丛枝真菌对Pb胁迫下烟草的解毒效应
高 杉, 郜海民, 赵佳楠, 袁祖丽
(1.河南农业大学生命科学学院,河南 郑州 450002; 2.河南中烟工业有限责任公司黄金叶生产制造中心,河南 郑州 450000)
丛枝真菌对Pb胁迫下烟草的解毒效应
高 杉1, 郜海民2, 赵佳楠1, 袁祖丽1
(1.河南农业大学生命科学学院,河南 郑州 450002; 2.河南中烟工业有限责任公司黄金叶生产制造中心,河南 郑州 450000)
为了探讨丛枝真菌解除Pb胁迫下烟草氧化胁迫毒性的效应,采用盆栽试验,以烟草(NicotianatabacumL.) 品种中烟100为材料,研究Pb胁迫下接种丛枝真菌对烟草根、叶Pb含量及叶片活性氧、丙二醛、丙酮醛含量及清除活性氧的抗氧化酶活性、酮醛转位酶活性和还原型谷胱甘肽含量的影响。结果表明,接种丛枝真菌后与同质量分数Pb胁迫相比,烟草根中Pb含量显著增加,叶片中Pb含量减少;叶片中过氧化氢含量和超氧阴离子产生速率、丙二醛含量均显著下降,超氧化物歧化酶、过氧化氢酶、抗坏血酸过氧化物酶、过氧化物酶活性相应降低;酮醛转位酶I的活性升高,还原型谷胱甘肽含量增加。接种丛枝真菌可减少烟草叶片中的氧化胁迫,降低叶片Pb含量,提高烟叶质量。
烟草;铅;丛枝真菌;解毒效应
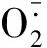
1 材料与方法
1.1试验材料及设计
1.1.1 试验材料 试验材料为烟草(NicotianatabacumL.)品种中烟100(原品系CF 965),由河南省农科院烟草研究中心提供。丛枝真菌为根内球囊霉菌(Glomusintraradices, GI),由北京市农林科学院植物营养与资源研究所中国丛枝菌根真菌种质研究库提供。供试土壤为郑州市农田耕作表层土。装盆前土壤过筛,每盆装干土1 500 g。
1.1.2 试验设计 根据《土壤环境质量标准》GB 15618的标准,本试验设计为0,300 mg·kg-1(未接种),300 mg·kg-1(接种),500 mg·kg-1(未接种)和500 mg·kg-1(接种),其中供试土壤以干土计,并以纯Pb计。
1.1.3 试验处理 烟苗采用漂浮育苗的方法培育。取长势一致的烟苗(3片烟叶)移栽,每盆移栽烟苗1株。处理组每盆接入5 g根内球囊霉菌接种物(孢子、菌丝体、侵染根数等)放在土表4~5 cm下,对照组不接种根内球囊霉菌。处理组和对照组均设置3个重复。重金属Pb以Pb(NO3)2的形式在移栽后7 d以污灌的方式施入,处理组和对照组均进行Pb胁迫处理。常规管理,生长60 d取样。
1.2测定内容及方法
1.2.1 根、茎、叶中Pb含量的测定 分别取各处理的根(根毛区及以下根段)、中部烟叶(自下向上第4和5片),将材料置于80 ℃烘箱中烘干72 h,分别取烘干磨碎(过40目筛)的烟草根、茎、叶各0.2 g,参照王瑞敏[20]的方法用电感耦合等离子体质谱法(ICP-MS)测定。
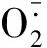
1.2.3 抗氧化酶活性的测定 取新鲜烟草中部叶片适量,液氮研磨后,参照WANG等[24]的方法测定SOD活性,参照XU等[25]的方法测定POD活性,参照NAKANO等[26]的方法测定APX活性,参照AEBI[27]的方法测定CAT活性。
1.2.4 MG含量、GSH含量和Gly I活性的测定 MG含量的测定:取0.5 g的烟草中部叶片,加入高氯酸研磨,上清液加活性炭脱色,在测定MG之前,用饱和碳酸钾溶液将上清液pH值调至中和,中和的上清液用于测定[28]。GSH含量的测定:取新鲜烟草中部叶片适量,DTNB法[21]测定。Gly I活性的测定:取0.1 g新鲜烟草中部叶片研磨后,加入反应体系进行测定[29]。
1.3数据处理
运用Excel 2003进行数据初步计算,采用DPS v 14.10数据处理软件进行单因素统计分析。
2 结果与分析
2.1Pb胁迫下接种GI对烟草根、茎、叶中Pb含量的影响
由表1可以看出,未接GI的处理,与对照相比,300和500 mg·kg-1的Pb胁迫下烟草叶内Pb含量分别增加了0.47,1.96倍,茎中Pb含量分别增加了1.79,2.35倍,根内Pb含量分别增加了3.69,8.28倍;而接种GI的处理,与同等胁迫质量分数未接种的烟草茎、叶内Pb含量相比下降显著,下降幅度为13.76%,16.61%和10.65%,11.35%,根内Pb含量则比未接种的增加了57.15%,62.92%。这表明通过接种GI,菌丝及其分泌物可将部分Pb固定在烟株根部,显著减少Pb向烟株地上部运输。

表1 Pb胁迫下接种GI对烟草根、茎、叶中Pb含量的影响Table 1 Effects of inoculation with Glomus intraradices on Pb content in tobacco leaves, stem and roots under Pb stress
注:不同大写字母表示在0.01水平上差异显著,不同小写字母表示在0.05水平上差异显著。下同。
Note:Capital letters show the significant at 0.01level, and lower-case letters show the significant difference at 0.05 level. The same as below.



