含羟基苯并吲哚的增强型荧光探针测定过氧化氢
刘长辉,刘 波,齐风佩,王海洋
(湖南城市学院 材料与化学工程学院 黑茶金花湖南省重点实验室,湖南 益阳 413000)
含羟基苯并吲哚的增强型荧光探针测定过氧化氢
刘长辉,刘 波,齐风佩,王海洋
(湖南城市学院 材料与化学工程学院 黑茶金花湖南省重点实验室,湖南 益阳 413000)
合成了含羟基苯并吲哚并研究其对过氧化氢的荧光响应,考察了溶剂种类及pH值等因素对检测体系的影响﹒在最佳实验条件下,过氧化氢将引发580 nm处荧光信号蓝移至560 nm处,且其荧光强度随过氧化氢浓度的增加而增强,线性范围为0.1~1.2 mmol/L,检出限为72 mol/L,探针响应不受其它干扰物的影响﹒该方法可用于消毒液及自来水样品中过氧化氢的准确测定﹒
过氧化氢;荧光探针;荧光增强;苯并吲哚
过氧化氢(H2O2)是一种强氧化剂,广泛用于医用消毒杀菌、农业杀虫抗菌、工业漂白发色和药物合成等领域,又因其具有漂白、防腐和除臭的效果,常被用作食品添加剂﹒然而,过量的H2O2有致癌性,严重危害人们的身体健康﹒因此,H2O2的高灵敏检测在医药、化工及环境分析等领域受到研究者的密切关注﹒目前,H2O2的检测方法主要有电化学法[1-3]、色谱法[4]、化学发光法[5]、生物发光法[6]、分光光度法[7-9]和荧光光度法[10-18]等﹒相比上述方法,荧光法具有前处理简便、灵敏度高、信号可调、快速及操作简单等优点而备受关注[19]﹒本文利用 H2O2易将酚羟基氧化为苯醌的反应机制[20-21],设计合成了含羟基苯并吲哚,构建了一种特异性测定痕量H2O2的方法,并应用于消毒液及自来水样品中H2O2含量的测定﹒
1 材料与方法
1.1 仪器与试剂
U-3010型紫外-可见分光光度计(日本岛津公司);F-4600型荧光光度计(日本岛津公司);Varian INOVA 400核磁共振仪(Varian公司);HP1100 LC/MSD质谱仪(Agilent公司)﹒
1,2,2-三甲基苯并吲哚,碘甲烷,3,4-二羟基苯甲醛均购自北京百灵威科技有限公司﹒所用试剂均为分析纯;实验用水为超纯水系统(Barnstead/Thermolyne Corp.,Dubuque,IA)制备的超纯水(18.2 MΩ·cm);溶剂购自上海国药试剂有限公司,使用前均通过标准方法进行纯化和干燥﹒样品均购自湖南省益阳市主要超市等﹒
1.2 含羟基苯并吲哚的合成
含羟基苯并吲哚的合成路线如图1所示﹒
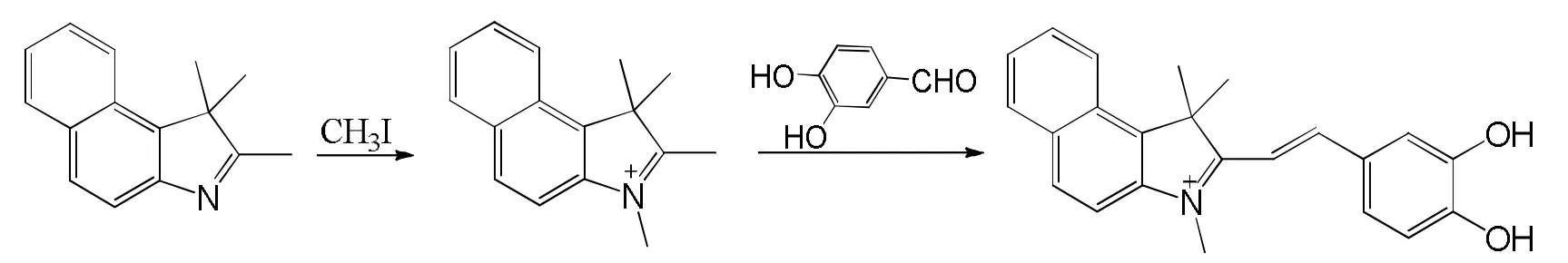
图1 含羟基苯并吲哚的合成路线图
[22]的方法合成:将 1.36 g(6.5 mmol)1,2,2-三甲基苯并吲哚溶于20 mL甲苯中,加入0.8 mL(13 mmol)碘甲烷,混匀回流10 h﹒反应完毕,冷却,抽滤,乙醚洗涤,旋干得白色固体1,2,3,3,-四甲基苯并吲哚,产率51.28%,未经纯化直接用作后续反应﹒
将176 mg(0.5 mmol)1,2,3,3,-四甲基苯并吲哚与165.6 mg(1.2 mmol)3,4-二羟基苯甲醛溶于15 mL甲苯中,在氮气保护下回流8 h﹒反应完毕,冷却,加入30 mL去离子水分液,水层用乙酸乙酯提取(50 mL×3)三次,有机层用饱和食盐水洗涤(50 mL×2)两次,合并有机相,无水硫酸镁干燥,旋干,硅胶柱层析后得深褐色固体含羟基苯并吲哚,产率17.02%﹒1H NMR(400 MHz, DMSO-d6, δ,ppm): 8.55(d, J=16.2, 2H), 8.40 (d, J=8.1, 2H), 8.32(d, J=8.4, 1H), 8.29(d, J=8.9, 1H), 8.25(d, J=8.1,1H), 7.90(d, J=7.9, 2H), 7.76(m, 2H), 7.66(m, 1H),5.38(s, 2H), 4.02(s, 3H), 2.11(s, 6H).13C NMR (100 MHz, CDCl3, δ, ppm): 186.0, 151.6, 143.2, 140.7,136.8, 134.4, 133.9, 131.9, 130.1, 129.5, 128.9,126.7, 118.5, 57.7, 39.3, 28.1. HRMS-ESI: m/z calcd M+, 344.167 5; found, 344.168 3.
