Camptothecin-based nanodrug delivery systems
Yan Wen, Yingze Wang, Xiaoli Liu, Wei Zhang, Xinhe Xiong, Zhongxiao Han, Xingjie Liang,CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Beijing 0090, China; University of Chinese Academy of Sciences, Beijing 0009, China; College of Biological Science and Engineering, Hebei University of Science and Technology, Shijiazhuang 0008, China; Department of Materials Science and Engineering, Herbert Wertheim College of Engineering, University of Florida, Gainesville, FL 6, USA; School of Biological Sciences and Medical Engineering, Southeast University, Nanjing 0096, China
REVIEW
Camptothecin-based nanodrug delivery systems
Yan Wen1,2*, Yingze Wang3*, Xiaoli Liu1, Wei Zhang1, Xinhe Xiong4, Zhongxiao Han5, Xingjie Liang1,21CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Beijing 100190, China;2University of Chinese Academy of Sciences, Beijing 100049, China;3College of Biological Science and Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, China;4Department of Materials Science and Engineering, Herbert Wertheim College of Engineering, University of Florida, Gainesville, FL 32611, USA;5School of Biological Sciences and Medical Engineering, Southeast University, Nanjing 210096, China
The drug camptothecin has a wide range of antitumor effects in cancers including gastric cancer, rectal and colon cancer, liver cancer, and lung cancer. Camptothecin-based drugs inhibit topoisomerase 1 (Topo 1), leading to destruction of DNA, and are currently being used as important chemotherapeutic agents in clinical antitumor treatment. However, the main obstacle associated with cancer therapy is represented by systemic toxicity of conventional anticancer drugs and their low accumulation at the tumor site. In addition, low bioavailability, poor water solubility, and other shortcomings hinder their anticancer activity. Different from traditional pharmaceutical preparations, nanotechnology-dependent nanopharmaceutical preparations have become one of the main strategies for different countries worldwide to overcome drug development problems. In this review, we summarized the current hotspots and discussed a variety of camptothecin-based nanodrugs for cancer therapy. We hope that through this review,more efficient drug delivery systems could be designed with potential applications in clinical cancer therapy.
Camptothecins; nanomedicine; cancer therapy; drug delivery system
Introduction
In 1966, Monroe E. Wall1first isolated camptothecin (CPT)from the stem of Camptotheca, a plant genus endemic to China. He discovered that CPT had a number of effects on malignant tumors such as gastrointestinal cancer, liver cancer, and leukemia. Later, in 1985, Y. H. Hsiang2found that CPT could block the synthesis of topoisomerase I (Topo 1), an enzyme closely related to cell division. Blockade of Topo 1 production by CPT prevents cancer cell growth, thus endowing this compound with unique anticancer properties.From this discovery, research on CPT entered a new stage. A large number of CPT derivatives and analogs emerged,among which irinotecan, topotecan, and 10-hydroxycamptothecin (HCPT) were approved for listing.Moreover, various active compounds are currently in the clinical stage. However, similar to most chemotherapeutic agents, application of CPT is limited by its inherentdeficiencies such as poor water solubility, low biocompatibility, toxic side effects on healthy tissue3, and a variety of complications4,5.
In recent years, scientists have been trying to overcome these deficiencies. The emergence of nanotechnology provides possibilities to address chemotherapy-associated drawbacks such as toxic side effects of anticancer drugs as well as their low water solubility. Due to the unique features of nanoparticles, including nanoscale size, high specific surface area, and controllable physical and chemical properties, the water solubility and stability of drugs can be improved, resulting in desirable pharmacokinetics and other parameters. In addition, nanopharmaceuticals can accumulate at the tumor site and regulate drug distribution due to their enhanced permeability and retention effect(EPR)6,7. The key advantages of nanomedicines are as follows: (1) improved water solubility and biocompatibility of the drug, (2) prolongation of tolerance time for the anticancer drug in the body by surface-modified nanoparticles, (3) precise accumulation of chemotherapeutics at the tumor site by targeting strategy, (4) stimulusresponsive release of payloads, and (5) reduced toxic side effects against normal cells and tissues7,8. In this regard, an effective combination of conventional chemotherapeutics with nanotechnology-based approaches to achieve efficient tumor treatment with low toxic side effects has become an important research area in cancer treatment and clinical application8-11.
