LPS诱导牛子宫内膜细胞miRNA差异表达分析
王 军 , 燕晓晓 , 丁 赫 , 王静芳 , 吕文发
(1.吉林农业大学动物科学技术学院 吉林省反刍动物繁育生物技术与健康养殖工程实验室 , 吉林 长春 130118 ;2.新疆阿勒泰畜牧兽医职业学校 , 新疆 阿勒泰 836599)
LPS诱导牛子宫内膜细胞miRNA差异表达分析
王 军1, 燕晓晓1, 丁 赫1, 王静芳2, 吕文发1
(1.吉林农业大学动物科学技术学院 吉林省反刍动物繁育生物技术与健康养殖工程实验室 , 吉林 长春 130118 ;2.新疆阿勒泰畜牧兽医职业学校 , 新疆 阿勒泰 836599)
MicroRNA (miRNA)在炎症反应中起重要作用,本试验用miRNA测序技术研究了细菌脂多糖(Lipopolysaccharide,LPS)诱导的牛子宫内膜细胞miRNA差异表达。用1μg/mL 的LPS处理牛子宫内膜细胞24 h,测定细胞上清液IL-6和IL-8分泌量,对细胞进行miRNA测序,并用荧光定量PCR验证测序结果。结果表明,LPS可诱导牛子宫内膜细胞11个miRNA表达上调、9个miRNA表达下调。差异表达miRNA的靶基因注释到生物过程的GO term共20个,注释到细胞组分的GO term共12个,注释到分子功能的GO term共20个;差异表达miRNA靶基因主要富集于PI3K-Akt和MAPK等信号通路。结果提示,miRNA在LPS诱导的牛子宫内膜细胞炎症反应中起重要作用,其作用与PI3K-Akt和MAPK等信号通路激活有关。
细菌脂多糖 ; 子宫内膜细胞 ; miRNA测序 ; 差异表达
奶牛子宫内膜炎是由病原微生物感染引起的一种产科疾病,其发病率达20%~40%,给奶牛业造成严重的经济损失[1]。研究奶牛子宫内膜炎的发病机制对于开发新型技术防治该病具有重要意义。LPS是革兰阴性菌细胞壁的主要成分,也是使细胞产生炎症反应的重要物质[2],LPS诱导奶牛子宫内膜细胞发生炎症反应的调节机制一直是研究热点[3-4]。miRNA是一类长度约为20~24个核苷酸长度的具有调控功能的非编码RNA,主要参与基因转录后水平的调控,在细胞增殖、凋亡、器官形成和机体发育等生理过程中发挥重要作用[5-6]。最近,越来越多的证据表明,miRNA参与炎症反应的调节[7-8],但miRNA在LPS诱导牛子宫内膜细胞发生炎症反应中的作用目前尚不清楚。本试验利用miRNA测序技术研究LPS感染牛子宫内膜细胞24 h后miRNA的差异表达,为揭示LPS诱导牛子宫内膜细胞发生炎症反应的调控机制奠定基础。
1 材料与方法
1.1 细胞培养 从屠宰场采集牛子宫角,采用组织块培养法,于37 ℃,5% CO2饱和湿度培养箱中培养子宫内膜细胞,每2 d更换一次完全培养液,待细胞长满培养瓶底壁80%时,用0.25%胰酶-EDTA消化细胞传代培养。
1.2 试验设计 选择3~5代生长状态良好的牛子宫内膜细胞,用1 μg/mL的 LPS (Sigma,美国)处理(LPS组),对照组用完全培养液培养(Ctr组),24 h后收集细胞及上清液,上清液用于IL-6和IL-8分泌量测定,细胞用于miRNA测序及后续荧光定量PCR验证。
1.3 IL-6和IL-8浓度测定 细胞上清液用3 000 r /min离心20 min后,分别用ELISA试剂盒(Life Technologies,美国)测定上清液中IL-6和IL-8浓度,具体操作按试剂盒说明书执行。