1.3 光谱测定
用N, N-二甲基甲酰胺(DMF)溶解DHB并定容得1.0 mmol/L的母液备用﹒将母液用DMF
PBS(10 mmol/L,pH=7.2,体积比1︰4)稀释至10 mol/L作为探针溶液﹒室温下,量取1.5 mL探针溶液,加入0.5 mL不同浓度H2O2,测其紫外-可见吸收光谱;以380 nm激发,测定其荧光光谱﹒激发和发射狭缝均为10 nm并保持不变﹒
1.4 样品中过氧化氢浓度测定
测定方法同1.3,用0.5 mL待测液替代H2O2标准溶液,利用标准工作曲线计算求得样品中H2O2的浓度,每份样品平行测定3次﹒
2 实验结果
2.1 探针DHB与过氧化氢反应的光谱性能
首先考察了探针含羟基苯并吲哚(DHB)与H2O2反应前后光物理性质的变化情况,结果如图2所示﹒
由图2A可知,DHB的最大吸收波长位于350 nm处,其与H2O2反应30 min后,最大吸收波长的吸光度增大﹒图2B说明,探针DHB展现一定强度的荧光信号,其与H2O2作用后的荧光信号显著增强﹒可能的原因是,H2O2将酚羟基氧化成苯醌,增大了体系的共轭效应,改变了体系的推拉电子效应﹒

图2 探针DHB(10 µM)与H2O2(1.0 mM)反应前后的吸收光谱(A)和荧光发射光谱(B)
2.2 溶剂对DHB与H2O2反应的影响
在工作中,考察了探针 DHB在不同溶剂中的荧光发射光谱,结果如图3所示﹒
由图3可知,探针DHB在PBS缓冲溶液(10 mmol/L,pH=7.2)中的荧光强度较弱,在乙腈、DMF及乙醇等有机溶剂中的荧光较强,但在DMF-PBS (体积比1︰4)的溶液体系中对H2O2展现最强的响应效果﹒可能的原因是探针 DHB具有较大的共轭体系,其在不同的溶剂体系中展现溶解度的差异﹒因此,在本工作中,检测体系均采用 DMF-PBS(体积比 1︰4)的混合溶液作为检测体系﹒

图3 探针DHB(10 µM)在不同溶剂中与H2O2(1.0 mM)反应后的荧光发射光谱
2.3 pH对DHB与过氧化氢反应的影响
为了确证探针DHB能够检测水体中H2O2,在DMF-PBS (体积比1︰4)缓冲溶液中,我们考察了pH对检测体系的影响,结果如图4所示﹒

图4 探针DHB(10 µM)在不同pH值条件下与H2O2(1.0 mM)反应的荧光变化情况
由图4可知,在pH值4~8的范围内,探针DHB保持恒定的荧光信号;加入H2O2并反应30 min后,其荧光强度在pH值4~8的范围内的显著增幅增加﹒然而,在强碱性条件下(pH>8),酚羟基被转化为苯氧负离子,过氧化氢也容易分解,导致荧光强度的增幅减小,灵敏度偏低﹒结果表明,DHB能够在较宽的pH范围内(pH值4~7.4)检测H2O2﹒
2.4 过氧化氢对DHB荧光光谱的影响
在DMF-PBS (体积比1︰4)溶液中加入H2O2,考察 H2O2浓度对检测体系的影响,结果如图 5所示﹒
由图5A可知,未加入H2O2时,探针DHB在580 nm处展现较弱的荧光信号;随着H2O2浓度的增加,560 nm处出现新的荧光信号且其荧光强度随H2O2浓度的增加而不断增强;当浓度为1.0 mmol/L时,荧光强度增加了3.1倍,且荧光强度在 0.1~1.2 mmol/L的范围内的变化程度与H2O2浓度呈现良好的线性关系,其回归方程为y=0.302x+0.071 3,线性相关系数R2=0.991 0,最低检测限为72 mol/L,如图5B所示﹒结果表明,探针DHB可检测痕量H2O2﹒

