Brain metastasis in advanced colorectal cancer: results from the South Australian metastatic colorectal cancer (SAmCRC)registry
Gonzalo Tapia Rico, Timothy J. Price, Christos Karapetis, Cynthia Piantadosi, Rob Padbury, Amitesh Roy,
Guy Maddern4,5, James Moore6, Scott Carruthers7, David Roder8, Amanda R. Townsend11Department of Medical Oncology, The Queen Elizabeth Hospital and University of Adelaide, Adelaide SA 5011, Australia;2Department of Medical Oncology, Flinders Medical Center and Flinders University, Adelaide SA 5042, Australia;3Department of Surgery, Flinders Medical Center, Bedford Park SA 5042, Australia; 4Department of Surgery, The Queen Elizabeth Hospital, Adelaide SA 5011, Australia; 5Department of Surgery, University of South Australia, Adelaide SA 5005,Australia; 6Department of Surgery, Royal Adelaide Hospital, Adelaide SA 5000, Australia; 7Department of Radiation Oncology, Royal Adelaide Hospital, Adelaide SA 5000, Australia; 8Department of Epidemiology, University of South Australia,Adelaide SA 5005, Australia
ORIGINAL ARTICLE
Brain metastasis in advanced colorectal cancer: results from the South Australian metastatic colorectal cancer (SAmCRC)registry
Gonzalo Tapia Rico1, Timothy J. Price1, Christos Karapetis2, Cynthia Piantadosi3, Rob Padbury3, Amitesh Roy2,
Guy Maddern4,5, James Moore6, Scott Carruthers7, David Roder8, Amanda R. Townsend11Department of Medical Oncology, The Queen Elizabeth Hospital and University of Adelaide, Adelaide SA 5011, Australia;2Department of Medical Oncology, Flinders Medical Center and Flinders University, Adelaide SA 5042, Australia;3Department of Surgery, Flinders Medical Center, Bedford Park SA 5042, Australia;4Department of Surgery, The Queen Elizabeth Hospital, Adelaide SA 5011, Australia;5Department of Surgery, University of South Australia, Adelaide SA 5005,Australia;6Department of Surgery, Royal Adelaide Hospital, Adelaide SA 5000, Australia;7Department of Radiation Oncology, Royal Adelaide Hospital, Adelaide SA 5000, Australia;8Department of Epidemiology, University of South Australia,Adelaide SA 5005, Australia
Objective: Brain metastasis is considered rare in metastatic colorectal cancer (mCRC); thus, surveillance imaging does not routinely include the brain. The reported incidence of brain metastases ranges from 0.6% to 3.2%.Methods: The South Australian mCRC Registry (SAmCRC) was analyzed to assess the number of patients presenting with brain metastasis during their lifetime. Due to small numbers, a descriptive analysis is presented.Results: Only 59 patients of 4,100 on the registry at the time of analysis had developed brain metastasis (1.4%). The clinical characteristics of those with brain metastasis were as follows: the median age was 65.3 years and 51% were female. Where the VKi-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation status of the tumor was known, the majority harbored a KRAS mutation (55%); 31 (53%) underwent craniotomy and 55 (93%) underwent whole-brain radiotherapy. The median survival time from diagnosis of brain metastasis was 4.2 months (95% confidence interval 2.9–5.5). Patients who underwent craniotomy and radiotherapy had superior survival compared to those who underwent whole-brain radiotherapy (8.5 months vs. 2.2 months,respectively). Data from the SAmCRC (a population-based registry) confirm that brain metastases are rare and the median time to development is approximately 2 years.Conclusions: Brain metastasis is a rare outcome in advanced CRC. Patients within the registry tended to be female, young in age,and harbored with higher rates of KRAS mutations. Whether routine surveillance brain scanning should be considered remains controversial given the relative rarity of developing brain metastases in mCRC and ultimately, most patients with central nervous system involvement die from their extracranial disease.
Brain metastasis; colorectal cancer; survival; surveillance
Introduction
Patterns of care for patients with metastatic colorectal cancer(mCRC) have evolved leading to longer survival with the median now reaching 30 months1,2. Brain metastasis is considered rare in mCRC and surveillance imaging does not routinely include the brain in neurologically asymptomaticpatients3,4. The reported incidence of brain metastasis ranges in the literature from 0.6% to 3.2%5. In this setting, brain metastases usually occur in advanced stages of the disease and are usually observed concomitant with liver involvement(50%) or lung metastases (80%)6,7.
