Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension
Filiz Kisaayak Collak, Ummuhan Demir, Seyma Ozkanli, Esra Kurum, Pinar Engin ZerkMolecular Biology and Genetics Department, Faculty of Engineering and Natural Sciences, Istanbul Medeniyet University,Istanbul 4700, Turkey; Pathology Department, Faculty of Medicine, Istanbul Medeniyet University, Istanbul, 4700, Turkey;Statistics Department, University of California Riverside, Riverside, CA 95, USA
ORIGINAL ARTICLE
Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension
Filiz Kisaayak Collak1, Ummuhan Demir1, Seyma Ozkanli2, Esra Kurum3, Pinar Engin Zerk21Molecular Biology and Genetics Department, Faculty of Engineering and Natural Sciences, Istanbul Medeniyet University,Istanbul 34700, Turkey;2Pathology Department, Faculty of Medicine, Istanbul Medeniyet University, Istanbul, 34700, Turkey;3Statistics Department, University of California Riverside, Riverside, CA 92521, USA
Objective: Yes associated protein 1 (YAP1) is a member of the Hippo pathway, acting as a transcriptional coactivator. To elucidate the role of YAP1 and phosphorylated (p)YAP1 in prostate cancer (PCa) tumorigenesis, we investigated their expression in clinical samples of PCa and cell lines.Methods: Fifty-four tumor, adjacent nontumor, and prostate intraepithelial neoplasia (PIN) tissues from patients with PCa after radical prostatectomy were selected from a retrospective cohort and studied using immunohistochemistry (IHC). Protein and mRNA expression levels of YAP1 were evaluated by Western blot analysis and quantitative real-time reverse transcription PCR,respectively, in cancer cell lines. Publicly available gene expression datasets were downloaded to analyze YAP1 mRNA and protein levels in PCa tissue samples.Results: IHC analysis of PCa tissues revealed that YAP1 staining intensities were moderate to weak in the nucleus and cytoplasm of tumor cells, whereas adjacent normal epithelia showed strong staining. We observed that benign prostates were characterized by higher expression levels of both nuclear (P=0.004) and cytosolic (P=0.005) YAP1. pYAP1 staining was weak in the cytoplasm and absent in the nucleus of all the tissues investigated. YAP1 expression was an indicator of extraprostatic extension (EPE). The level of YAP1 was negatively correlated with the level of the androgen receptor (AR) in The Cancer Genome Atlas dataset and Western blot analysis of cell lines.Conclusions: Our study suggested that YAP1 expression is heterogeneous in PCa tissue samples; therefore, YAP1 might play different roles in different aspects of PCa progression. This might involve AR–YAP1 interplay in PCa.
YAP1; pYAP1; PCa
Introduction
Prostate cancer (PCa) is the most diagnosed cancer type and the second leading cause of cancer death in men. Yes associated protein (YAP) is a transcription co-activator that is negatively regulated by the Hippo tumor suppressor pathway. This pathway has a role in cell proliferation and apoptosis. The Hippo pathway is stimulated by cell-to-cell contact and induces cell proliferation in normal cells until the cell population reaches a certain density. When the cell density is sufficiently high, the pathway activates an inhibitory switch to stop proliferation and activate apoptosis.This proliferation-apoptosis switch is regulated by cell adhesion, cell polarity, cytoskeletal dynamics, andmechanical forces. The Hippo pathway regulates organ size in the same manner1. The core component of the Hippo pathway in mammals is a protein kinase cascade. This cascade starts with mammalian sterile twenty-like 1/2(MST1/2) kinases. These kinases are activated by several intra and extracellular stimuli. The second kinase in the cascade is large tumor suppressor 1/2 (LATS1/2), which is phosphorylated by MST1/2. MOB kinase activator 1A(MOB1) acts as a cofactor for LATS1. The effector proteins in this cascade are YAP and transcriptional coactivator with a PDZ-binding domain (TAZ). In their unphosphorylated form, these proteins translocate into the nucleus and act as transcriptional cofactors. Upon phosphorylation by LATS1/2, YAP and TAZ accumulate in the cytoplasm and are directed to degradation2. The cytoplasmic localization of YAP is facilitated by binding of the 14-3-3 proteins to the phosphorylated form of YAP. YAP only localizes to the nucleus when the pathway is inactive, and controls the expression of differentiation, tissue growth, pro-proliferative,and anti-apoptotic genes, together with several transcription factors. Runt-related transcription factor 2 (Runx2), TEA domain (TEAD), tumor protein p73 (p73), human epidermal growth receptor 4 (ErbB4), SMAD family member 7(Smad7), and SMAD family member 1 (Smad1) are among the transcription factors that YAP can bind3,4. There are several upstream modulators of the pathway. Merlin, a cytoskeletal protein encoded by Neurofibromatosis 2 (NF2), is a positive regulator of the pathway, and therefore is an inactivator of YAP. Ras-association domain family (RASSF)proteins also activate MST1/25. Other than these well-known regulators, E-cadherin, G protein-coupled receptors(GPCRs), and the leukemia inhibitory factor receptor (LIFR)have been reported to play roles in modulation of YAP activity, dependent or independent of the Hippo pathway6.
