α-突触核蛋白通过Rab5B下调海马神经元膜表面NMDA受体含量及其介导的Ca2+内流和内向电流
于文娇 杨巍巍,3,4 李 昕,3,4 李旭冉 陈 敏 于 顺,3,4*
(1.首都医科大学宣武医院神经生物学研究室,北京市老年病医疗研究中心,北京 100053;2.首都医科大学帕金森病临床诊疗与研究中心,北京 100053;3.帕金森病北京市重点实验室和教育部神经变性病重点实验室,北京 100053;4.国家老年疾病临床医学研究中心,北京 100053;5.桂林医学院附属医院神经科学实验室,广西桂林 541001)
·Alpha-突触核蛋白的致病机制·
α-突触核蛋白通过Rab5B下调海马神经元膜表面NMDA受体含量及其介导的Ca2+内流和内向电流
于文娇1, 2杨巍巍1, 2,3,4李 昕1, 2,3,4李旭冉1, 2陈 敏1, 5*于 顺1, 2,3,4*
(1.首都医科大学宣武医院神经生物学研究室,北京市老年病医疗研究中心,北京 100053;2.首都医科大学帕金森病临床诊疗与研究中心,北京 100053;3.帕金森病北京市重点实验室和教育部神经变性病重点实验室,北京 100053;4.国家老年疾病临床医学研究中心,北京 100053;5.桂林医学院附属医院神经科学实验室,广西桂林 541001)
目的研究α-突触核蛋白(α-synuclein,α-Syn)对原代海马神经元膜表面N-甲基-D-天门冬氨酸(N-methyl-D-aspartate,NMDA)受体含量和功能的影响及其机制。方法细胞外添加基因重组α-Syn,使其进入原代培养神经元细胞内,观察敲减Rab5B基因前后神经元膜表面NMDA受体(NMDAR)和Rab5B表达的变化;NMDA激活NMDAR,活细胞工作站测定神经元Ca2+内流变化,全细胞膜片钳记录内向跨膜电流变化。结果α-Syn增加神经元Rab5B的表达,减少膜表面NMDAR含量,并因而抑制NMDA引起的Ca2+内流及内向跨膜电流;敲减Rab5B可逆转α-Syn对NMDAR的下膜作用及功能的影响。结论α-Syn通过上调Rab5B而下调海马神经元膜表面NMDAR含量及其介导的Ca2+内流及内向电流。
α-突触核蛋白;NMDA受体;Rab5B;神经元;海马
α-突触核蛋白(α-synuclein,α-Syn)异常表达存在于老化和某些神经系统退行性疾病如阿尔茨海默病(Alzheimer’s disease,AD)、帕金森病(Parkinson’s disease,PD)、路易体痴呆(dementia with Lewy body,DLB)患者的脑中[1]。老化和上述变性脑均存在记忆功能下降或障碍的表现[2]。海马是与记忆密切相关的神经结构。以往研究[3-6]表明,海马神经元表面与记忆密切相关的谷氨酸受体——N-甲基-D-天门冬氨酸受体(N-methyl-D-aspartate receptors,NMDARs)在老化和变性脑中发生显著的变化,但其机制不清楚。笔者以往研究[7]表明,α-Syn的异常表达可以促进多巴胺神经元膜表面NMDARs的内在化,导致膜表面NMDARs减少,这一作用与α-Syn上调Rab5B表达有关。Rab5B是一种小的GTPases蛋白,是NMDARs内在化所必需的蛋白[8]。鉴于老化和变性脑的海马结构存在α-Syn的异常积聚,笔者推测,海马神经元膜表面NMDARs的变化可能与α-Syn有关。本研究将利用原代培养大鼠海马神经元观察α-Syn对神经元膜表面NMDARs内在化及功能的影响,并分析Rab5B的作用。
1 材料与方法
1.1 材料