Central nervous system (CNS) metastases are a late manifestation of mCRC and confer significant morbidity and poorer median survival on such patients, with a life expectancy without treatment of approximately 4 to 6 weeks.The vast majority of mCRC patients that develop brain metastases are treated with palliative intent, since most are expected to die from their extracranial disease8,9. To date,there is no consensus on their management, which is usually determined by the presence of disseminated disease, patient performance status, and previous response to treatments.Many affected patients will suffer considerable loss of autonomy due to neurocognitive and functional deficits,as well as morbidity associated with steroids and anticonvulsants. Only a small number of patients who can have surgical resection with or without postoperative wholebrain radiotherapy (WBRT) are considered, with more encouraging outcomes [median overall survival (mOS) of approximately 8 months]10-12. Over the last few years,stereotactic radiosurgery has become another treatment modality with outcomes appearing to be comparable to those of surgical resection for patients with oligometastases that are small and present minimal mass effects. The advantages of this treatment are its minimal invasiveness, low rate of posttreatment complications, and a high rate of local tumor control13.
The incidence of brain metastases in oncology is gradually increasing, which can be explained in part by earlier detection with new imaging techniques and in part by longer survival due to advances in systemic treatments for metastatic disease. In this study, we have analyzed the South Australian mCRC population-based registry to assess the frequency of brain metastasis, the timing of presentation, as well as potential risk factors in the South Australian population,which may together guide those mCRC patients who are at a higher risk of developing brain metastases.
Patients and methods
The SAmCRC registry is a state-wide, population-based database of all patients diagnosed with synchronous or metachronous mCRC since February 2006. The registry is managed by a committee of surgeons, oncologists,epidemiologists, and clinical trial and data collection experts.The clinical membership is drawn from the state’s major public hospitals (Flinders Medical Center, Royal Adelaide Hospital, and The Queen Elizabeth Hospital) and associated private hospitals. The SAmCRC registry data were analyzed to assess the number of patients detected to have developed brain metastasis during their lifetime. For this study, we included data which were collected between February 2, 2006 and March 31, 2016. Patient characteristics were reported and overall survival was analyzed using the Kaplan-Meier method. Statistical analysis was performed using SPSS version 18.0 (IBM software) and GraphPad Prism version 5(GraphPad Software, San Diego).The local Human Research Ethics Committee (TQEH/FMC/LMH) approved the study protocol (Ethics reference number is 2006133).
Results
Population
At the time of analysis, more than 4, 000 mCRC patients were enrolled into the registry between February 2, 2006 and March 31, 2016. Only 59 patients developed brain metastasis(1.4% of our total population). The median follow-up time was 23 months at the time of analysis. Demographic and CRC data are summarized in Table 1. The clinical characteristics of those with brain metastases compared to those across the whole registry were as follows: median age at diagnosis, 65.3 years vs. 70.8 years; 51% vs. 43% women;metastatic sites at initial diagnosis, 54% lung (32 patients hadlung involvement) vs. 15%, 47% liver (28 patients had hepatic secondaries documented) vs. 35%, and 7% bone vs.2.6%. The primary site of CRC was 29% right-sided colon and 70% left-sided CRC (34% rectum, 36% left colon/sigmoid) for the brain metastasis group. This was in contrast to those across the whole registry, where there was 36% right primary and 28% rectum. The left colon prevalence was identical for both groups (36%). Where the KRAS status was known (27 patients in our cohort), 55% of them had KRAS exon 2 mutations compared to 42% across the whole registry. Regarding prior surgery for lung/liver metastases, 25% (15/59) underwent liver resection and lung metastases were resected from only 5 patients (8%). The median number of prior lines of chemotherapy before diagnosis of brain metastasis was 2 (range: 0–4 lines).