Onivyde, a nanoliposomal dosage form of irinotecan, was approved by the FDA for pancreatic cancer treatment in 2015, and is currently being tested clinically for other malignancies12,13. In this manner, carrier-assisted CPT nanodrug delivery systems have been studied extensively. In addition, prodrug-coupled nanodrug delivery system is more suitable for effective transport and tracking of CPT-based drugs. Carrier-free, self-assembled pure nanodrug delivery systems provide more efficient drug doses for better therapeutic effects. In this review, we present all three nanodrug delivery systems based on CPTs (Figure 1).
Carrier-aided nanodrug delivery system
Normally, drugs undergo rapid metabolism in the body and lose their pharmacological activity. Therefore, it is important to improve their effective accumulation in the lesion. Based on the remarkable research achievements made in material science and pharmacy, nanomaterials with different sizes,structures, and surface properties have been developed including liposomes, polymeric nanoparticles, and inorganic nanomaterials such as iron oxide, carbon nanotubes, and silica14. These carriers can be widely used for delivering CPT drugs in vivo. The therapeutic effect of the drug is improved by the systemic (intravenous) or in situ route of administration. In addition, existing surface modification techniques can also affect the pharmacokinetic behavior and biodistribution of nanoparticles. For example, PEGylation protects drug-loaded nanoparticles from adsorption in the bloodstream, thereby achieving a long circulation cycle in the body and resulting in enhanced delivery at the tumor site through the EPR effect. Furthermore, the nanoparticle surface can be modified with active ligands to target specific cells. At present, many nanocarriers of CPT drugs have been used in clinical trials (Table 1).
Polymer-based nanocarriers for delivering CPTs
Delivery systems for transporting CPT drugs based on polymers as building blocks can be divided into two groups:natural polymers (such as proteins, and polysaccharides),and synthetic polymers (such as PLGA-PEG and PCL-PEG).Due to its natural presence in humans as well as its unique shape and excellent biocompatibility, the transferrin nanocarrier has attracted substantial interest. Chen et al24.prepared surface-modified transferrin nanoparticles of irinotecan, containing the specific targeting polypeptide PROM1, to achieve targeted delivery in colorectal cancer.Min et al.25loaded CPT into glycol-modified chitosan to prepare a nanoscale drug delivery system that shows prolonged blood circulation time and tumor targeting ability,for use in the treatment of human breast cancer. In addition,human serum albumin (HSA) is a multi-gene family protein that exists in the circulatory system with an average molecular weight of 66 kDa and a blood concentration of 50 mg/mL. It has low toxicity, high biocompatibility, and suitable biodegradation rate, and has therefore been widely used in drug delivery systems. Wang et al.26prepared HSA-modified HCPT-containing nanoparticles, FA-HSA-nHCPTNPs, in which the drug loading was 7.8%.

Figure 1 Schematic illustration of established camptothecins-based nanodrug platforms.

Table 1 Carrier-aided nanodrug delivery system for camptothecins nanomedicine in clinical trials. Alternative names for the products are given in brackets
Owing to the diversity of chemical properties, synthetic polymer nanocarriers are a promising tool for nanotechnologybased therapy. Svenson et al.27prepared CPT nanoparticles CRLX101, based on cyclodextrin as a carrier. Preclinical data indicated that CRLX101 showed a complete tumor response of 55.6% at day 125 after treatment at a dose of 10 mg/kg,whereas no complete tumor responses were observed in irinotecan-treated mice. Because of its antitumor properties such as inhibition of tumor cell proliferation and angiogenesis,CRLX101 has entered the clinical stage21. Hamaguchi et al.23have developed NK012 into clinical phase II. SN-38 was first connected to polyglutamate through an ester bond, and then assembled with polyethylene glycol as a shell to form nanopolymeric micelles (Figure 2). The size of the resulting nanoparticles was about 20 nm, even in different patients who have shown a stable effect23. The copolymer was also considered a promising carrier. For example, Lee et al.28loaded SN-38 onto poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (Pluronic F-108) and poly(PEG-b-PCL) to form nanoparticles. The drug loading capacity was (20.73±0.66)% and good biodistribution was observed, which improved the drug’s ability to kill tumor cells. Apart from these, PDMA-block-poly(εcaprolactone) (PDMA-b-PCL)28, poly-lactide-co-glycolidepoly-Ethylene glycol-folate (PLGA-PEG-FOL) conjugate29and other polymers have the potential to improve cell penetration and enhance the antitumor effect of CPT drugs.