健康出生12 h内Wistar新生大鼠,购自中国人民解放军军事医学科学实验动物中心,实验动物许可证号:SCXK-2014-004。杜恩斯组织匀浆器(Wheaton Kimble公司,美国);Neurobasal A培养基、B27、DMEM培养基(Gibco公司,美国);胎牛血清(FBS,Berlin公司,美国);NMDA、MK-801(Sigma公司,美国);Rab5B反义寡核苷酸(百恩维公司,中国);NMDAR NR1抗体(Abcam公司,美国);Rab5B抗体(Santa Cruz公司,美国);Fluo4-AM(东仁化学公司,日本)。
Hank’s平衡盐溶液A(HBSS,不含Ca2+、Mg2+)(mmol/L):NaCl 0.14,KCl 0.54×10-2,葡萄糖0.006,KH2PO40.44×10-3,Na2HPO40.33×10-3,调整pH至7.2;Hank’s平衡盐溶液B(HBSS,不含Mg2+)(mmol/L):NaCl 0.14,KCl 0.54×10-2,葡萄糖 1,KH2PO40.44×10-3,Na2HPO40.33×10-3,CaCl20.13×10-2,调整pH至7.2。电极内液(mmol/L):CsCl 70,NaCl 10,4-羟乙基哌嗪乙磺酸(HEPES)10,EGTA 10,ATP 2,GTP 0.2,调整pH至7.3;细胞外液(mmol/L):NaCl 150,KCl 5,CaCl21.4,葡萄糖 10,HEPES 5,河豚毒素(TTX)0.000 3,木防仪苦毒素(picrotoxin)0.1,NBQX 0.01,士的宁(trychnine)0.1,调整pH至7.3,调整渗透压至330 mOsm。
活细胞工作站(Leica公司,德国);玻璃微电极拉制仪(Narishige公司,日本);膜片钳放大器(Multiclamp 700B)(HEKA公司,德国),数据采样板(Digidata 1322A)和数据采集分析软件(pClamp 9.2)(Molecular Devices公司,美国);MiniAnalysis软件包(Synaptosoft公司,美国)。
1.2 方法
1.2.1 重组人α-Syn的制备
基因重组人α-Syn的表达和纯化根据本室已建立的方法进行[7]。纯化后的蛋白分别用SDS-PAGE和Western blotting法进行鉴定,BCA法定量后备用。
1.2.2 海马原代神经元培养
原代神经元培养方法根据已建立的方法[9]进行:将新生鼠断头,剥离鼠脑后于显微镜下分离并剪碎双侧海马,胰蛋白酶消化30 min后,用含10%(体积分数)FBS的DMEM培养基终止消化。玻璃滴管火焰抛光后,轻柔吹打分散细胞,计数细胞密度。以2 × 105个/cm2密度接种到多聚赖氨酸包被的35 mm培养皿中,于37 ℃,5%(体积分数) CO2的培养箱内培养。待神经元贴壁后,改用Neurobasal A、B27无血清培养基继续培养,每3 d换半液,培养10~12 d。
1.2.3 Rab5B反义寡核苷酸的制备
Rab5B反义寡核苷酸(antisense sequence,AS)及扰乱顺序的寡核苷酸(scrambled sequence,SS),其序列分别是:5′-GCTGTGCTTCTGCTAGTCATTTCAAGAG AATGACTAGCAGAAGCACAGCTTTTTTCTCGAGG-3′和5′-GATCCCTCGAGAAAAAAGCTGTGCTTCTGCTA GTCATTCTCTTGAAATGACTAGCAGAAGCACAGC-3′。
1.2.4 细胞免疫荧光检测
细胞用PBS漂洗后,预冷的4%(质量分数)多聚甲醛固定细胞30 min,含有1%(体积分数)Triton-X 100的PBST溶液室温孵育30 min,1%(质量分数) BSA封闭1 h。加入抗NMDAR NR1抗体(1∶1 000)或抗Rab5B抗体(1∶1 000),于4 ℃反应过夜。PBST洗3次,每次10 min。加入荧光二抗(1∶5 000),室温反应2 h。PBST洗3次,每次10 min。激光共聚焦显微镜观察。