图5 探针DHB(10 µM)与H2O2作用后荧光发射光谱(A)及其荧光强度比值((F-F0)/F0)与H2O2浓度的变化曲线(B)
2.5 干扰实验
在 DMF-PBS(体积比 1︰4)缓冲溶液中,在pH值为7.2,H2O2浓度为1.0 mmol/L的条件下,我们研究了探针 DHB与可能存在的干扰物作用后的荧光发射光谱及强度变化,如图6所示﹒
由图6可知,探针DHB与水体中可能的干扰物,如 Cl-,Na+,F-,K+,Ca2+,CO32-,Fe2+,Mg2+,Zn2+,Ni2+,Cu2+,Pb2+,Fe3+,ClO-,等作用后,体系的荧光强度变化可以忽略不计﹒与其形成鲜明对比的是,H2O2的加入显著增强了体系的荧光强度,说明探针DHB对H2O2展现特异性响应能力﹒尤其是,在干扰物共存的条件下,探针DHB与H2O2作用后仍展现很强的荧光信号﹒结果表明,探针DHB对 H2O2展现较高的选择性以及较强的抗干扰能力,为H2O2的特异性定量检测提供了保证﹒

图6 探针DHB(10 µmol/L)与干扰物(1.0 mmol/L)作用后荧光响应(A)及干扰物存在下与H2O2作用后荧光响应(B)
2.6 样品分析
在 DMF-PBS(体积比 1︰4)缓冲溶液中,在pH值为7.2条件下,将探针DHB用于医用双氧水消毒剂及自来水中H2O2的定量检测,结果如表1所示﹒从表1可以看出,探针DHB对医用双氧水消毒剂中H2O2的加标回收率为98.7%~102.2%,对自来水中H2O2的加标回收率为99.7%~103.0%,获得满意的测定结果﹒因此,探针 DHB可有效测定实际样品中的H2O2﹒
3 结论
根据H2O2氧化苯酚为苯醌的机制,建立了含羟基苯并吲哚的增强型荧光法检测 H2O2的新体系﹒在DMF-PBS(体积比1︰4)缓冲溶液及pH值为7.2的条件下,H2O2浓度在0.1~1.2 mmol/L范围内与检测体系的荧光强度变化呈线性关系,检测限为72 mol/L﹒该方法操作简单、选择性高、抗干扰能力强,可应用于消毒液及自来水等样品中痕量 H2O2的测定,加标回收率分别为98.7%~102.2%和 99.7%~103.0%,获得满意的测定结果﹒
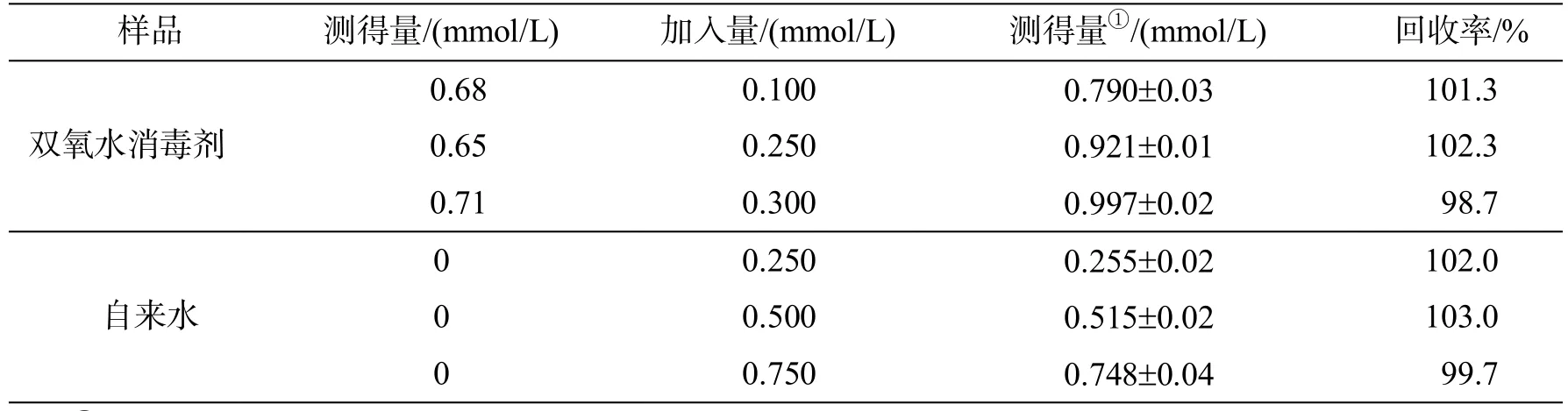
表1 实际样品中测定H2O2
参考文献:
[1]QIN X, WANG H, MIAO Z,et al. A novel non-enzyme hydrogen peroxide sensor based on catalytic reduction property of silver nanowires[J]. Talanta, 2015, 139(2): 56-61.
[2]LI X, LIU X, WANG W,et al. High loading pt nanoparticles on functionalization of carbon Nanotubes for fabricating nonenzyme hydrogen peroxide sensor[J]. Biosensors and Bioelectronics, 2014,59(3): 221-226.
[3]HABIBI B, JAHANBAKHSHI M. A novel nonenzymatic hydrogen peroxide sensor based on the synthesized mesoporous carbon and silver nanoparticles nanohybrid[J]. Sensor and Actuators B- Chemistry, 2014, 203(7): 919-925.