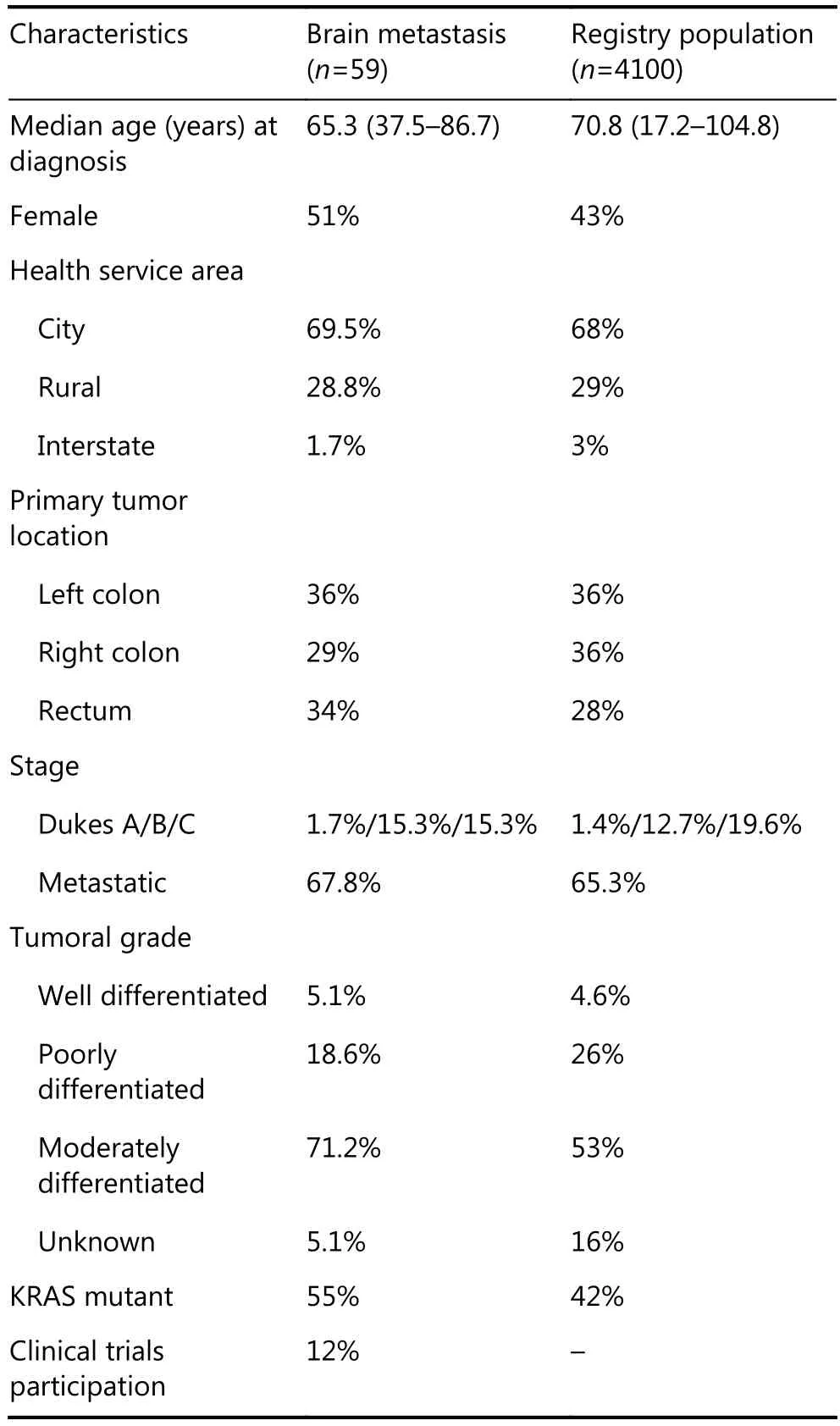
Table 1 Patient’s characteristics
Overall and brain metastases-free survival
Complete details and follow-up data were available for 58 patients. The median interval between diagnosis of the primary cancer and identification of brain metastasis was 1.9 years (range: 0–6 years). The mOS from diagnosis of brain metastasis was 4.2 months 95%CI 2.9–5.5 months (Figure 1).Excluding 8 patients that presented with synchronous cerebral metastasis, the median time to CNS metastases was 2.05 years (range: 0.3–6.0 years) and median survival was 3.8 months (95%CI 2.0–5.7 months). Thirty-one patients (53%)underwent craniotomy and 93% underwent WBRT. The mOS for patients that underwent craniotomy and radiotherapy was 8.5 months (95% CI 6.9–10 months)compared to 2.2 months (95% CI 1.5–2.9 months) with WBRT alone (Figure 2).