1.2.5 细胞内Ca2+浓度的测定
细胞培养皿置于活细胞工作站载物台上,激发光波长为488 nm,发射光波长为515 nm,固定参数后每10 s扫描一次,扫描180s作为基线。加入NMDA(100μmol/L)及甘氨酸(10μmol/L),继续扫描目的细胞内的Ca2+荧光强度改变,图像由随机软件分析处理(Leica公司,德国)。胞内的Ca2+荧光强度变化用Fluo-4与Ca2+结合后的荧光强度比值表示。计算公式:荧光强度变化=Ft/F0(Ft:添加NMDA后第t秒神经元内的荧光复合物的荧光强度,F0:基线水平的神经细胞内的荧光复合物的荧光强度[7])。
1.2.6 全细胞膜片钳记录细胞膜电流及给药
采用常规全细胞记录模式[10],记录细胞的电信号,输入膜片钳放大器,而后经由模数转换仪输入到计算机,经10 Hz滤波,采样频率为2~3 Hz。记录信号的监测、储存及通过记录点击给予细胞的指令电压以及分析处理由Axon软件pClamp 8.0和Clampfit 8.0完成。全细胞记录在22~24 ℃室温环境下进行,连续记录30 min以上。
通过软件控制正压给药系统(DAD-12 Superfusion System)的通道灌注NMDA(100 μmol/L)、Glycine(10 μmol/L)。使用HL-2型恒流泵进行细胞外灌注冲洗细胞,以终止药物作用。
1.3 统计学方法

2 结果
2.1 Rab5B参与α-Syn引起的神经元膜表面NMDAR 亚单位NR1的内在化
免疫荧光标记显示,没有α-Syn处理的神经元(PBS组),NR1主要分布于神经元的膜表面。外加α-Syn处理后,神经元膜表面NR1显著减少,胞质中的NR1明显增多,提示NR1发生内在化。单纯转染Rab5B SS和Rab5B AS的神经细胞,NR1也主要分布于神经元的膜上。用α-Syn处理Rab5B SS转染神经元,膜表面的NR1显著减少,胞质中的NR1明显增多,而在Rab5B AS转染神经元,α-Syn使NR1内在化的作用消失,提示α-Syn促进NMDAR NR1的内在化的作用需要Rab5B的参与(图1)。
2.2 Rab5B参与α-Syn抑制NMDA受体介导的Ca2+内流
活细胞工作站结果显示(n=5),NMDA引起 [Ca2+]i快速而显著增加,NMDA的这一作用被NMDA受体特异性阻断剂MK-801完全阻断。事先用α-Syn处理神经元后,则 NMDA引起的[Ca2+]i增加的作用明显受到抑制,而用Rab5B-AS抑制Rab5B表达后,α-Syn抑制[Ca2+]i增加的作用被消除(图2)。
2.3 Rab5B参与α-Syn抑制NMDA受体介导的跨膜内向电流
将钳制电压设定为-75 mV条件下时,NMDA受体介导的内向电流随受体特异性激动剂NMDA浓度的增加而增加(图3)。α-Syn处理细胞后,NMDA受体介导的内向电流受到抑制,且此抑制作用在Rab5B AS转染细胞后消失(图4)。
3 讨论
本研究结果表明,利用外加重组α-Syn处理海马神经元后,可使神经元膜表面NMDARs亚单位NR1减少,胞质含量增加。鉴于NR1是构成NMDAR的必需亚单位,其在神经元膜表面的减少意味着完整的具有功能的NMDARs发生了内在化。笔者以往的研究[11]表明,将α-Syn添加到细胞外,α-Syn可以迅速进入细胞并引起细胞内α-Syn含量增加,由此推测,细胞外添加α-Syn导致的NMDARs的内在化,很可能是α-Syn进入细胞内引起的。作为支持这一推测的证据,在α-Syn过表达的神经细胞中,NMDARs也发生内在化。由于Rab5B蛋白是内吞机制中的关键蛋白,能够促进细胞膜表面蛋白的内在化[8],笔者推测,α-Syn引起的海马神经元膜表面NMDARs的内在化可能有Rab5B的参与。为了证明这一点,笔者利用小干扰RNA敲减Rab5B,观察α-Syn对NMDARs内在化的影响。结果显示,敲减Rab5B后,α-Syn引起的NMDARs内在化明显受到抑制。