Lipid-based nanocarriers for delivering CPTs
Liposomes are vesicles composed of a phospholipid bilayer with an aqueous phase as the core. This structure allows loading of therapeutic drugs either into the hydrophobic region of the liposome (the partition of the lipid layer) or in the hydrophilic part (the aqueous phase). This feature makes liposomes very promising nanocarriers30. A new technique was first demonstrated for stabilizing the embedded drugs using liposomes by encapsulating irinotecan (CPT-11) into long-term circulating liposomal nanoparticles in 1999. After that the most critical achievement is that irinotecan nanoliposomes (Onivyde) was approved by the FDA for the treatment of metastatic pancreatic cancer in 201531. In addition, the presence of polyethylene glycol (PEG) on the nanocarrier surface can prevent absorption of liposomes by the vascular endothelial system (RES). Atyabi et al.32found that the blood concentration of PEGylated nanoliposomes carrying SN-38 is nearly 50%, higher than that of non-PEGylated liposomes, and their accumulation in the liver,spleen, lungs, kidneys, and other organs is lower than that of non-PEGylated nanomicelles. Recently, Zhang et al.33obtained (HCPT-Ch-LDH)@LS nanostructures by liposome surface modification through a co-assembling strategy, thus providing better water solubility and sustained release of HCPT compared to those of unmodified-liposome nanoparticles.
Inorganic nanoparticles for delivering CPTs
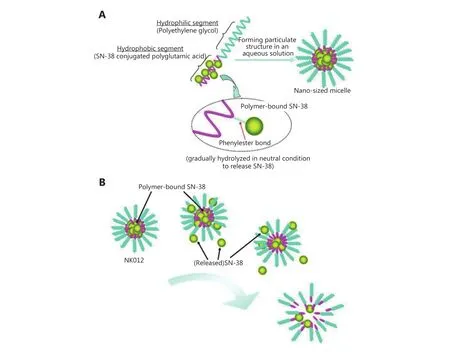
Figure 2 Schematic structure of NK012 and release of SN-38 from NK012. Reprinted with permission from Ref [23]; Copyright © 2010,American Association for Cancer Research.
Inorganic nanoparticles are widely used in tumor imaging,radiotherapy, and drug delivery34-39. Single-walled carbon nanotubes have low immunocompetence and show effective cell endocytosis, and were used by Lee et al.40as carriers of SN-38 for targeted delivery and controlled drug release. Liu et al.41developed a lipid carrier coated with mesoporous silica (LB-MSNP), which shows better biocompatibility and therapeutic effects than those shown by liposomes and free drugs, and is expected to be used as the first-line medication in the treatment of pancreatic duct cancer (PDAC). This group also attached the iRGD polypeptide to the liposome surface in order to enhance the efficacy of irinotecan and reduce tumor metastasis42. In addition, Song et al.43utilized PEG-modified hollow tantalum oxide with the payload SN-38 (H-TaOx-PEG@SN-38) for magnetic resonance imaging(MRI) and single-photon emission computed tomography(SPECT) multimodal imaging, as well as to achieve the combined effect of radiotherapy and chemotherapy. Muniesa et al.44also designed a nanomaterial with CPT and mesoporous silica, thereby achieving a response in the glutathione environment.
Prodrug-coupled nanodrug delivery systems
At present, prodrug-coupled nanodrugs are one of the successful drug delivery systems in clinical treatment. A prodrug is a compound that is metabolized to a pharmacologically active drug after administration.Nanotherapeutics are designed by covalently linking prodrugs with nanosized carriers composed of antibody molecules, lipids, proteins, polysaccharides, polypeptides, or polymers, which can improve bioavailability when a drug itself is poorly absorbed, and thus reduce the severe unintended and undesirable side effects of the drug45.