The role of YAP1 in oncogenesis is still controversial.Although YAP1 was initially proven to be proto-oncogenic and its expression is increased in breast cancer7, hepatocellular carcinoma8, colorectal cancer9, gastric cancer10,pancreatic cancer11, and esophageal squamous cell carcinoma12, other studies showed that YAP1 expression is decreased, such as in breast13and prostate14cancers, claiming that the protein functions as a tumor suppressor.
There are few studies in the literature analyzing YAP1 level in PCa tissue samples. Sheng et al.15evaluated 32 PCa, 15 benign prostatic hyperplasia (BPH), and 15 para-PCa (para-PCa) tissues. They showed that YAP1 was abundant in cancer tissue, absent in BPH, and scarce in para-PCa tissues. Their results indicated that the YAP1 level is positively correlated with the Gleason score, tumor, node, metastasis (TNM)staging, and prostate-specific antigen (PSA) levels. Zhang et al.16compared 7 primary therapy-naive PCa tissues with 13 castrate-resistant PCa tissues in terms of YAP1 levels. Little or no YAP1 was observed in primary samples; however, YAP1 was activated and overexpressed in castrate-resistant cases.
To determine the relevance of the Hippo/YAP pathway in PCa pathogenesis, we sought to evaluate the expression and subcellular localization of YAP1 and phosphorylated YAP1(pYAP1) in prostate tumors. We also studied the clinical significance of YAP1 expression and its correlation with clinicopathological parameters of PCa. Furthermore, to investigate the relationship between YAP1 and the androgen receptor (AR), we analyzed their expression levels using publicly available data sets as well as by Western blot analysis in a panel of benign and malignant prostate cell lines.
Materials and methods
Patients and tissue samples
Upon approval of the protocol by the research and ethics committees, the pathology files from the Pathology Department of the Istanbul Medeniyet University Hospital were reviewed. Paraffin blocks were obtained from tissues of prostatectomy cases (2013 to 2015). The sample set included 54 PCa, prostate intraepithelial neoplasia (PIN), and cancer adjacent benign tissues that were routinely fixed in 10%neutral buffered formalin and embedded in paraffin.
Immunohistochemistry
The tissues were evaluated both histochemically using hematoxylin-eosin staining and immunohistochemically using YAP1 and pYAP1 antibodies. Sections of 4 μm were cut from paraffin blocks that were previously coated with poly-L-lysine. Specimen slides were then deparaffinized with xylene for 10 min and rehydrated. Hydrogen peroxide, phosphate buffered saline (PBS), and a nonspecific blocking reagent(Leica Biosystems, Newcastle upon Tyne, UK) were applied as antigen retrieval solutions. The tissue sections were incubated at room temperature for approximately 90 min with primary antibodies (anti-YAP1, diluted 1:400; antipYAP1, diluted 1:50; catalog No. 12395 and 4911,respectively; Cell Signaling Technology, Danvers, MA, USA)to detect YAP1 and pYAP1 (phosphorylated at Ser127). This step was followed by detection using the Bond Polymer Refine kit for 25 min on a Bond-Max Autostainer (Leica Biosystems), visualization with diaminobenzidine chromogen, and counterstaining with hematoxylin.Cytoplasmic and nuclear staining of YAP1 indicated a positive sample. The findings were evaluated via quantitative morphometric analysis.
Evaluation of immunostained samples
All cases were reviewed by two pathologists (S.O. and P.Z.)who were blinded to the origin of the samples. For each patient, formalin-fixed paraffin-embedded tissue blocks containing prostatic adenocarcinoma, PIN, and cancer adjacent normal tissue in one block were prepared. YAP1 and pYAP1 expression was evaluated in the nucleus and cytoplasm of the tumor, PIN, and tumor adjacent normal epithelia. The intensity was scored as: 0 (negative), 1 (weak),2 (intermediate), and 3 (strong). Discrepancies in scores were discussed to obtain a consensus.
Gene expression and protein profiles from public databases
Several publicly available PCa datasets were used for the analysis. All the data were downloaded from cBioPortal(www.cbioportal.org)17,18. The prostate adenocarcinoma[The Cancer Genome Atlas (TCGA); provisional] data set was analyzed to correlate YAP1 and AR at the mRNA and protein levels. The prostate adenocarcinoma (Fred Hutchinson CRC, Nat Med 2016) data set was used to analyze YAP1 mRNA in matched localized and metastatic samples. Data about copy number alterations of YAP1 were retrieved from prostate adenocarcinoma, metastatic(Michigan, Nature 2012), prostate adenocarcinoma(MSKCC, Cancer Cell 2010), and prostate adenocarcinoma(Fred Hutchinson CRC, Nat Med 2016) data sets. The statistical analysis of the downloaded data was performed in SPSS 22 (IBM, Armonk, NY, US).