如前所述,神经元膜表面NR1亚单位的减少意味着膜表面功能性NMDARs的减少。由于NMDARs在通道开放情况下可以介导Na+和Ca2+的内流和由此引起的内向电流,笔者分别利用活细胞工作站和膜片钳技术研究了α-Syn处理和非处理神经元NMDARs特异性激动剂NMDA对Ca2+内流和内向跨膜电流的影响。在无α-Syn处理的神经元,NMDA引起细胞内Ca2+浓度快速而显著增加,同时可以记录到NMDARs介导的内向电流,NMDA的这一作用呈浓度依赖性。而在α-Syn处理神经元,NMDA引起细胞内Ca2+浓度增加以及NMDAR介导的内向电流明显受到抑制。这一结果提示,α-Syn引起了NMDARs功能的变化,这一变化很可能是通过促进NMDARs的内在化从而导致神经元膜表面功能性的NMDARs减少。由于α-Syn的上述作用可因敲减Rab5B而被明显抑制,因此推测Rab5B参与了α-Syn引起的NMDARs内在化过程。
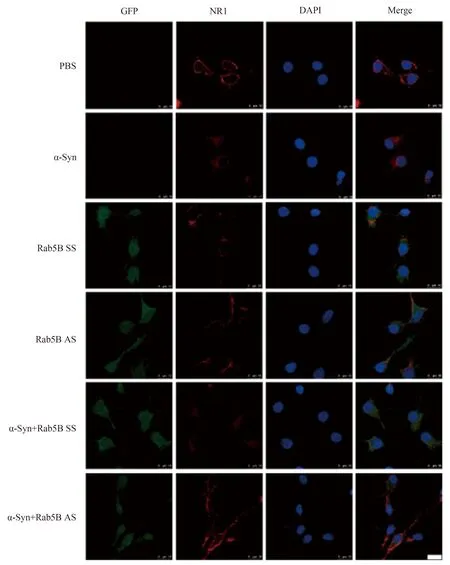
图1 Rab5B在α-Syn所致细胞膜表面NR1内在化中的作用Fig.1 The role of Rab5B in α-Syn-induced NR1 internalization
Representative immunofluorescence images showing NR1 (red),Rab5B SS or AS (green),and DAPI (blue).Cells were incubated separately with α-Syn (10 μmol/L) for 24 h or LV-GFP-Rab5B AS for 3 days,with or without added α-Syn.Other batches of cells were treated with corresponding vehicles,PBS and LV-GFP-Rab5B SS,either in the presence or absence of α-Syn.Each batch of cells was then stained for immunofluorescence microscopy and nuclei were counterstained with DAPI.Images were captured under a 100×objective with equal exposure times to allow direct comparison.Each of the three independent replicates was assessed from three to ten random fields of view.n=5 in each group,bar=10 μm.NR1: N-methyl-D-aspartate receptors1;PBS: phosphate buffered saline;α-Syn: α-synuclein;SS: scrambled sequence;AS: antisense sequence;GFP: green fluorescent protein.
