. Biotechnology Bioengineering, 2013, 110(7): 1 874-1 883.
[61] LAHJOUJI K, STORMS R, XIAOZhi-zhuang, et al. Biochemical and molecular characterization of a cellobiohydrolase fromTrametesversicolor[J]. Applied Microbiol Biotechnology, 2007, 75(2): 337-346.
[62] DEVENDRAN S, ABDEL-HAMID A M, EVANS A F, et al. Multiple cellobiohydrolases and cellobiose phosphorylases cooperate in the ruminal bacteriumRuminococcusalbus8 to degrade cellooligosaccharides[J]. Scientific Reports,2016, 6: 35 342.
[63] BOONVITTHYA N,BOZONNET S,BURAPATANA V, et al. Comparison of the heterologous expression ofTrichodermareeseiendoglucanase II and cellobiohydrolase II in the yeastsPichiapastorisandYarrowialipolytica[J]. Molecular Biotechnology, 2013, 54(2): 158-169.
[64] DANA C M, DOTSONFAGERSTROM A, ROCHE C M, et al. The importance of pyroglutamate in cellulase Cel7A[J]. Biotechnology & Bioengineering, 2014, 111(4): 842-847.
Researchprogressofcellobiohydrolases
YUAN Mao-yi1,YE Fa-yin1, LEI Lin1,ZHAO Guo-hua1,2*
1(College of Food Science, Southwest University, Chongqing 400715, China)2(Chongqing Special Food Programme and Technology Research Center, Chongqing 400715,China)
Cellulose is the richest renewable resource in the world, and it has been a difficult and hot spot for research to break down into small molecular sugars and turn them into fuel or fine chemicals.Cellobiohydrolase is one of the key enzymes of cellulose biodegradation, which belongs to excision enzyme. It acts on the end of the chain of crystalline cellulose and cuts the separated beta-1,4-glycosidic bond, finally releasing cellobiose.In this article, the source, classification, structure, the action mechanism, enzymatic properties of cellobiohydrolases,molecular evolution, production situation of commercial enzymes and their application characteristics are summarized. In addition, the research perspective on cellobiohydrolasesis proposed.
cellobiohydrolase; enzymatic properties; structure; catalytic mechanism; application
10.13995/j.cnki.11-1802/ts.014791
硕士研究生(赵国华教授为通讯作者,E-mail: zhaoguohua1971@163.com。)。
果蔬典型加工过程中品质功能劣变与保质减损及其调控机理(2016YFD0400204-2);重庆市特色食品工程技术研究中心能力提升项目(cstc2014pt-gc8001)
2017-05-17, 改回日期:2017-06-20