Only a few nanoparticles conjugated with CPT, such as N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers46,47,polyglutamic acid (PGA), polyethylene glycol (PEG)48-50, and carboxymethyldextran polyalcohol polymer51,52have entered clinical practice so far. Prodrug-coupled nanodrug delivery systems have also been used in clinical trials (Table 2). These block copolymers are assembled to form micelles. Drug coupling to the polymer carrier can improve the water solubility of hydrophobic drugs, thus making it easier to inject them in patients. Although there are many polymers that can be used for drug delivery, only few of them can be used in the clinic due to their toxicity and immunogenicity,resulting in less effective drug delivery45.
In addition, most nanoparticles conjugated with CPT using polymers show tumor targeting dependent on tumor vascular permeability (EPR effect53). Han et al.54connected herceptin (an antibody to HER2) to the surface of MAP-CPT nanoparticles via a boronic acid bond to achieve targeted combination therapy for HER2-overexpressing breast cancer.Peng et al.55conjugated the anticancer drug CPT with a polypeptide that was self-assembled to form nanofibers that could release CPT by hydrolysis. Zhang et al.56prepared a combination of irinotecan and fatty acids to achieve a high drug load with 77.2% irinotecan content, high drug availability, and enrichment at the tumor site.
Carrier-free pure nanodrug delivery systems
While drug-loaded nanocarriers have many advantages, there are still some concerns regarding potential issues8,59,60in the fields of environment, health, and safety61,62. Furthermore,almost all carriers have no therapeutic efficacy by themselves63,64. It is complicated to establish a proper manufacturing process65for drug-loaded nanocarriers, and most of them demonstrate low drug-loading capacity66. Even worse, many carriers may cause high toxicity and serious inflammation in the kidneys and other organs61,63,64.Therefore, development of alternative targeted delivery strategies with minimum use of inert materials is highly desirable67. There is no doubt that carrier-free pure nanodrugs will thus become candidates in the next generation of drugs63,68-71.
Hitoshi Kasai68proposed the use of CPT (SN-38) to form a dimer and first assembled it to form nanoparticles successfully. These nanoparticles showed reduced side effects due to the absence of other components, and the drugloading capacity was nearly 100%. Since then, pure drugs with the ability to self-assemble into nanodelivery systems have opened a new chapter in drug delivery. Subsequently,disulfide-linked CPT and irinotecan nanoparticles achieved a response to glutathione and drug release in a specified area72.Improvement of the water solubility of CPT and multiple attacks against tumor cells could be achieved through coassembly of CPT and other drug components. In a previous work, Chen et al.73provided a new strategy for the combination of drugs by co-loading HCPT and doxorubicin(DOX)73,74, which resulted in a synergistic effect in overcoming tumor drug resistance. Wen et al.75combined HCPT with dihydroporphyrin (Ce6). These two components co-formed a nanorod structure by π-π conjugation and hydrophobic interaction. This hybrid nanodrug not only circumvents the extreme hydrophobicity of HCPT (with a solubility at least 100-fold higher than that of free HCPT in water), but also integrates two tumor treatment modalities into one system. It provided a simple and green solution to develop pure carrier-free nanodrugs that combine two treatment modalities, chemotherapy and photodynamic therapy, into a single platform to circumvent the drawbacksof traditional small molecules and to achieve highly potent antitumor capacity, which could be easily expanded to other drugs and modalities. The rationale of this facile and green strategy for carrier-free pure nanodrugs might provide new opportunities for the development of combinatorial therapeutics for tumors75.

Table 2 Prodrug-coupled nanodrug delivery system for camptothecins nanomedicine in clinical trials. Alternative names for the products are given in brackets
Conclusions
Due to their unique physical and chemical properties, CPT drugs have received widespread attention in the field of pharmaceutical preparations. However, there are many obstacles for nanodrugs in their journey from the laboratory to the clinical stage. Although CPT drug delivery systems have been extensively studied, most nanodrug-dependent nanopharmaceuticals are limited by the potentially toxic side effects of nanomaterials and in vivo metabolism and controllable problems. Thus, nanodrugs that can be used without nanocarriers, relying on self-assembled drug molecules, are thought to be the new generation of pharmaceutical preparations of clinical value, but they still need to be tested. As a general rule, the simpler and easier the development of a system is, the better are its chances of reaching the clinic.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA.Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc. 1966; 88: 3888-90.