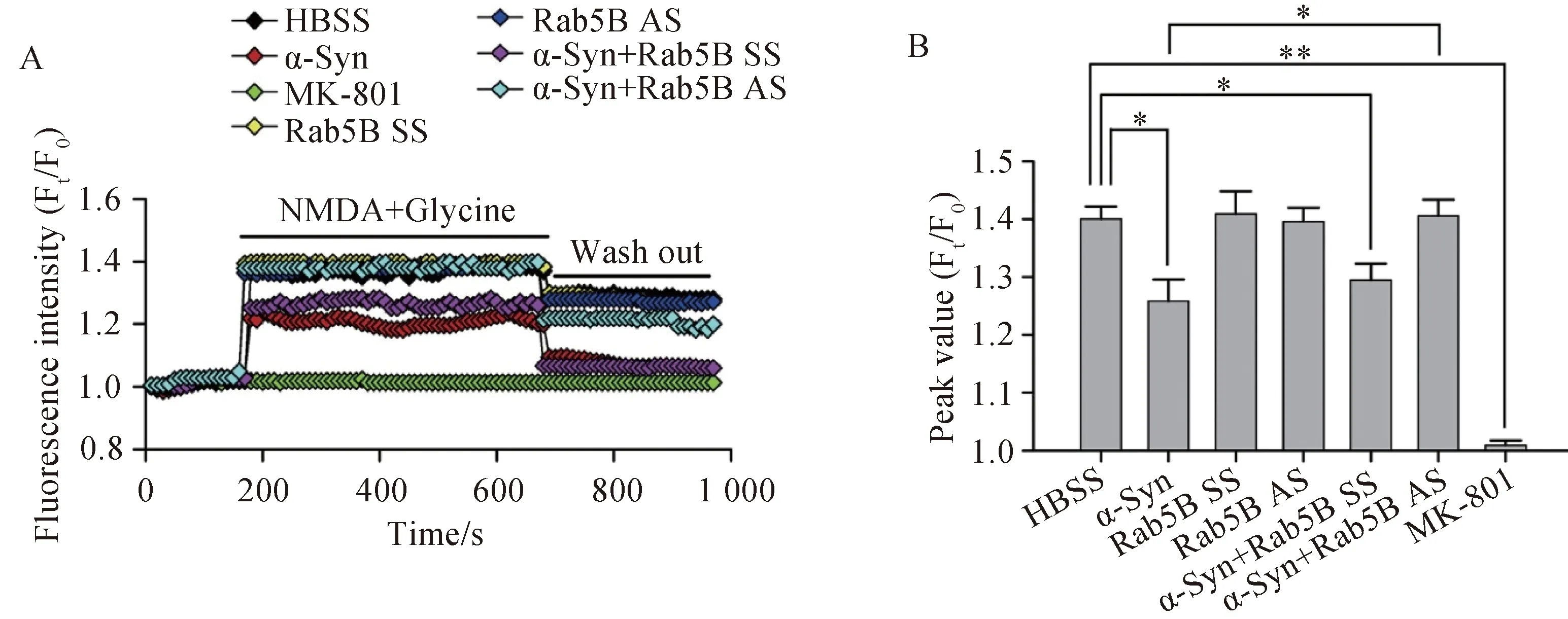
图2 α-Syn对于NMDA引起的[Ca2+]i增加的影响Fig.2 Effect of α-Syn on NMDA-induced elevation of [Ca2+]i
Cells were transfected with Rab5B SS or Rab5B AS for three days,and then treated with α-Syn (10μmol/L) for 24 h before observing NMDAR-mediated Ca2+influx.Cells were stained Ca2+indicator fluo4-AM.A: Dynamic changes in [Ca2+]i as indicated by fluorescence intensity (Ft/F0);B: Statistical peak values of [Ca2+]i before and after NMDA stimulation.n=5 in each group;*P<0.05,**P<0.01;α-Syn: α-synuclein;NMDA:N-methyl-D-aspartate;HBSS:Hank’s balanced salt solution;SS: scrambled sequence;AS: antisense sequence;Ft: fluorescence of the indicator at t second;F0: fluorescence of the indicator at 0 second;[Ca2+]i: Ca2+influx.