1.4 miRNA测序及分析 利用TRIZol法提取细胞总RNA,经检测,RNA质量合格后,直接将miRNA两端加上接头,然后反转录合成cDNA,随后经PCR扩增和切胶回收等环节获得cDNA文库。使用Agilent 2100和Q-PCR明确文库质量合格后,进行HiSeq/MiSeq测序。将测序序列与miRBase 20.0数据库中牛的已知miRNAs比对分析,得到miRNA表达结果,分别使用log2比率(火山图)和散点图比较两组共同表达的miRNA表达量差异,并将差异表达的miRNA进行聚类分析,利用miRBase数据库中的软件及数据对差异表达的miRNA进行靶基因预测,利用Blast等软件对靶基因进行GO分析和KEGG分析。
1.5 荧光定量PCR 为验证miRNA测序的可靠性,随机选择4个miRNA,采用polyA加尾法进行miRNA的荧光定量PCR验证。利用TRIZol法提取细胞总RNA,通过实时荧光定量 PCR测定miRNA表达量。miRNA引物及内参U6的引物信息见表1。实时荧光定量 PCR总反应体系中含SYBR PremixExTaq10 μL、ROX reference dye 0.4 μL、上下游引物各0.8 μL、cDNA 2 μL和灭菌去离子水6 μL。反应条件:预变性95 ℃ 30 s、变性95 ℃ 5 s、退火温度持续34 s、延伸95 ℃ 15 s,共40个循环。利用2-△△Ct法分析miRNA相对表达量。
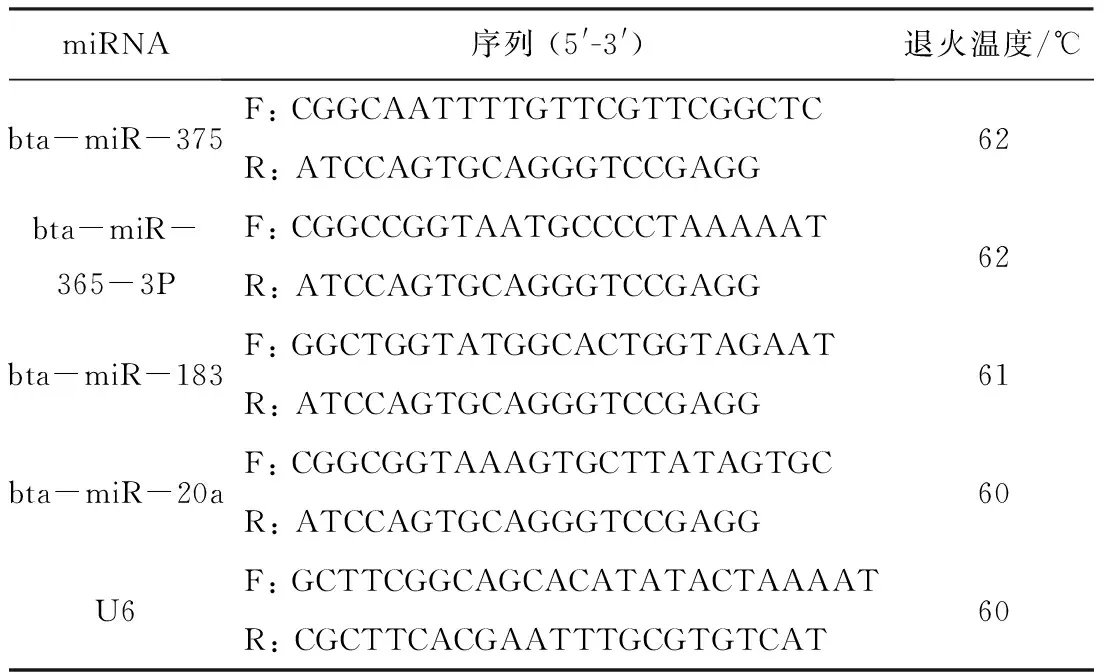
表1 miRNA荧光定量PCR引物信息
1.6 统计分析 利用SPSS18.0软件对试验结果进行分析,t检验法比较分析Ctr组和LPS组之间的差异,显著水平为Plt;0.05。
2 结果与分析
2.1 细胞培养液中IL-6和IL-8水平 图1表明,LPS处理24 h可显著提高奶牛子宫内膜细胞IL-6和IL-8分泌量(Plt;0.01),提示LPS处理成功。
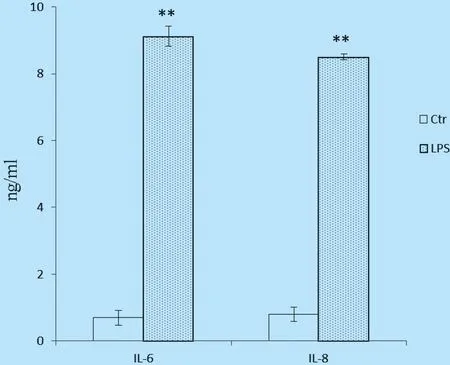
图1 不同处理组细胞培养液上清IL-6和IL-8浓度
2.2 miRNA测序结果 测序结果表明,LPS处理24 h后牛子宫内膜细胞有20个miRNA差异表达,其中11个miRNA上调、9个miRNA下调(见中插彩版图2A、C、D)。随机选取的4个miRNA荧光定量PCR结果也与miRNA测序结果一致(见中插彩版图2B),表明测序结果的准确性。差异表达miRNA的靶基因GO分析结果表明,差异基因注释到生物过程、细胞组分和分子功能的GO term分别为20,12个和20个(图3)。差异表达miRNA靶基因KEGG分析结果表明,LPS诱导牛子宫内膜细胞差异表达miRNA的靶基因主要富集于PI3K-Akt等信号通路(见中插彩版图4)。

图3差异表达miRNA靶基因GO分析(LPSvsCtr)
注:BP:生物过程; CC:细胞组成; MF:分子功能
3 讨论
LPS可诱导牛子宫内膜细胞IL-6、IL-8和IL-1β等炎症因子表达量上升,引发炎症反应,但其表达调控机制目前尚不清楚。本试验利用miRNA测序技术研究了LPS对牛子宫内膜细胞miRNA表达的影响,发现11个miRNA表达上调和9个miRNA表达下调,并明确了这些差异表达miRNA靶基因富集的信号通路。研究结果不仅为明确miRNA参与LPS诱导牛子宫内膜细胞发生炎症反应的调节提供证据,也为研究LPS诱导牛子宫内膜细胞炎症反应的分子机制提供参考。