Western blot analysis
Total cell lysates were prepared on ice-cold lysis buffer (20 mM HEPES (pH 7.4), 150 mM NaCl, 0.5% Nonidet P-40, 1 mM EDTA, 5 mM NaF, protease inhibitors, and phosphatase inhibitors). Protein concentrations were determined by the Lowry method (Bio-Rad, Hercules, CA, USA). Forty micrograms of protein in the cell lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and blocked with PBST (PBS with 0.1% Tween 20) containing 5% (w/v) skimmed milk powder. Signals were detected using a WesternBright Sirius chemiluminescent horseradish peroxidase (HRP) substrate (Advansta Corporation, Menlo Park, CA, USA). Antibodies and working dilutions for Western blot were: anti-AR (1:1000, EMD Millipore,Darmstadt, Germany), anti-YAP1, (1:1000, Cell Signaling Technology), and anti-β-Actin (1:1000, Cell Signaling Technology). Immunoreactive protein bands were scanned using a digital chemiluminescent imaging system (Azure c300, Azure Biosystems, Dublin, CA, USA). The housekeeping protein β-Actin served as a loading control.
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from each group of PCa cell lines using a Quick RNA MiniPrep Kit (Zymoresearch, Irvine, CA,USA). The total RNA was quantified using a UV spectrophotometer (DS-11, DeNowix, Wilmington, DE,USA), and 1 μg RNA was used for the reverse transcription reaction to obtain cDNA. cDNA synthesis was performed using a SensiFAST cDNA Synthesis Kit (Bioline, London,UK) according to the manufacturer's instructions. The qPCR reactions were performed with 1 μL of cDNA to amplify the YAP1 and AR mRNA, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference gene, using the RotorGene Q cycler (Qiagen, Hilden, Germany). The cycling conditions were as follows: 95 °C for 10 sec, 95 °C for 15 sec,and 60 °C for 1 min (40 cycles). The forward (F) and reverse(R) primer sequences for the YAP1, AR, and GAPDH mRNA obtained from Thermofisher were as follows: YAP1F: 5′-TGCGTAGCCAGTTACCAACACTG-3′, YAP1R: 5′-TCGAGA GTGATAGGTGCCACTG-3′, ARF: 5′-ATCCTCATATGGCCC AGTGTCAAG-3′, ARR: 5′-GCTCTCTAAACTTCCCGTGG CATA-3′, GAPDHF: 5′-GGAGCGAGATCCCTCCAAAT-3′-and GAPDHR: 5′-GGCTGTTGTCATACTTCTCATGG-3′.
Statistical analysis
For the statistical analysis of staining intensity between and within YAP1 and pYAP1 in the cytoplasm and nucleus of tumors, benign tissues, and PIN areas, the Kruskal–Wallis H-test and posthoc pairwise comparison tests were performed. The Kruskal–Wallis H-test is a nonparametric method used to test the differences between two or more groups of an independent variable on an ordinal dependent variable. In our analysis, the ordinal dependent variable was the staining intensity, and depending on the comparison that was pursued, the independent variables would be the protein type (YAP1 or pYAP1), cell compartment (nucleus or cytoplasm), and tissue type (tumor, benign, PIN). When the Kruskal–Wallis H test indicated a statistical difference between the groups of independent variables, we concluded that at least one of the groups of the independent variables was different from the others. In addition, if this test showed that there was a difference between the groups of the independent variables, to identify which group or groups were significantly different, we performed a posthoc pairwise comparison test to explore these differences. When comparing the YAP1 staining intensity with the clinicopathological parameters, staining intensity was grouped as“positive” or “negative”. The staining intensities 3 (strong)and 2 (intermediate) were considered positive staining,whereas 1 (weak) and 0 (negative) were considered negative staining. Statistical significance was assumed when P < 0.05.
Results
Expression and subcellular localization of YAP1 and pYAP1 in human PCa tissues
We investigated YAP1 and pYAP1 expression using immunohistochemistry in PCa, PIN, and cancer adjacent benign tissue of 54 radical prostatectomy specimens. YAP1 was highly expressed both in the cytoplasm and nucleus of the benign tissue, whereas it was moderately expressed in PIN and cancer tissues in both cellular locations (Figure 1A–C). YAP1 expression was decreased in PIN and cancer tissues; benign tissue adjacent to cancer tissue exhibited stronger cytoplasmic and nuclear expression (Table 1, P <0.05). pYAP1 expression levels were low in the cytoplasm and negative in the nucleus of most cases (Figure 1D and E).There was no statistically significant difference in the expression of pYAP1 in the cytoplasm of all tissue types(Table 1, P > 0.05). The staining intensities of pYAP1 were different from each other in the cytoplasm and nucleus of all tissue types (benign, PIN, and cancer) investigated (Table 1,P < 0.05).