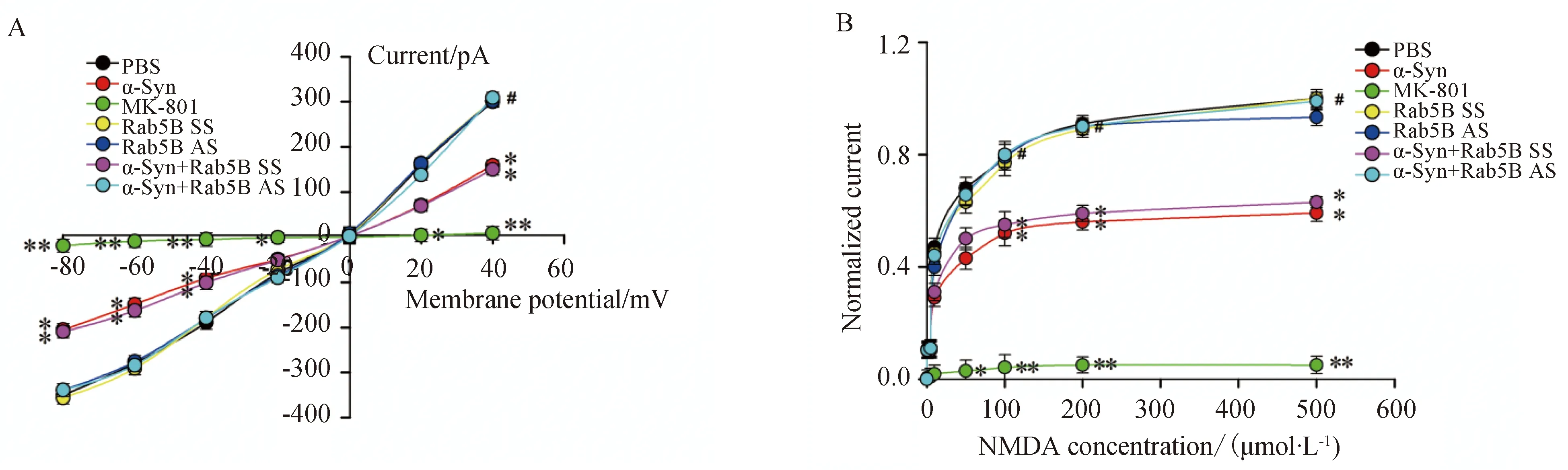
图3 α-Syn对不同浓度的NMDA诱发内向跨膜电流的影响Fig.3 The effect of α-Syn on currents evoked by NMDA of different concentrations
Whole cell patch clamp experiments were used to record NMDA receptor-mediated currents in cultured hippocampal neurons.Batches of cells were treated as described above.A,BCurrents in α-Syn-treated cells(10 μmol/L) were decreased at a range of agonist concentrations (0,10,20,50,100,200 and 500μmol/L) and returned to robust levels on addition of LV-GFP-AS (n=5,*P<0.05,**P<0.01vsPBS-treated group,#P<0.05vsα-Syn group);α-Syn: α-synuclein;NMDA:N-methyl-D-aspartate;SS: scrambled sequence;PBS: phosphate buffered saline;AS: antisense sequence.
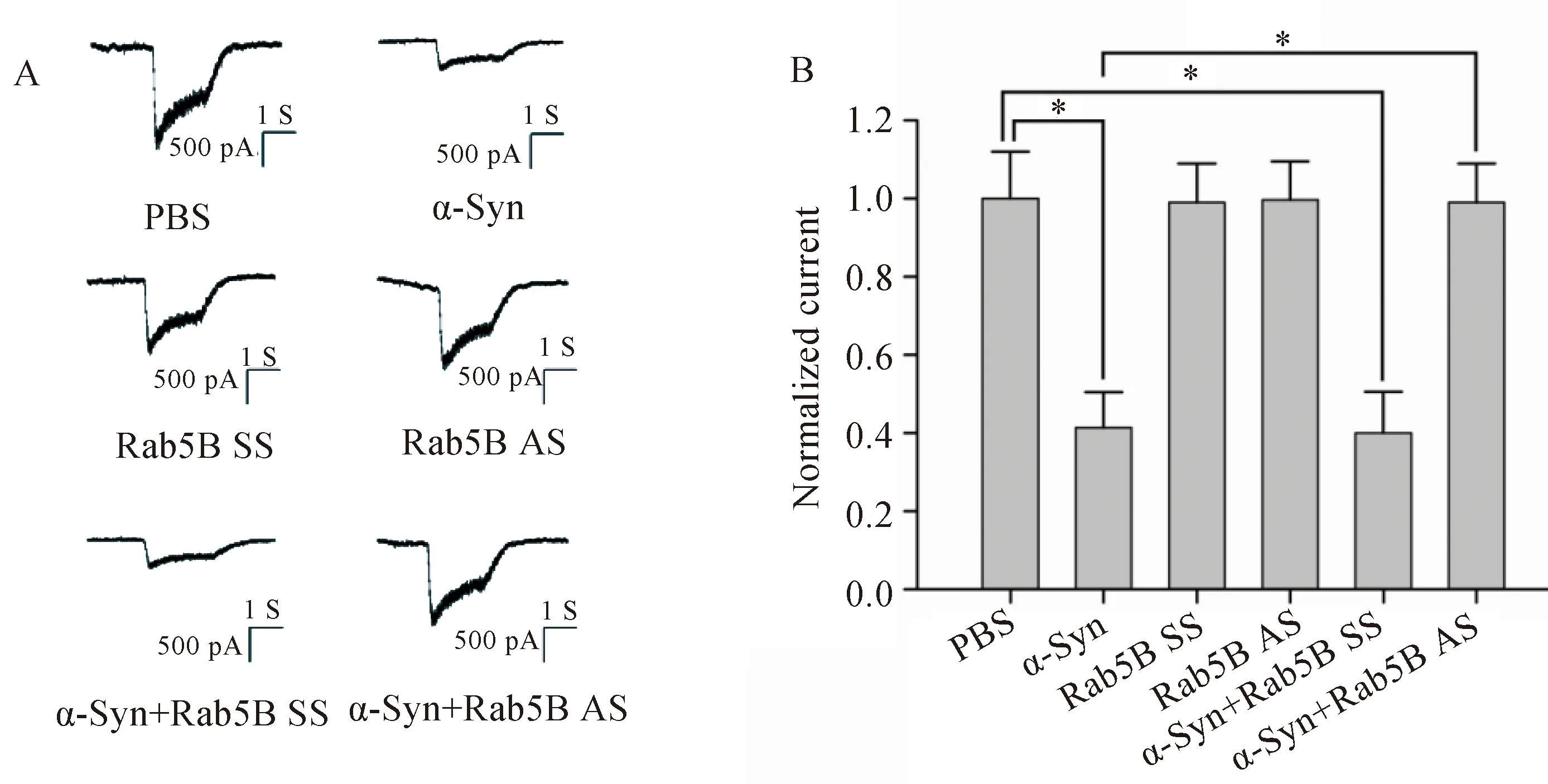
图4 α-Syn抑制NMDA受体介导的内向电流Fig.4 α-Syn reduced NMDAR-mediated inward currents
A: Trace of NMDA receptor-mediated currents in each batch of cells.B: Bar graphs shows the normalized currents after NMDA stimulation.n=5 in each group;*P<0.05;α-Syn: α-synuclein;NMDA:N-methyl-D-aspartate;PBS: phosphate buffered saline;SS: scrambled sequence;AS: antisense sequence.