[4]HONG J, MAGUHN J, FREITAG D,et al. Determination of H2O2and organic peroxide by high-performance liquid chromatography with post-column UV irradiation, Derivatization and fluorescence detection[J]. Fresenius Jurnal of Analytical Chemistry, 1998, 361(2): 124-128.
[5]LEE D, KHAJA S, VELASQUEZ-CASTANO J,et al. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles[J]. Nature Materials, 2007(6): 765-769.
[6]VANDE BITTNER G C, BERTOZZI C R, CHANG C J. A strategy for dual-analyte luciferin imaging: In vivo bioluminescence detection of hydrogen peroxide and caspase activity in a murine model of acute inflammation[J]. Journal of the America Chemistry Society, 2013, 135(5): 1783-1795.
[7]MAJI S K, MANDAL A K, NGUYEN K T,et al. Cancer cell detection and therapeutics using peroxidase-active nanohybrid of gold nanoparticle-loaded mesoporous silica-coated graphene[J].ACS Applied Materials and Interfaces, 2015, 7(18): 9807-9816.
[8]SUN C, CHEN X, XU J,et al. Fabrication of an inorganic-organic hybrid based on an iron-substituted polyoxotungstate as a peroxidase for colorimetric immunassays of H2O2 and cancer cells[J]. Journal of Materials Chemistry A, 2013,15(1): 4699-4705.
[9]ZHANG Y, TIAN J, LIU S, et al. Novel application of cofe layered double hydroxide nanoplates for colorimetric detection of H2O2 and glucose[J]. Analyst, 2012, 137(6): 1325-1328.
[10]ZHAO W, LI Y, YANG S, et al. Target-activated modulation of dual-color and two-photon fluorescence of graphene quantum dots for in vivo imaging of hydrogen peroxide[J]. Analytical Chemistry, 2016, 88(9): 4833-4840.