Correlation of YAP1 and pYAP1 expression with the clinicopathological features of PCa
The relationships between YAP1 expression and the clinicopathological characteristics of PCa were analyzed by ordinal regression. We did not find a significant relationship between the expression of nuclear and cytoplasmic YAP1 and patients’ (positive) surgical margin, seminal vesicle invasion,Gleason score, and perineural invasion. However, we observed that high nuclear and cytosolic YAP1 levels were associated with extraprostatic extension (EPE) (P < 0.04) in PCa (Table 2). The odds ratio for EPE was 3.61, indicating that in the presence of EPE, YAP1 was highly expressed.
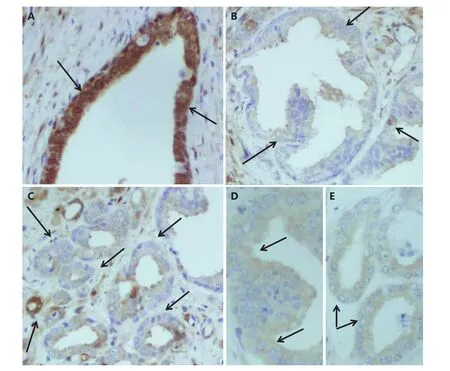
Figure 1 Representative photographs of cytoplasmic and nuclear YAP1 immunostaining. (IHC staining, 20×). (A) Benign. (B) PIN. (C) Cancer tissue areas of PCa patient. (D) pYAP1 immunostaining of the same patient’s PIN. (E) pYAP1 immunostaining of the same patient’s cancer tissue area. The areas are indicated with arrows.
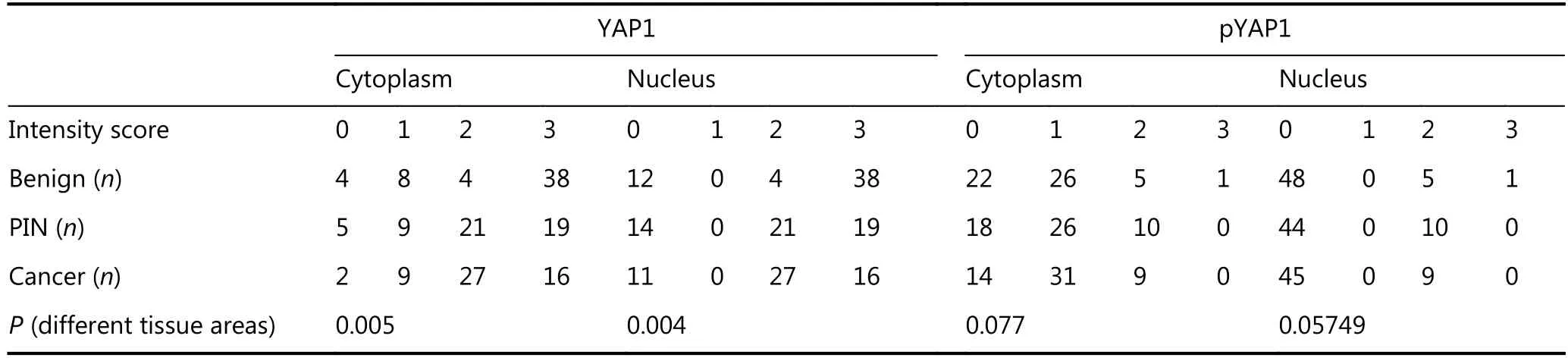
Table 1 YAP1 and pYAP1 immunostaining in different tissue areas and cellular compartments
Investigation of YAP1 aberrations in PCa tissue samples using public databases
The correlation of high YAP1 staining with EPE led us to consider that YAP1 may have a role in metastasis. We selected the prostate adenocarcinoma (Fred Hutchinson CRC, Nat Med 2016) data set because it harbors primary PCa and matching metastatic tissue. We analyzed YAP1 mRNA expression in 13 matched local and metastatic PCa tissue samples. There was no difference in terms of YAP1 mRNA expression between the local and matched metastatic samples(Figure 2A). Next, we obtained the copy number alteration(CNA) data for YAP1 in three different PCa datasets. The results indicated that CNA for YAP1 was much more common in metastatic samples than in primary tumors(Figure 2B, C). These aberrations were very heterogeneous,displaying both gains and losses at the YAP1 locus.