本研究为老化和某些神经退行性疾病脑的海马神经元膜表面NMDARs变化的机制提供了实验依据。
[1] Yu S,Ueda K,Chan P.Alpha-synuclein and dopamine metabolism[J].Mol Neurobiol,2005,31(1-3): 243-254.
[2] Aarsland D,Creese B,Politis M,et al.Cognitive decline in Parkinson disease[J].Nat Rev Neurol,2017,13(4):217-231.
[3] Horak M,Holubova K,Nepovimova E,et al.The pharmacology of tacrine at N-methyl-d-aspartate receptors[J].Prog Neuropsychopharmacol Biol Psychiatry,2017,75:54-62.
[4] Hall H,Reyes S,Landeck N,et al.Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease[J].Brain,2014,137(Pt 9): 2493-2508.
[5] Ullman M T,Pullman M Y.A compensatory role for declarative memory in neurodevelopmental disorders[J].Neurosci Biobehav Rev,2015,51: 205-222.
[6] Foster T C,Kyritsopoulos C,Kumar A.Central role for NMDA receptors in redox mediated impairment of synaptic function during aging and Alzheimer’s disease[J].Behav Brain Res,2017,322(Pt B):223-232.
[7] Cheng F,Li X,Li Y,et al.alpha-Synuclein promotes clathrin-mediated NMDA receptor endocytosis and attenuates NMDA-induced dopaminergic cell death[J].J Neurochem,2011,119(4):815-825.