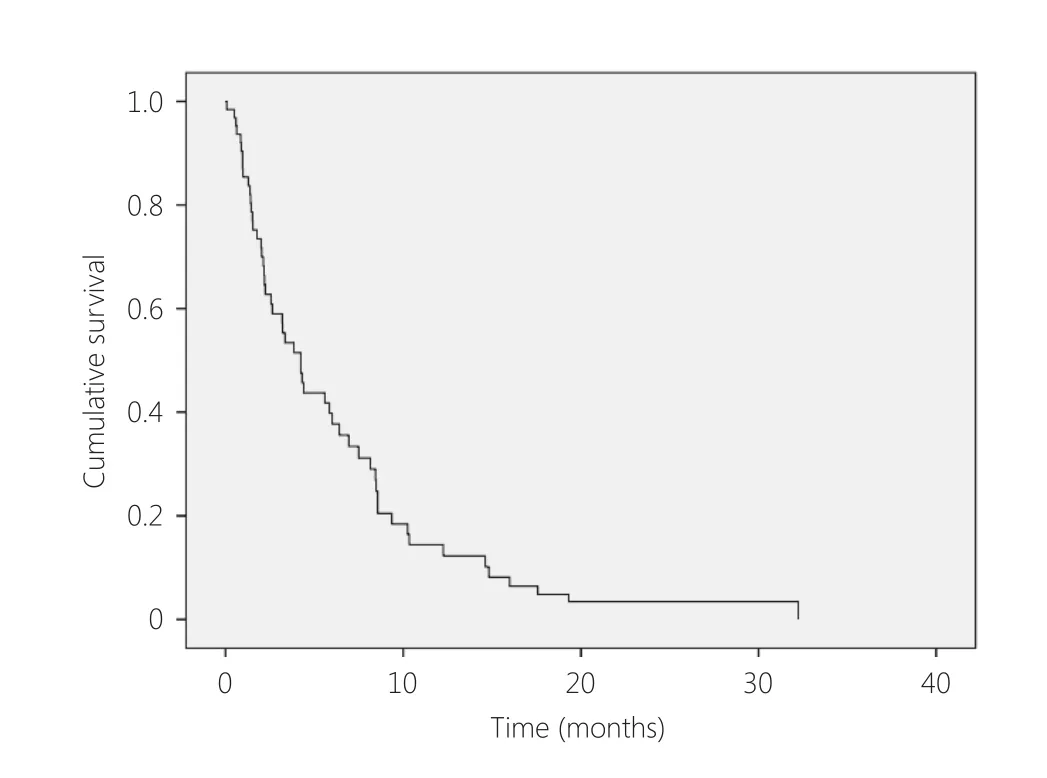
Figure 1 OS from diagnosis of brain metastases
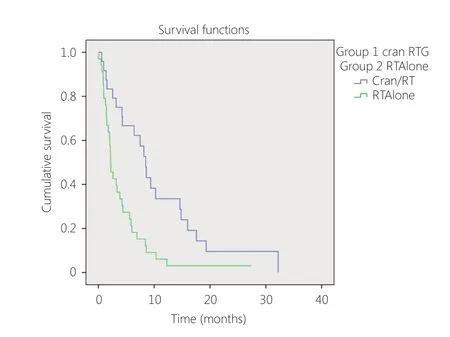
Figure 2 Survival craniotomy/radiotherapy vs. radiotherapy alone
Discussion
A diagnosis of brain metastases from CRC is an uncommon clinical situation and the risk factors for developing CNS secondaries are poorly understood. Although the number of studies reviewing brain metastases from CRC has been limited to date, the incidence is estimated to be between 0.6%and 3.2%. However, the small sample size and statistical variations of these studies, the differences in diagnostic methods, and the lack of neurological symptoms in some patients make it very difficult to establish the true incidence of brain metastases. The incidence of CNS metastases observed in our study (1.4%) is similar to what has been previously observed5. It is thought that the incidence of brain metastases recorded from all solid tumors has steadily increased over the last two decades due to the increase in survival of patients, better radiological diagnostic methods,and in particular, the widespread use of magnetic resonance imaging (MRI) to detect small metastases. However, in the specific case of CRC, the incidence seems to have remained somewhat unchanged, as supported by the outcome of our analysis5,14.