Investigation of AR-YAP1 correlation in PCa tissue samples using public databases and PCa cell lines
AR is a crucial transcription factor in PCa progression and an overall therapy target. We investigated the AR-YAP1 correlation using TCGA data. Interestingly, AR and YAP1 mRNA levels were positively correlated (Figure 3A), while AR and YAP1 were negatively correlated at the protein level(Figure 3B). We obtained similar results in several PCa cell lines (Figure 4A, B, and C). We assessed the level of YAP1 and AR proteins using western blotting. The level of YAP1 in AR-negative PCa cell lines (DU145 and PC3) and in an immortalized primary epithelial cell line (RWPE1) was higher compared to that in the AR-positive cell lines (LNCaP,LNCaP-abl, VCaP, DuCaP) (Figure 4A). However, YAP1 and AR mRNA levels were positively correlated (LNCaP, LNCaP-abl, DU145, PC3, and RWPE1) and these results were in agreement with the results obtained from the datasets(Figure 4B and C). The exceptions to this were the DuCaPand VCaP cell lines, in which AR and YAP1 were negatively correlated at both the mRNA and protein levels.
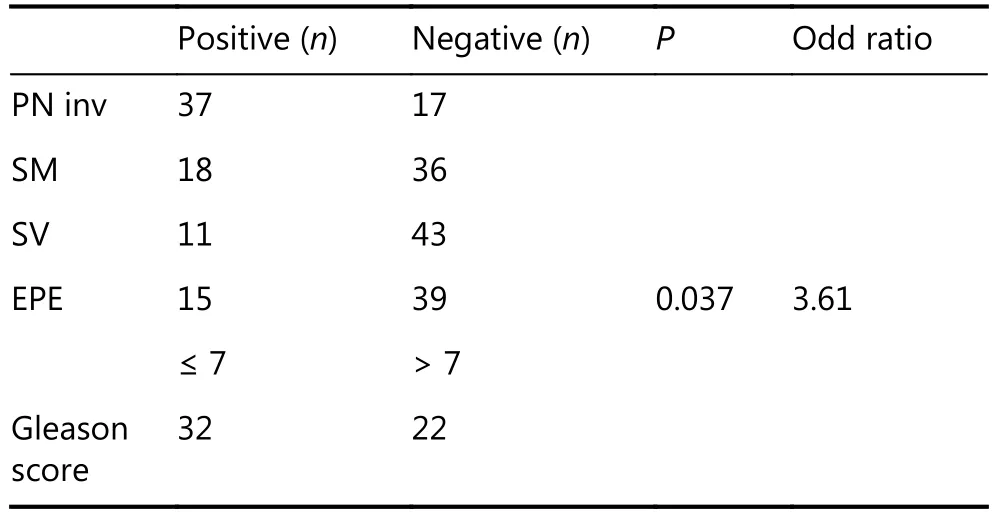
Table 2 Correlation of YAP1 immunostaining with clinicopathological parameters
Discussion
In this study, we investigated the expression patterns and localizations of YAP1 and pYAP1 in PCa, and compared them with PIN and tumor adjacent benign tissue using immunohistochemistry. After immunohistochemical staining, we scored nuclear and cytoplasmic YAP1 and pYAP1 staining from 0 to 3, considering no staining as 0, low staining as 1, moderate staining as 2, and strong staining as 3.We correlated the staining intensity with the clinicopathological parameters of patients, which suggested that YAP1 is an indicator of EPE. Overexpression of YAP1 has been observed in cervical tumors19, breast cancer7, and PCa15. However, decreased or absent expression of YAP1 has also been reported in breast13and PCa14. In an early study investigating the YAP1-cancer association conducted by Zhao et al.20, elevated and nuclear-localized YAP1 was observed in PCa. The number of patients and their PCa status were not mentioned in the study, which made it difficult to compare their data with ours. Similar to a previous evaluation of YAP1 expression in PCa by Hu et al.14,we also observed that YAP1 expression was decreased in PCa tissues; normal prostate tissues adjacent to cancer exhibited stronger nuclear and cytoplasmic expression of YAP1.
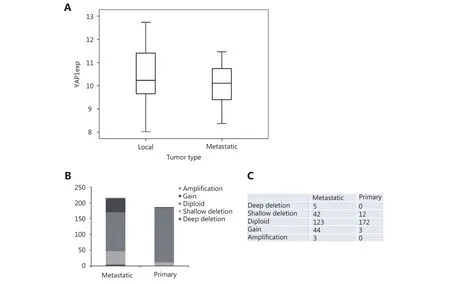
Figure 2 CNA and mRNA expression of YAP1 in primary and metastatic PCa samples. (A) There is no statistically significant difference between mRNA values of YAP1 in 13 primary PCa sample and their matched metastatic lesions. (B) Metastatic PCa samples show high CNA of YAP1 compared to primary PCa samples. (C) Absolute numbers of cases from B.