[8] Arnett A L,Bayazitov I,Blaabjergm,et al.Antisense oligonucleotide against GTPase Rab5b inhibits metabotropic agonist DHPG-induced neuroprotection[J].Brain Res,2004,1028(1): 59-65.
[9] 王鹏,李昕,陈予东,等.α-突触核蛋白寡聚体抑制大鼠原代培养神经元突起早期生长[J].首都医科大学学报,2014,35 (5): 587-591.
[10] Wu H Y,Yuen E Y,Lu Y F,et al.Regulation of N-methyl-D-aspartate receptors by calpain in cortical neurons[J].J Biol Chem,2005,280(22): 21588-21593.
[11] Yin J,Han J,Zhang C,et al.C-terminal part of alpha-synuclein mediates its activity in promoting proliferation of dopaminergic cells[J].J Neural Transm (Vienna),2011,118(8):1155-1164.
α-SynucleinreducessurfaceexpressionsofNMDAreceptorsandNMDA-invokedCa2+influxandinwardcurrentbyupregulationofRab5Binculturedprimaryhippocampalneurons
Yu Wenjiao1,2,Yang Weiwei1,2,3,4,Li Xin1,2,3,4,Li Xuran1,2,Chen Min1,5*,Yu Shun1,2,3,4*
(1.DepartmentofNeurobiology,XuanwuHospital,CapitalMedicalUniversity,BeijingInstituteofGeriatrics,Beijing100053,China;2.ClinicalCenterforParkinson’sDisease,CapitalMedicalUniversity,Beijing100053,China;3.BeijingKeyLaboratoryforParkinson’sDiseaseandKeyLaboratoryofNeurodegenerativeDiseases,MinistryofEducation,Beijing100053,China;4.NationalClinicalResearchCenterforGeriatricDisorders,Beijing100053;5.LaboratoryofNeuroscience,AffiliatedHospitalofGuilinMedicalUniversity,Guilin541001,GuangxiZhuangAutonomousRegion,China)
ObjectiveTo investigate the effects of α-synuclein(α-Syn) on surface expressions and functions of N-methyl-D-aspartate (NMDA) receptors (NMDARs) in cultured primary hippocampal neurons.MethodsIncreased intracellular α-Syn levels were realized by extracellular addition of recombinant human α-Syn to the culture medium of rat primary hippocampal neurons.Expressions of surface NMDARs and Rab5B were observed by fluorescence immunocytochemistry and Western blotting.Rab5B antisense oligonucleotides were applied to suppress Rab5B expression.Live cell imaging system and whole-cell patch-clamp recording were used to record NMDA-evoked Ca2+influx and inward currents.ResultsNeurons treated with α-Syn displayed increased Rab5B,decreased surface NMDARs,and reduced NMDA-evoked Ca2+influx and inward currents.The above α-Syn effects were inhibited by suppression of Rab5B expression.Conclusionα-Syn reduces the surface expression and functions of NMDARs by increasing Rab5B expression.
α-synuclein;NMDA receptor;Rab5B;neuron;hippocampus
国家自然科学基金(81371200,81071014,81401042),北京市医院管理局“使命”计划专项经费资助(SML20150803),北京市科学技术委员会资助(Z161100005116011,Z171100000117013),北京市卫生和计划生育委员会“老年重大疾病关键技术研究”(PXM2017_026283_000002),广西自然科学基金(2014GXNSFAA118197)。This study was supported by National Natural Science Foundation of China (81371200,81071014,81401042),Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150803),Beijing Municipal Science &Technology Commission (Z161100005116011,Z171100000117013),Beijing Municipal Commission of Health and Family Planning (PXM2017_026283_000002),Natural Science Foundation of Guangxi (2014GXNSFAA118197).
*Corresponding authors,E-mail:yushun103@163.com,chenmin790830@163.com
时间:2017-12-13 21∶29
http://kns.cnki.net/kcms/detail/11.3662.R.20171213.2129.066.html
10.3969/j.issn.1006-7795.2017.06.018]
Q189
2017-10-23)
编辑 陈瑞芳