Brain metastases in mCRC are generally associated with advanced stage and most diagnoses of CNS secondaries are metachronous. In the literature, brain metastases from mCRC are reported mainly in patients with lung and liver metastases: between 55% to 85% of brain metastasis diagnoses are associated with lung involvement and between 22% and 60% with hepatic secondaries-these numbers are also consistent with our results where we observed a high rate of lung involvement in the brain metastasis group (54%)compared to the whole registry (15%)8,9,15. The high percentage of concurrent or antecedent pulmonary metastases supports the hematogenous dissemination theory proposed in 1928 by Ewing16, according to which tumor cells,after reaching the lungs, eventually travel through the blood,flow into the left side of heart, and then embolize to other organs, including the brain. Some authors have postulated that a diagnosis of pulmonary metastases in mCRC patients may confer an increase in the risk of developing CNS metastases, supporting Ewing’s theory17. Chyun18also noted that the lag time from primary CRC diagnosis to pulmonary metastases occurred three times earlier among patients who subsequently developed brain metastases. In our registry, we found also that approximately half of the diagnoses of brain metastases were associated with co-existing hepatic involvement (47%), although this was not dramatically higher than those of the whole registry population (35%).Cascino19and Temple7reported similar findings with 50%and 76% of patients, respectively, presenting with evidence of liver metastases at the time of their CNS diagnosis. Other authors have proposed an opposite theory whereby different molecular features of the cancer explain the pattern of brain metastases. Across the literature, KRAS mutations in mCRC correlate with poor OS and increased incidence of both lung and brain metastases20-22. In our study, noting the small number in whom the KRAS mutation status was known, 55%of patients with brain metastases presented with KRAS-mutated cancers compared to 43% of the overall population,in keeping with this hypothesis. The impact of other somatic mutations on the development of brain metastases in the mCRC context is currently unclear. Some authors have proposed mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and v-raf murine sarcoma viral oncogene homologe B1 (BRAF)as predictors of CNS involvement in mCRC patients;however, the evidence is scarce and even contradictory20,22,23.BRAF and PIK3CA statuses were not available in our registry for the brain metastasis group, again based on small numbers.
An important finding of our analysis was that the median time between the diagnosis of mCRC and the development of brain metastases was 1.9 years (range: 0–6 years), which is consistent with the timeframe reported in other publications.There is increasing evidence suggesting that brain metastases are observed more commonly in left-sided CRC (about 60%of cases) than in proximal colon cancers24,25. Our results were consistent with the association between primary tumor location and the development of brain metastases, although it would appear that the higher rate observed with left-sided primaries may be driven by the rectal subgroup. Although less common, Hammoud26reported that patients with brain metastases from metastatic proximal colon cancers were associated with shorter survival times compared with distal mCRC patients with CNS involvement (3 vs. 6 months, P <0.005). Small numbers in our series did not allow us to conduct a comparison of survival alongside primary cancers.
The mOS from the diagnosis of brain metastasis in our series was 4.2 months (95% CI 2.9–5.5 months). This poor survival is similar to what has been reported in the existing literature. In our study, one possible explanation for this ominous prognosis is the fact that 54% of our patients presented with lung metastases, and in 47% of cases, there was liver involvement. It must also be noted that these patients were pre-treated with a median number of 2 lines of chemotherapy prior to the diagnosis of brain metastases(range: 0–4 lines). It is reported that mCRC patients with uncontrolled extracranial metastases have worse outcomes compared to mCRC brain patients who have no extracranial lesions27. Unfortunately, we do not collect data in our registry on patient progression and hence, we cannot report on the control of extracranial disease at the time of brain metastasis.
Another possible explanation for such a limited survival could be the low proportion of patients who underwent aggressive therapies for their brain metastases. None of our patients received stereotactic radiotherapy and just half underwent surgical resection. Surgery combined with WBRT is proven to be the most effective treatment for isolated CRC brain metastasis in patients who are ideal surgical candidates10-12,26. Farnell28, for instance, published interesting work looking at the survival rates of 150 mCRC patients treated with different treatment modalities and determining the main characteristics in long-term survivors.He reported a mOS of 10.5, 11.25, 4, and 2 months for patients receiving a combination of surgery and radiotherapy,surgery alone, radiotherapy alone, and best supportive care only, respectively. Twenty-four patients survived > 1 year after brain metastasis diagnosis. Among these long-term survivors, 79% were treated surgically and 38% presented with no additional systemic disease. These aggressive interventions are only reasonable for patients with minimal CNS disease burden in terms of number of metastases, and where surgically accessible. Thus, patients with better initial prognosis with their CNS disease are more likely to receive aggressive intervention.