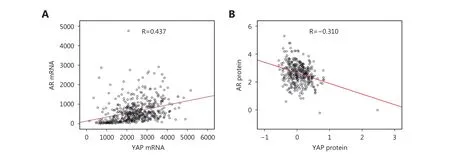
Figure 3 Correlation of YAP1 and AR. (A) Correlation of YAP1 and AR in mRNA level is positive. The correlation coefficients are R=0.437 and R=0.326 according to Spearman rho and Pearson correlation, respectively (P < 0.0001). (B) Correlation of YAP1 and AR in protein level is negative. The correlation coefficients are R=–0.236 and R=–0.310 according to Spearman rho and Pearson correlation, respectively (P <0.0001).
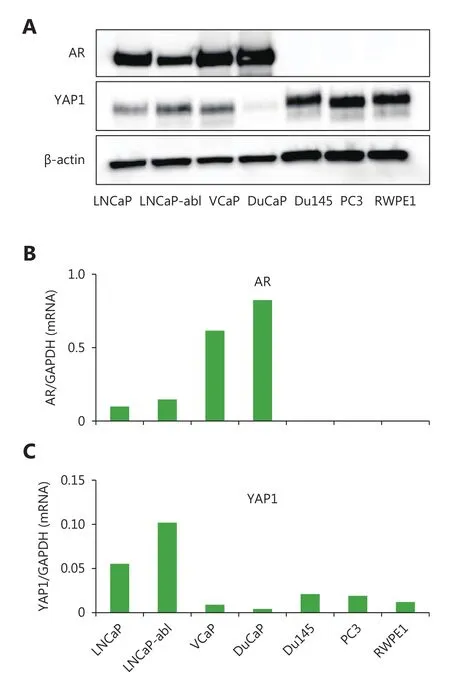
Figure 4 Expression of YAP1 and AR in protein and mRNA level and their correlation in PCa cell lines. (A) Western blot analysis of YAP1 and AR revealed that the YAP1 protein was high in AR negative (DU145, PC3 and RWPE1) prostate cell lines. (B) Real time PCR analysis of AR mRNA level in prostate cell lines (The order of cell lines represented in the graph is the same as in figure C). (C)Real time PCR analysis of YAP1 mRNA level in prostate cell lines.
YAP1 is located on chromosome 11q22 and was originally reported to be a candidate oncogene in mouse mammary tumors21. Amplification of the 11q22 chromosomal locus was observed in glioblastomas22, oral squamous-cell carcinomas23, and in cancers of the pancreas24, lung25,ovary26, and cervix27. However, Overholtzer et al.21could not detect YAP amplification in more than 100 sporadic human breast cancers using qPCR analysis of microscopically dissected specimens. Loss of heterozygosity (LOH) was reported in early genetic studies of breast cancer and PCa28.The decrease of YAP1 expression could be explained by LOH in our study groups; however, further investigations should be done to test this hypothesis. In contrast to our results, no differences were found between localized tumors and control tissues taken from BPH patients in the study by Jiang et al.29.However, using other patients’ tissues as controls may represent a complication when interpreting the outcome. In contrast to our observations, immunohistochemical analysis of 31 prostate tumors and matching normal tissues revealed that YAP1 was overexpressed in PCa30. The assessment of YAP1 expression in normal prostate (n=9) and PCa tissues(n=22) using IHC by Kuser-Abali et al.31showed that YAP1 was amply stained in both cases. Staining of the tissues was mainly nuclear, was not uniform, and its intensity was heterogeneous, differing from no-staining, low-staining, and overstaining within the same samples and among the samples. We also observed similar staining patterns in our working cohort, which might be caused by the complexity of Hippo-YAP1 regulation and the mechanisms involved in different cases. To understand these mechanisms, we analyzed the AR-YAP1 correlation using a publicly available TCGA dataset. AR is a central player in PCa progression and is the main regulator of gene transcription in prostate tissue.Therefore, it is important to determine if YAP1 and AR levels are correlated. At the mRNA level, AR and YAP1 were positively correlated. However, at the protein level, this correlation was negative. This further proved that AR-YAP1 interplay is complex and that not only transcriptional, but post-transcriptional mechanisms are also involved in their regulation. Further research is required to elucidate these mechanisms.
YAP1 is a transcription cofactor and is believed to act as an oncogene in the nucleus by promoting cell proliferation and inhibiting apoptosis. Phosphorylation of YAP1, particularly at serine 127, generates a 14-3-3 binding site that leads to YAP1 moving from the nucleus to the cytoplasm32-34. YAP1 becomes inactivated by this relocalization and its ability to act as a co-activator is diminished32,35. We could not relate the dysregulation of YAP1 expression to its phosphorylation status and the cytoplasmic versus nuclear localization of pYAP1 because the staining intensity of pYAP1 was 1 in the cytoplasm and 0 in nucleus of all normal, PIN, and cancerous tissues. Consistent with the tissue samples, both normal cells(RWPE1) and cancer cells expressed pYAP1 and YAP1 in varying degrees.