2.Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I.J Biol Chem. 1985; 260: 14873-8.
3.Wall ME. Camptothecin and taxol: discovery to clinic. Med Res Rev. 1998; 18: 299-314.
4.O’Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. Eur J Cancer. 1998;34: 1500-8.
5.Potmesil M. Camptothecins: from bench research to hospital wards. Cancer Res. 1994; 54: 1431-9.
6.Maeda H, Greish K, Fang J. The EPR effect and polymeric drugs: a paradigm shift for cancer chemotherapy in the 21st century. Adv Polym Sci. 2006; 193: 103-21.
7.Xin Y, Yin MM, Zhao LY, Meng FL, Luo L. Recent progress on nanoparticle-based drug delivery systems for cancer therapy.Cancer Biol Med. 2017; 14: 228-41.
8.Wicki A, Witzigmann D, Balasubramanian V, Huwyler J.Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015; 200: 138-57.
9.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR.Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016; 33: 2373-87.
10.D'Mello SR, Cruz CN, Chen ML, Kapoor M, Lee SL, Tyner KM.The evolving landscape of drug products containing nanomaterials in the United States. Nat Nanotechnol. 2017; 12: 523-9.
11.Li X, Zhang XN, Li XD, Chang J. Multimodality imaging in nanomedicine and nanotheranostics. Cancer Biol Med. 2016; 13:339-48.
12.Zhang HJ. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016; 9: 3001-7.
13.Passero FC Jr, Grapsa D, Syrigos KN, Saif MW. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabinebased therapy. Expert Rev Anticancer Ther. 2016; 16: 697-703.
14.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013; 12: 991-1003.
15.Batist G, Gelmon KA, Chi KN, Miller WH, Chia SKL, Mayer LD, et al. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res.2009; 15: 692-700.
16.Infante JR, Keedy VL, Jones SF, Zamboni WC, Chan E, Bendell JC,et al. Phase I and pharmacokinetic study of IHL-305 (PEGylated liposomal irinotecan) in patients with advanced solid tumors.Cancer Chemother Pharmacol. 2012; 70: 699-705.
17.Tardi P, Choice E, Masin D, Redelmeier T, Bally M, Madden TD.Liposomal encapsulation of topotecan enhances anticancer efficacy in murine and human xenograft models. Cancer Res. 2000; 60:3389-93.
18.Verschraegen CF, Gilbert BE, Huaringa AJ, Newman R, Harris N,Leyva FJ, et al. Feasibility, phase I, and pharmacological study of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced malignancies in the lungs. Ann N Y Acad Sci. 2000; 922:352-4.
19.Pal A, Khan S, Wang YF, Kamath N, Sarkar AK, Ahmad A, et al.Preclinical safety, pharmacokinetics and antitumor efficacy profile of liposome-entrapped SN-38 formulation. Anticancer Res. 2005;25: 331-42.
20.Ko AH, Tempero MA, Shan YS, Su WC, Lin YL, Dito E, et al. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabinerefractory metastatic pancreatic cancer. Br J Cancer. 2013; 109:920-5.
21.Gaur S, Wang YF, Kretzner L, Chen LL, Yen T, Wu XW, et al.Pharmacodynamic and pharmacogenomic study of the nanoparticle conjugate of camptothecin CRLX101 for the treatment of cancer. Nanomedicine. 2014; 10: 1477-86.
22.Ghamande S, Lin CC, Cho DC, Shapiro GI, Kwak EL, Silverman MH, et al. A phase 1 open-label, sequential dose-escalation study investigating the safety, tolerability, and pharmacokinetics of intravenous TLC388 administered to patients with advanced solid tumors. Invest New Drugs. 2014; 32: 445-51.
23.Hamaguchi T, DoiT, Eguchi-Nakajima T, Kato K, Yamada Y,Shimada Y, et al. Phase I study of NK012, a novel SN-38-incorporating micellar nanoparticle, in adult patients with solid tumors. Clin Cancer Res. 2010; 16: 5058-66.