Given the low incidence of brain metastases in mCRC,there are no studies looking at the cost-effectiveness of brain imaging in this setting, as opposed to other solid tumors that frequently metastasize to the brain (lung cancer or melanoma)29. With regard to screening for intracranial metastases in mCRC, no consensus has been reached concerning when to use contrast-enhanced computed tomography (CT) or MRI30. The latter is currently the investigation of choice given its significant sensitivity/specificity compared to CT in the detection of metastatic disease within the brain and in determining the location and number of metastases, especially small lesions < 5 mm in diameter31,32.
In our cohort, the patients who developed brain metastases tended to be younger, be female, present with more lung involvement, and present with primary tumors that were more often rectal and KRAS-mutant. Clinicians should be mindful of including imaging of the brain in their surveillance protocol for mCRC patients with the aforementioned features in the later stages of disease. The potential benefits of an early diagnosis of brain metastases in patients with good performance status may allow treatment options such as surgical resection and stereotactic radiotherapy techniques to be considered, and ultimately,increase life expectancy in very selected patients.
There are limitations of our registry cohort as reported.Differences in performance status and disease extent within the brain, and systemically between those patients proceeding to craniotomy and radiotherapy and those having radiotherapy alone may have played a part in the survival difference reported. Unfortunately, these data are not recorded in our registry. Furthermore, complete details on molecular results are also not available based on the differences in practice over the lifetime of the registry.
Conclusions
Brain metastases remain a rare diagnosis in mCRC patients,with an incidence of 1.4% from our registry results. Female,KRAS mutations, rectal primary cancer, and young age seem to confer an increased risk for the development of CNS metastases, although the numbers of patients with brain metastases are too small to truly draw any significant conclusion. Although routine surveillance scanning remains controversial in this context, we suggest considering brain imaging in patients with these characteristics as early detection and proper staging may lead to better selection of patients for more aggressive modalities of treatment and potentially improved outcomes.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Sanoff HK, Sargent DJ, Campbell ME, Morton RF, Fuchs CS,Ramanathan RK, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008; 26: 5721-7.
2.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004; 22: 1209-14.
3.Benson AB, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 3. 2014. J Nat Comprehens Cancer Network. 2014; 12: 1028-59.
4.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: Esmo clinical practice guidelines for diagnosis,treatment and follow-up. Ann oncol. 2014; 25 Suppl 3: iii1-9.
5.Christensen TD, Spindler KLG, Palshof JA, Nielsen DL. Systematic review: Brain metastases from colorectal cancer—incidence and patient characteristics. BMC Cancer. 2016; 16: 260.
6.Mongan JP, Fadul CE, Cole BF, Zaki BI, Suriawinata AA, Ripple GH, et al. Brain metastases from colorectal cancer: Risk factors,incidence, and the possible role of chemokines. Clin Colorectal Cancer. 2009; 8: 100-5.
7.Temple DF, Ledesma EJ, Mittelman A. Cerebral metastases. From adenocarcinoma of the colon and rectum. N Y State J Med. 1982;82: 1812-4.
8.Damiens K, Ayoub JPM, Lemieux B, Aubin F, Saliba W, Campeau MP, et al. Clinical features and course of brain metastases in colorectal cancer: An experience from a single institution. Current Oncol. 2012; 19: 254-8.
9.Noura S, Ohue M, Shingai T, Fujiwara A, Imada S, Sueda T, et al.Brain metastasis from colorectal cancer: Prognostic factors and survival. J Surg Oncol. 2012; 106: 144-8.
10.Kye BH, Kim HJ, Kang WK, Cho HM, Hong YK, Oh ST. Brain metastases from colorectal cancer: The role of surgical resection in selected patients. Colorectal Dis. 2012; 14: e378-85.
11.Wroński M, Arbit E. Resection of brain metastases from colorectal carcinoma in 73 patients. Cancer. 1999; 85: 1677-85.
12.Aprile G, Zanon E, Tuniz F, Iaiza E, De Pauli F, Pella N, et al.Neurosurgical management and postoperative whole-brain radiotherapy for colorectal cancer patients with symptomatic brain metastases. J Cancer Res Clin Oncol. 2009; 135: 451-7.
13.Fokas E, Henzel M, Hamm K, Surber G, Kleinert G, Engenhart-Cabillic R. Multidisciplinary treatment of brain metastases derived from colorectal cancer incorporating stereotactic radiosurgery:Analysis of 78 patients. Clin Colorectal Cancer. 2011; 10: 121-5.
14.Smedby KE, Brandt L, Bäcklund ML, Blomqvist P. Brain metastases admissions in sweden between 1987 and 2006. Br J Cancer. 2009;101: 1919-24.