The results suggested that YAP1 is an indicator of EPE,which led us to question whether YAP1 might have a role in metastasis. When we analyzed PCa tissue and its matched metastatic tissue, there was no difference in the YAP1 mRNA level. However, we need to keep in mind that posttranscriptional mechanisms may be involved in the analyzed sample set. Kang et al.10observed that in gastric cancer and normal gastric mucosa, the YAP1 mRNA and protein level did not correlate. Although the mean YAP1 mRNA expression was not higher in tumor tissue than in paired adjacent normal tissue, YAP1 was abundant in cancer tissue, whereas it was rare in normal adjacent tissue. In a recent study by Lee et al.,36where the transition in PCa progression from a local tumor to lymph node metastasis was modeled by transplanting mice with PC3 cells orthotopically,YAP1 expression was reported to be different in primary tumors and lymph nodes. In the primary tumor, fewer cells expressed YAP1 compared with the number of YAP1-positive cells in the lymph nodes. As a result, the staining intensity of YAP1 was higher in the metastatic samples. They suggested that biophysical cues present in the lymphatics and/or lymph nodes contributed to high YAP1 expression in metastases. It would be of particular relevance to examine YAP1 expression at the protein level in matched local and metastatic PCa tissue specimens to better understand its function in PCa metastasis.
Although the YAP1 staining intensity was generally higher in normal adjacent tissue compared to that in the cancerous areas in our patient cohort of primary PCa, YAP1 was still present and was moderately expressed in the cancer tissues.Further in vitro functional analyses in PCa cell models as well as animal studies should be performed to better understand the role of YAP1 in PCa tumorigenesis.
Acknowledgements
This work is financially supported by the The Scientific and Technological Research Council of Turkey (TUBITAK, Grant No. 114S419) and Istanbul Medeniyet University Scientific Research Grants (Grant No. FBA-2014-293).
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Camargo FD, Gokhale S, Johnnidis JB, Fu DD, Bell GW, Jaenisch R,et al. YAP1 ıncreases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007; 17: 2054-60.
2.Guo LW, Teng LS. YAP/TAZ for cancer therapy: opportunities and challenges (review). Int J Oncol. 2015; 46: 1444-52.
3.Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008; 135: 4059-69.
4.Kodaka M, Hata Y. The mammalian Hippo pathway: Regulation and function of YAP1 and TAZ. Cell Mol Life Sci. 2015; 72:285-306.
5.Matallanas D, Romano D, Hamilton G, Kolch W, O’Neill E. A Hippo in the ointment: MST signalling beyond the fly. Cell Cycle.2008; 7: 879-84.
6.Moroishi T, Hansen CG, Guan K-L. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015; 15: 73-9.
7.Kim SK, Jung WH, Koo JS. Yes-associated protein (YAP) is differentially expressed in tumor and stroma according to the molecular subtype of breast cancer. Int J Clin Exp Pathol. 2014; 7:3224-34.
8.Kim GJ, Kim H, Park YN. Increased expression of yes-associated protein 1 in hepatocellular carcinoma with stemness and combined hepatocellular-cholangiocarcinoma. PLoS One. 2013; 8: e75449.
9.Wang LJ, Shi SJ, Guo ZY, Zhang X, Han SX, Yang AG, et al.Overexpression of YAP and TAZ Is an ındependent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One. 2013; 8: e65539.
10.Kang W, Tong JHM, Chan AWH, Lee T-L, Lung RWM, Leung PPS, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011; 17: 2130-9.
11.Diep CH, Zucker KM, Hostetter G, Watanabe A, Hu C, Munoz RM, Von Hoff DD, et al. Down-regulation of yes associated protein 1 expression reduces cell proliferation and clonogenicity of pancreatic cancer cells. PLoS One. 2012; 7: e32783.
12.Muramatsu T, Imoto I, Matsui T, Kozaki K-I, Haruki S, Sudol M,et al. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011; 32: 389-98.
13.Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008; 15: 1752-9.
14.Hu XY, Jia YY, Yu JJ, Chen J, Fu Q. Loss of YAP protein in PCa is associated with Gleason score increase. Tumori. 2015; 101: 189-93.
15.Sheng X, Li W-B, Wang D-L, Chen K-H, Cao J-J, Luo Z, et al. YAP is closely correlated with castration-resistant PCa, and downregulation of YAP reduces proliferation and induces apoptosis of PC-3 cells. Mol Med Rep. 2015; 12: 4867-76.
16.Zhang L, Yang SP, Chen XC, Stauffer S, Yu F, Lele SM, et al. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of PCa cells. Mol Cell Biol. 2015; 35:1350-62.