24.Chen JLY, Tsai YC, Tsai MH, Lee SY, Wei MF, Kuo SH, et al.Prominin-1-specific binding peptide-modified apoferritin nanoparticle carrying irinotecan as a novel radiosensitizer for colorectal cancer stem-like cells. Part Part Syst Charact. 2017; 34:1600424.
25.Min KH, Park K, Kim Y-S, Bae SM, Lee S, Jo HG, et al.Hydrophobically modified glycol chitosan nanoparticlesencapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J Control Release. 2008; 127: 208-18.
26.Wang WC, Liang H, Sun BH, Xu JL, Zeng Z, Zhao XJ, et al.Pharmacokinetics and tissue distribution of folate-decorated human serum albumin loaded with nano-hydroxycamptothecin for tumor targeting. J Pharm Sci. 2016; 105: 1874-80.
27.Svenson S, Wolfgang M, Hwang J, Ryan J, Eliasof S. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J Control Release. 2011; 153: 49-55.
28.Lee SY, Yang CY, Peng CL, Wei MF, Chen KC, Yao CJ, et al. A theranostic micelleplex co-delivering SN-38 and VEGF siRNA for colorectal cancer therapy. Biomaterials. 2016; 86: 92-105.
29.Ebrahimnejad P, Dinarvand, R, Sajadi, A. Preparation and in vitro evaluation of actively targetable nanoparticles for SN-38 delivery against HT-29 cell lines. Nanomedicine. 2010; 6: 478-85.
30.Waterhouse DN, Yapp D, Verreault M, Anantha M, Sutherland B,Bally MB. Lipid-based nanoformulation of irinotecan: dual mechanism of action allows for combination chemo/angiogenic therapy. Nanomedcine. 2011; 6: 1645-54.
31.Ur Rehman SS, Lim K, Wang-Gillam A. Nanoliposomal irinotecan plus fluorouracil and folinic acid: a new treatment option in metastatic pancreatic cancer. Expert Rev Anticancer Ther. 2016; 16:485-92.
32.Atyabi F, Farkhondehfai A, Esmaeili F, Dinarvand R. Preparation of pegylated nano-liposomal formulation containing SN-38: In vitro characterization and in vivo biodistribution in mice. Acta Pharm.2009; 59: 133-44.
33.Zhang YF, Wu WX, Mi YM, Li HP, Hou WG. Engineering of (10-hydroxycamptothecin intercalated layered double hydroxide)@liposome nanocomposites with excellent water dispersity. J Phys Chem Solids. 2017; 108: 125-32.
34.Huang H-C, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release.2011; 155: 344-57.
35.Rivera Gil P, Hühn D, del Mercato LL, Sasse D, Parak WJ.Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol Res. 2010; 62: 115-25.
36.Ross RW, Zietman AL, Xie WL, Coen JJ, Dahl DM, Shipley WU, et al. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin Imaging. 2009; 33: 301-5.
37.Na HB, Song IC, Hyeon T. Inorganic nanoparticles for MRI contrast agents. Adv Mater. 2009; 21: 2133-48.
38.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, et al.Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008; 2: 889-96.
39.Xu ZP, Zeng QH, Lu GQ, Yu AB. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci. 2006; 61:1027-40.
40.Lee PC, Chiou YC, Wong JM, Peng CL, Shieh MJ. Targeting colorectal cancer cells with single-walled carbon nanotubes conjugated to anticancer agent SN-38 and EGFR antibody.Biomaterials. 2013; 34: 8756-65.
41.Liu XS, Situ A, Kang YN, Villabroza KR, Liao YP, Chang CH, et al.Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer. ACS Nano. 2016; 10: 2702-15.
42.Liu XS, Lin P, Perrett I, Lin J, Liao YP, Chang CH, et al. Tumorpenetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer. J Clin Invest. 2017; 127:2007-18.
43.Song GS, Chao Y, Chen YY, Liang C, Yi X, Yang GB, et al. All-in-One Theranostic Nanoplatform Based on Hollow TaOx for Chelator-Free Labeling Imaging, Drug Delivery, and Synergistically Enhanced Radiotherapy. AdvFunct Mat. 2016; 26: 8243-54.
44.Muniesa C, Vicente V, Quesada M, Sáez-Atiénzar S, Blesa JR,Abasolo I, et al. Glutathione-sensitive nanoplatform for monitored intracellular delivery and controlled release of Camptothecin. RSC Adv. 2013; 3: 15121-31.