15.Nieder C, Pawinski A, Balteskard L. Colorectal cancer metastatic to the brain: Time trends in presentation and outcome. Oncology.2009; 76: 369-74.
16.Ewing J. Neoplastic diseases. A treatise on tumors. Am J Med Sci.1928; 176: 278.
17.Hwang TL, Close TP, Grego JM, Brannon WL, Gonzales F.Predilection of brain metastasis in gray and white matter junction and vascular border zones. Cancer. 1996; 77: 1551-5.
18.Chyun Y, Hayward E, Lokich J. Metastasis to the central nervous system from colorectal cancer. Med Pediatr Oncol. 1980; 8: 305-8.
19.Cascino TL, Leavengood JM, Kemeny N, Posner JB. Brain metastases from colon cancer. J Neurooncol. 1983; 1: 203-9.
20.Yaeger R, Cowell E, Chou JF, Gewirtz AN, Borsu L, Vakiani E, et al.Ras mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121: 1195-203.
21.Zang YW, Gu XD, Xiang JB, Chen ZY. Brain metastases from colorectal cancer: Microenvironment and molecular mechanisms.Int J Mol Sci. 2012; 13: 15784-800.
22.Tie J, Lipton L, Desai J, Gibbs P, Jorissen RN, Christie M, et al. Kras mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res. 2011; 17:1122-30.
23.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of braf mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer.Cancer. 2011; 117: 4623-32.
24.Hugen N, Van De Velde CJ, De Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann oncol. 2014; 25: 651-7.
25.Sundermeyer ML, Meropol NJ, Rogatko A, Wang H, Cohen SJ.Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer. 2005; 5: 108-13.
26.Hammoud MA, McCutcheon IE, Elsouki R, Schoppa D, Patt YZ.Colorectal carcinoma and brain metastasis: Distribution, treatment,and survival. Ann Surg Oncol. 1996; 3: 453-63.
27.Da Silva AN, Nagayama K, Schlesinger DJ, Sheehan JP. Gamma knife surgery for brain metastases from gastrointestinal cancer. J Neurosurg. 2009; 111: 423-30.
28.Farnell GF, Buckner JC, Cascino TL, O’Connell MJ, Schomberg PJ,Suman V. Brain metastases from colorectal carcinoma: The long term survivors. Cancer. 1996; 78: 711-716.
29.Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int.2013; 4: S209-19.
30.Barajas RF, Jr, Cha S. Imaging diagnosis of brain metastasis. Prog Neurolog Surg. 2012; 25: 55-73.
31.Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of mri compared to cct in patients with brain metastases. J Neuro-Oncol.1999; 44: 275-81.
32.Åkeson P, Larsson E-M, Kristoffersen DT, Jonsson E, Holtås S.Brain metastases-comparison of gadodiamide injection-enhanced mr imaging at standard and high dose, contrast-enhanced ct and non-contrast-enhanced mr imaging. Acta Radiol. 1995; 36: 300-6.
Cite this article as: Tapia Rico G, Price TJ, Karapetis C, Piantadosi C,Padbury R, Roy A, et al. Brain metastasis in advanced colorectal cancer:results from the South Australian metastatic colorectal cancer (SAmCRC)registry. Cancer Biol Med. 2017; 14: 371-6. doi: 10.20892/j.issn.2095-3941.2016.0068
Amanda R. Townsend
E-mail: Amanda.townsend@health.sa.gov.au
July 15, 2017; accepted August 7, 2017.
Available at www.cancerbiomed.org
Copyright © 2017 by Cancer Biology & Medicine
 Cancer Biology & Medicine2017年4期
Cancer Biology & Medicine2017年4期
- Cancer Biology & Medicine的其它文章
- Brain metastasis in non-small cell lung cancer (NSCLC)patients with uncommon EGFR mutations: a report of seven cases and literature review
- Complete pathologic response after chemoradiotherapy in a patient with rectal squamous cell carcinoma: a case report
- Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension
- Thermogenic protein UCP1 and UCP3 expression in nonsmall cell lung cancer: relation with glycolysis and anaerobic metabolism
- Profile of the breast cancer susceptibility marker rs4245739 identifies a role for miRNAs
- Promoter methylation of Wnt/β-Catenin signal inhibitor TMEM88 is associated with unfavorable prognosis of nonsmall cell lung cancer