17.Cerami E, Gao JJ, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov.2012; 2: 401-4.
18.Gao JJ, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013; 6: pl1.
19.Liu TB, Liu YD, Gao HY, Meng FL, Yang SS, Lou G. Clinical significance of yes-associated protein overexpression in cervical carcinoma. Int J Gynecol Cancer. 2013; 23: 735-42.
20.Zhao B, Wei XM, Li WQ, Udan RS, Yang Q, Kim J, et al.Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev.2007; 21: 2747-61.
21.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, et al.Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006; 103:12405-10.
22.Weber RG, Sommer C, Albert FK, Kiessling M, Cremer T.Clinically distinct subgroups of glioblastoma multiforme studied by comparative genomic hybridization. Lab Invest. 1996; 74: 108-19.
23.Snijders AM, Schmidt BL, Fridlyand J, Dekker N, Pinkel D, Jordan RCK, et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma.Oncogene. 2005; 24: 4232-42.
24.Bashyam MD, Bair R, Kim YH, Wang P, Hernandez-Boussard T,Karikari CA, et al. Array-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancer. Neoplasia. 2005; 7: 556-62.
25.Dai Z, Zhu W-G, Morrison CD, Brena RM, Smiraglia DJ, Raval A,et al. A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum Mol Genet. 2003; 12: 791-801.
26.Lambros MBK, Fiegler H, Jones A, Gorman P, Roylance RR, Carter NP, et al. Analysis of ovarian cancer cell lines using array-based comparative genomic hybridization. J Pathol. 2005; 205: 29-40.
27.Imoto I, Tsuda H, Hirasawa A, Miura M, Sakamoto M, Hirohashi S, et al. Expression of cIAP1, a target for 11q22 amplification,correlates with resistance of cervical cancers to radiotherapy.Cancer Res. 2002; 62: 4860-6.
28.Dahiya R, McCarville J, Lee C, Hu WX, Kaur G, Carroll P, et al.Deletion of chromosome 11p15, p12, q22, q23-24 loci in human PCa. Int J Cancer. 1997; 72: 283-8.
29.Jiang N, Hjorth-Jensen K, Hekmat O, Iglesias-Gato D, Kruse T,Wang C, et al. In vivo quantitative phosphoproteomic profiling identifies novel regulators of castration-resistant PCa growth.Oncogene. 2015; 34: 2764-76.
30.Kim T-D, Jin F, Shin S, Oh S, Lightfoot SA, Grande JP, et al.Histone demethylase JMJD2A drives prostate tumorigenesis through transcription factor ETV1. J Clin Invest. 2016; 126: 706-20.
31.Kuser-Abali G, Alptekin A, Lewis M, Garraway IP, Cinar B. YAP1 and AR interactions contribute to the switch from androgendependent to castration-resistant growth in PCa. Nat Commun.2015; 6: 8126.
32.Zhao B, Li L, Lei QY, Guan K-L. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev.2010; 24: 862-74.
33.Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008; 135: 1081-8.
34.Lei Q-Y, Zhang H, Zhao B, Zha Z-Y, Bai F, Pei X-H, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and ıs ınhibited by the hippo pathway. Mol Cell Biol. 2008; 28:2426-36.
35.Nishioka N, Inoue K-I, Adachi K, et al. The hippo signaling pathway components lats and YAP pattern Tead4 activity to distinguish mouse trophectoderm from ınner cell mass. Dev Cell.2009; 16: 398-410.
36.Lee HJ, Diaz MF, Price KM, Ozuna JA, Zhang S, Sevick-Muraca EM, et al. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat Commun. 2017; 8: 14122.
Cite this article as: Collak FK, Demir U, Ozkanli S, Kurum E, Zerk PE.Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension. Cancer Biol Med. 2017; 14: 405-13. doi: 10.20892/j.issn.2095-3941.2017.0083
Filiz Kisaayak Collak
E-mail: filiz.collak@medeniyet.edu.tr
July 11, 2017; accepted November 9, 2017.
Available at www.cancerbiomed.org
Copyright © 2017 by Cancer Biology & Medicine
 Cancer Biology & Medicine2017年4期
Cancer Biology & Medicine2017年4期
- Cancer Biology & Medicine的其它文章
- A race to uncover a panoramic view of primary liver cancer
- The ascent of immune checkpoint inhibitors: is the understudy ready for a leading role?
- Cell cycle regulation and anticancer drug discovery
- Camptothecin-based nanodrug delivery systems
- Brain metastasis in advanced colorectal cancer: results from the South Australian metastatic colorectal cancer (SAmCRC)registry
- Promoter methylation of Wnt/β-Catenin signal inhibitor TMEM88 is associated with unfavorable prognosis of nonsmall cell lung cancer