45.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006; 6: 688-701.
46.Williams CC, Thang SH, Hantke T, Vogel U, Seeberger PH,Tsanaktsidis J, et al. RAFT-derived polymer-drug conjugates:poly(hydroxypropyl methacrylamide) (HPMA)-7-ethyl-10-hydroxycamptothecin (SN-38) conjugates. ChemMedChem. 2012;7: 281-91.
47.Bissett D, Cassidy J, de Bono JS, Muirhead F, Main M, Robson L, et al. Phase I and pharmacokinetic (PK) study of MAG-CPT (PNU 166148): a polymeric derivative of camptothecin (CPT). Br J Cancer. 2004; 91: 50-5.
48.Awada A, Garcia AA, Chan S, Jerusalem GH, Coleman RE, Huizing MT, et al. Two schedules of etirinotecan pegol (NKTR-102) in patients with previously treated metastatic breast cancer: a randomised phase 2 study. Lancet Oncol. 2013; 14: 1216-25.
49.Patnaik A, Papadopoulos KP, Tolcher AW, Beeram M, Urien S,Schaaf LJ, et al. Phase I dose-escalation study of EZN-2208 (PEGSN38), a novel conjugate of poly(ethylene) glycol and SN38,administered weekly in patients with advanced cancer. Cancer Chemother Pharmacol. 2013; 71: 1499-506.
50.Scott LC, Yao JC, Benson AB III, Thomas AL, Falk S, Mena RR, et al. A phase II study of pegylated-camptothecin (pegamotecan) in the treatment of locally advanced and metastatic gastric and gastrooesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2009; 63: 363-70.
51.Veltkamp SA, Witteveen EO, Capriati A, Crea A, Animati F,Voogel-Fuchs M, et al. Clinical and pharmacologic study of the novel prodrug delimotecan (MEN 4901/T-0128) in patients with solid tumors. Clin Cancer Res. 2008; 14: 7535-44.
52.Soepenberg O, de Jonge MJ, Sparreboom A, de Bruin P, Eskens FA,de Heus G, et al. Phase I and pharmacokinetic study of DE-310 in patients with advanced solid tumors. Clin Cancer Res. 2005; 11:703-11.
53.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006; 11: 812-8.
54.Han H, Davis ME. Single-antibody, targeted nanoparticle delivery of camptothecin. Mol Pharm. 2013; 10: 2558-67.
55.Peng MY, Qin SY, Jia HZ, Zheng DW, Rong L, Zhang XZ. Selfdelivery of a peptide-based prodrug for tumor-targeting therapy.Nano Res. 2015; 9: 663-73.
56.Zhang CQ, Jin SB, Xue XD, Zhang TB, Jiang YG, Wang PC, et al.Tunable self-assembly of Irinotecan-fatty acid prodrugs with increased cytotoxicity to cancer cells. J Mater Chem B. 2016; 4:3286-91.
57.Homsi J, Simon GR, Garrett CR, Springett G, De Conti R,Chiappori AA, et al. Phase I trial of poly-L-glutamate camptothecin(CT-2106) administered weekly in patients with advanced solid malignancies. Clin Cancer Res. 2007; 13: 5855-61.
58.Yurkovetskiy AV, Fram RJ. XMT-1001, a novel polymeric camptothecin pro-drug in clinical development for patients with advanced cancer. Adv Drug Deliv Rev. 2009; 61: 1193-202.
59.Kawasaki ES, Player A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine.2005; 1: 101-9.
60.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013; 12: 958-62.
61.Vega-Villa KR, Takemoto JK, Yáñez JA, Remsberg CM, Forrest ML,Davies NM. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008; 60: 929-38.
62.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008; 3: 423-8.
63.Huang P, Wang DL, Su Y, Huang W, Zhou YF, Cui DX, et al.Combination of small molecule prodrug and nanodrug delivery:amphiphilic drug-drug conjugate for cancer therapy. J Am Chem Soc. 2014; 136: 11748-56.
64.Yu DS, Peng P, Dharap SS, Wang Y, Mehlig M, Chandna P, et al.Antitumor activity of poly(ethylene glycol)-camptothecin conjugate: the inhibition of tumor growth in vivo. J Control Release. 2005; 110: 90-102.