本试验发现的20个差异表达miRNA,虽然在牛子宫内膜炎上鲜有报道,但对其他物种的研究显示,多个miRNA参与炎症反应。miR-20a等参与miRNA靶向调控SIRPα表达介导巨噬细胞的炎症反应过程[9],miR-375不仅可调节食管和支气管上皮细胞IL-13的表达[10],而且可调节胸腺基质淋巴细胞生成素的产生[11]。结核分枝杆菌感染鼠树突状细胞时,miR-99b 可调节IL-6、IL-12和 IL-1β等炎症细胞因子的释放[12];let-7c 可通过靶向调控STAT3调控肺泡巨噬细胞炎症反应[13]。此外,我们发现LPS诱导牛子宫内膜细胞差异表达miRNA的靶基因主要富集于PI3K-Akt和MAPK等信号通路,以往报道支持这一发现。已有研究表明,LPS可通过激活多种细胞PI3K-Akt或MAPK信号通路而引发炎症反应[14-15]。本研究虽然揭示了miRNA在LPS诱导牛子宫内膜细胞炎症反应中的作用,但其作用机制有待深入研究。
4 结论
miRNA在LPS诱导的牛子宫内膜细胞炎症反应中起重要作用,其作用与PI3K-Akt和MAPK等信号通路激活有关。
[1] Sheldon I M, Jgoetze C. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle[J]. Biology of reproduction, 2009, 81(6): 1 025-1 032.
[2] Fu Y, Liu B, Feng X,etal. Lipopolysaccharide increases Toll-like receptor 4 and downstream Toll-like receptor signaling molecules expression in bovine endometrial epithelial cells[J]. Veterinary Immunology amp; Immunopathology, 2013, 151(1-2): 20-37.
[3] DE Moraes C N, Maia L, D E Oliveira E,etal. Shotgun proteomic analysis of the secretome of bovine endometrial mesenchymal progenitor/stem cells challenged or not with bacterial lipopolysaccharide [J]. Veterinary immunology and immunopathology, 2017, 187: 42-47.
[4] Piras C, Guo Y, Soggiu A,etal. Changes in protein expression profiles in bovine endometrial epithelial cells exposed to E. coli LPS challenge [J]. 2017, 13(2): 392-405.
[5] Saraiva C, Esteves M, Bernardino L. MicroRNA: basic concepts and implications for regeneration and repair of neurodegenerative diseases [J]. Biochemical pharmacology, 2017, pii: S0006-2952(17)30485-9. doi: 10.1016/j.bcp.2017.07.008. [Epub ahead of print]
[6] Alberti C, Cochella L. A framework for understanding the roles of miRNAs in animal development [J]. 2017, 144(14): 2 548-2 559.