[11]WEINSTAIN R, SAVARIAR E N, FELSEN C N, et al. In vivo targeting of hydrogen peroxide by activatable cell-penetrating peptides[J]. Journal of the America Chemistry Society, 2014,136(3): 874-877.
[12]LIPPERT A R, KESHARI K R, URHANEWICZ K J, et al. A hydrogen peroxide-responsive hyperpolarized 13C mri constrast agent[J]. Journal of the America Chemistry Society, 2011,133(11): 3776-3779.
[13]YUAN L, LIN W, ZHAO S, et al. A unique approach to development of near-infrared fluorescent sensors for in vivo imaging[J]. Journal of the America Chemistry Society, 2012,134(32): 13510-13523.
[14]ABO M, MINAKAMI R, MIYANO K, et al. Visualization of phagosomal hydrogen peroxide production by a novel fluorescent probe that is localized via SNAP-Tag labeling[J]. Analytical Chemistry, 2014, 86(12): 5983-5990.
[15]ZHANG W, LIU W, LI P, et al. Rapid-response fluorescent probe for hydrogen peroxide in living cells based on increased polarity of C-B bonds[J]. Analytical Chemistry, 2015, 87(19):9825-9828.
[16]ZHANG K M, DOU W, LI P X, et al. A counarin-based two-photon probe for hydrogen peroxide[J]. Biosensors and Bioelectronics, 2015, 64: 542-546.
[17]LIPPERT A R, VAN DE BITTNER G C, CHANG C J. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems[J]. Accounts of Chemical Research, 2011, 44(9): 793-804.
[18]LI H, YAO Q, FAN J, et al. A two-photon NIR-to-NIR fluorescent probe for imaging hydrogen peroxide in living cells[J].Biosensors and Bioelectronics, 2017, 94: 536-543.
[19]GUO Z, NAM S, PARK S, et al. A highly selective ratiometric near-infrared fluorescent cyanine sensor for cysteine with remarkable shift and its application in bioimaging[J]. Chemical Science, 2012, 3(9): 2760-2765.
[20]YU F, LI P, SONG P, et al. Facilitative functionalization of cyanine dye by an on-off fluorescent switch for imaging of H2O2 oxidative stress and thiols reducing repair in cells and tissues[J].Chemical Communication, 2012, 48, 4980-4982.
[21]BENNISTON A C, COPLEY G, ELLIOTT K J, et al.Redox-controlled fluorescence modulation in a bodipy quinone dyad[J]. European Journal of Organic Chemistry, 2008, 2008(16):2705-2713.
[22]SHAO N, ZHANG Y, CHEUNG S, et al. Copper ion-selective fluorescent sensor based on the inner filter effect using a spiropyran derivative[J]. Analytical Chemistry, 2005, 77(22):7294-7303.
(责任编校:陈健琼)
An Enhanced Fluorescent Probe for the Determination of Hydrogen Peroxide Using Benzoindoles Containing Hydroxy
LIU Chang-hui,LIU Bo,QI Feng-pei,WANG Hai-yang
(College of Materials and Chemical Engineering, Hunan Provincial Key Lab of Dark Tea and Jin-hua, Hunan City University, Yiyang, Hunan 413000, China)
Benzoindoles containing hydroxyl (DHB) was synthesized and applied for the fluorescence response to hydrogen peroxide (H2O2), and the effects of solvents and pH values were studied on the detection system. Under the optimum conditions, the increased concentration of H2O2caused the fluorescence signal of DHB shifted from 580 nm to 560 nm, and that at 560 nm enhanced gradually in the linear range of 0.1 mM to 1.2 mM with detection limit of 0.72 M. This method can be used for the determi nation of trace concentration of H2O2in disinfectant and tapwater samples with satisfactory results and good selectivity over other interference.
hydrogen peroxide; fluorescence probe; fluorescence enhancement; benzoindoles
O657
A
10.3969/j.issn.1672-7304.2017.03.0015
1672–7304(2017)03–0065–05
2017-05-08
国家自然科学基金项目(21675042);湖南省教育厅科研项目(15B043);黑茶金花湖南省重点实验室开放基金项目(HC6120)
刘长辉(1976-),男,湖南衡阳人,副教授,博士,主要从事化学分析与生物传感研究﹒E-mail: changzi915@126.com.