65.Tan WB, Zhang Y. Surface modification of gold and quantum dot nanoparticles with chitosan for bioapplications. J Biomed Mater Res A. 2005; 75: 56-62.
66.Cao ZQ, Yu QM, Xue H, Cheng G, Jiang SY. Nanoparticles for drug delivery prepared from amphiphilic PLGA zwitterionic block copolymers with sharp contrast in polarity between two blocks.Angew Chem Int Ed Engl. 2010; 49: 3771-6.
67.Li YN, Yang YL, An FF, Liu Z, Zhang XJ, Zhang XH. Carrier-free,functionalized pure drug nanorods as a novel cancer-targeted drug delivery platform. Nanotechnology. 2013; 24: 015103
68.Kasai H, Murakami T, Ikuta Y, Koseki Y, Baba K, Oikawa H, et al.Creation of pure nanodrugs and their anticancer properties. Angew Chem Int Ed. 2012; 51: 10315-8.
69.Li W, Yang YL, Wang C, Liu Z, Zhang XJ, An FF, et al. Carrier-free,functionalized drug nanoparticles for targeted drug delivery. Chem Commun. 2012; 48: 8120-2.
70.Cheetham AG, Zhang PC, Lin YA, Lock LL, Cui HG.Supramolecular nanostructures formed by anticancer drug assembly. J Am Chem Soc. 2013; 135: 2907-10.
71.Li W, Zhang XJ, Zhou MJ, Tian BS, Yu CT, Jie JS, et al. Functional core/shell drug nanoparticles for highly effective synergistic cancer therapy. Adv Healthc Mater. 2014; 3: 1475-85.
72.He DX, Zhang W, Deng HZ, Huo SD, Wang Y-F, Gong NQ, et al.Self-assembling nanowires of an amphiphilic camptothecin prodrug derived from homologous derivative conjugation. Chem Commun. 2016; 52: 14145-8.
73.Chen F, Zhao YY, Pan YM, Xue XD, Zhang X, Kumar A, et al.Synergistically enhanced therapeutic effect of a carrier-free HCPT/DOX nanodrug on breast cancer cells through improved cellular drug accumulation. Mol Pharm. 2015; 12: 2237-44.
74.Zhao YY, Chen F, Pan YM, Li ZP, Xue XD, Okeke CI, et al.Nanodrug formed by coassembly of dual anticancer drugs to inhibit cancer cell drug resistance. ACS Appl Mater Interfaces.2015; 7: 19295-305.
75.Wen Y, Zhang W, Gong NQ, Wang YF, Guo HB, Guo WS, et al.Carrier-free, self-assembled pure drug nanorods composed of 10-hydroxycamptothecin and chlorin e6 for combinatorial chemophotodynamic antitumor therapy in vivo. Nanoscale. 2017; 9:14347-56.
Cite this article as: Wen Y, Wang Y, Liu X, Zhang W, Xiong X, Han Z, et al.Camptothecin-based nanodrug delivery systems. Cancer Biol Med. 2017; 14:363-70. doi: 10.20892/j.issn.2095-3941.2017.0099
*These authors have contributed equally to this work
Xiaoli Liu and Xingjie Liang
E-mail: liuxiaoli@nanoctr.cn and liangxj@nanoctr.cn
August 14, 2017; accepted November 9, 2017.
Available at www.cancerbiomed.org
Copyright © 2017 by Cancer Biology & Medicine
 Cancer Biology & Medicine2017年4期
Cancer Biology & Medicine2017年4期
- Cancer Biology & Medicine的其它文章
- A race to uncover a panoramic view of primary liver cancer
- The ascent of immune checkpoint inhibitors: is the understudy ready for a leading role?
- Cell cycle regulation and anticancer drug discovery
- Brain metastasis in advanced colorectal cancer: results from the South Australian metastatic colorectal cancer (SAmCRC)registry
- Promoter methylation of Wnt/β-Catenin signal inhibitor TMEM88 is associated with unfavorable prognosis of nonsmall cell lung cancer
- Profile of the breast cancer susceptibility marker rs4245739 identifies a role for miRNAs