[7] Zhang L, Huang C, Guo Y,etal. MicroRNA-26b Modulates the NF-kappaB Pathway in Alveolar Macrophages by Regulating PTEN [J]. 2015, 195(11): 5 404-5 414.
[8] Nahid M A, Satoh M, Chan E K. Interleukin 1beta-Responsive MicroRNA-146a Is Critical for the Cytokine-Induced Tolerance and Cross-Tolerance to Toll-Like Receptor Ligands [J]. Journal of innate immunity, 2015, 7(4): 428-440.
[9] Zhu D, Pan C, Li L,etal. MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein alpha[J]. The Journal of allergy and clinical immunology, 2013, 132(2): 426-436.
[10] Lu T X, Lim E J, Wen T,etal. MiR-375 is downregulated in epithelial cells after IL-13 stimulation and regulates an IL-13-induced epithelial transcriptome [J]. Mucosal immunology, 2012, 5(4): 388-396.
[11] Bleck B, Grunig G, Chiu A,etal. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells [J]. Journal of immunology (Baltimore, Md : 1950), 2013, 190(7): 3 757-3 763.
[12] Singh Y, Kaul V, Mehra A,etal. Mycobacterium tuberculosis controls microRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity [J]. The Journal of biological chemistry, 2013, 288(7): 5 056-5 061.
[13] Yu J H, Long L, Luo Z X,etal. Anti-inflammatory role of microRNA let-7c in LPS treated alveolar macrophages by targeting STAT3 [J]. Asian Pacific journal of tropical medicine, 2016, 9(1): 72-75.
[14] Zhou P H, Hu W, Zhang X B,etal. Protective Effect of Adrenomedullin on Rat Leydig Cells from Lipopolysaccharide-Induced Inflammation and Apoptosis via the PI3K/Akt Signaling Pathway ADM on Rat Leydig Cells from Inflammation and Apoptosis [J]. Mediators of inflammation, 2016, doi: 10.1155/2016/7201549.
[15] Tsou Y A, Tung Y T, Wu T F,etal. Lactoferrin interacts with SPLUNC1 to attenuate lipopolysaccharide-induced inflammation of human nasal epithelial cells via down-regulated MEK1/2-MAPK signaling [J]. Biochemistry and cell biology = Biochimie et biologie cellulaire, 2017, 95(3): 394-399.
Lipopolysaccharide-InducedDifferentialExpressionofmiRNAsinBovineendometrialcells
WANG Jun1, YAN Xiao-xiao1, DING He1, WANG Jing-fang2, LV Wen-fa1
(1.Jilin Province Engineering Laboratory for Ruminant Reproductive Biotechnology and Healthy Production,College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118,China;2.Xinjiang Altay Animal Husbandry and Veterinary Schools Vocational Schools,Altay 836599,China)
MicroRNA (miRNA) plays important roles in inflammation response, the present study was conducted to study differential expression of miRNA in bovine endometrial cells induced by Lipopolysaccharide (LPS) using miRNA sequencing method. Firstly, bovine endometrial cells were treated with 1.0 μg/ml of LPS for 24 h, then concentration of IL-6 and IL-8 in cell supernatant and miRNA expression pattern of cell were determined using ELISA Kit and miRNA sequencing. Finally, real-time PCR was used to verify results of miRNA sequencing. The results showed that LPS stimulation led to differential expression of 20 miRNAs with 11 up-regulated and 9 down-regulated miRNAs. Target genes of the 20 miRNAs were referred to 20 Go terms for biological processes, 12 Go terms for cellular component and 20 Go terms for molecular function. Results of KEGG analysis showed that the target genes were significantly enriched in PI3K-Akt and AMPK signaling pathway. These results indicated that miRNA play important roles in the inflammation response of bovine endometrial cells induced by LPS, which may be achieved by the activation of PI3K-Akt and MAPK signaling pathway.
LPS ; endometrial cells ; miRNA sequencing ; differential expression
LV Wen-fa
S823
A
0529-6005(2017)10-0010-03
2017-07-24
国家自然科学基金面上项目(31672420);吉林省科技厅项目(20150519018JH ,20150204074NY ,20160209001NY)
王军(1979-),男,副教授,博士,主要从事动物繁殖调控研究,E-mail: junwang2004@126.com
吕文发,E-mail: wenfa2004@163.com