表2 Pb胁迫下接种GI对烟草叶片H2O2含量、产生速率和MDA含量的影响Table 2 Effect of inoculation with Glomus intraradices on H2O2 content,production rate of and MDA content in tobacco leaves under Pb stress
2.3Pb胁迫下接种GI对烟草叶片抗氧化酶活性的影响


表3 Pb胁迫下接种GI对烟草叶片抗氧化酶活性的影响Table 3 Effect of inoculation with Glomus intraradices on autioxidant enzyme activities in tobacco leaves under Pb stress U·g-1
2.4Pb胁迫下接种GI对烟草叶片MG和GSH的含量及GlyI活性的影响
从表4看出,与对照组相比,在300 mg·kg-1和500 mg·kg-1Pb胁迫下烟草叶片中的MG含量分别增加了1.17和2.80倍,GSH含量分别增加了0.31和1.74倍,Gly I的活性分别升高了0.75和1.13倍,这可能是由于Pb胁迫导致MG含量增加,植株内通过提高Gly I活性和GSH含量来解除MG毒性。而接种GI的烟草叶中Gly I的活性与同等胁迫(300和500 mg·kg-1Pb)未接种的相比,分别增强了7.14%, 26.47%,GSH含量分别增加39.73%, 15.94%,MG含量极显著降低,分别降低了40.36%, 43.45%。这一方面可能是叶中Pb含量减少,Pb胁迫毒性相应减少,MG含量减少;另一方面可能是接种GI后,Gly I活性增高,加速了MG代谢和减少,从而减少了对烟草造成的毒害。

表4 Pb胁迫下接种GI对烟草叶片MG和GSH含量及Gly I活性的影响Table 4 Effect of inoculation with Glomus intraradices on methylglyoxal and glutathione contentand activity of glyoxalase I in tobacco leaves under Pb stress
3 讨论与结论
Pb是植物生长的非必需元素,但却很容易被植物吸收,且多数积累在根系,仅少量进入地上部[30]。相关研究表明,AMF菌丝能分泌大量GRSP,使重金属(尤其是Pb, Cd, Pb等)在根部形成络合物,从而阻挡重金属进入植物体内[31]。本研究结果显示,Pb胁迫下,烟草根茎叶中Pb含量迅速增加,引起细胞内ROS相应增加;接种AMF后,根中Pb含量显著增加,茎叶中Pb含量却减少。这可能是因为AMF与烟草形成菌根共生体后可将大量Pb固定在根部,减少向叶片转移,使叶片中Pb积累量减少,最终MDA含量显著降低,减轻了膜脂过氧化程度,缓解Pb胁迫对烟草造成的危害[32]。

本研究表明,烟草接种GI后,能在一定程度上减少烟叶中Pb含量,增强叶片的抗氧化胁迫能力,提高烟叶品质。这不仅为深入了解烟草与GI互作解除Pb胁迫毒性的分子机制提供一定理论依据,而且对农业生产实践具有一定的指导意义。
[1] 周泽义.中国蔬菜重金属污染及控制[J].资源生态环境网络研究动态,1999,10(3):21-27.
[2] 桑爱云,张黎明,曹启民,等.土壤重金属污染的植物修复研究现状与发展前景[J].热带农业科学,2006,26(1)75-76.
[3] 周敏.环境Pb污染与Pb毒危害 [J].中国煤炭工业医学杂志,2004,19(4):267-268.
[4] IRVING D E, BAIRD V M. Heat production and respiration by broccoli florets during senescence at 20 ℃[J]. New Zealand Journal of Crop and Horticultural Science, 1996,24:199-202.
[5] HOSSAIN M A, HASANUZZAMAN M ,FUJITA M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer to-lerance to cadmium stress [J]. Physiology and Molecular Biology of Plants, 2010, 16(3): 259-272.
[6] NAVARI-IZZO F. Thylakoid-bound and stromal antioxidative enzymes in wheat treated with excess copper [J]. Physiologia Plantarum, 1998, 104(4): 630-638.
[7] YADAV S K, SINGLA-PAREEK S L, RAY M, et al.Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione [J]. Biochemical and Biophysical Research Communications, 2005, 337: 61-67.
[8] HOSSAIN Z, LOPEZ-CLIMENT M F, ARBONA V, et al. Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage [J]. J Plant Physiol, 2009,166: 1391-1404.
[9] CHAPLEN F W R, CAMERON D C, FAHL W E. Detection of methylglyoxal as a degradation product of DNA and deoxyri-bonucleotides treated with strong acid [J]. Anal Biochem, 1998, 236: 262-269.
[10] DESAI. Enhanced tolerance and antitumor efficacy by docetaxel-loaded albumin nanoparticles [J]. Drug Delivery,2010,23(8):1-11.
[11] THORNALLEY. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life [J]. Biochemical Journal,1990, 269(1): 1-11.
[12] WRIGHT S. UPADHYAYA A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi [J]. Plant and Soil, 1998, 198(1): 97-107.
[13] 弓明钦,陈应龙,仲崇禄.菌根研究及应用[M]. 北京: 中国林业出版社, 1997.
[14] 陈卫莉,赵晓改,王浩,等.Pb胁迫下接种丛枝菌根真菌对茶树解毒能力的影响[J].河南科学, 2014 (4): 511-515.
[15] 湛蔚,刘洪光,唐明.菌根真菌提高杨树抗溃疡病生理生化机制的研究[J]. 西北植物学报,2010, 30(12): 2437-2443.
[16] 石蕾,贺学礼.不同施P水平下AM真菌对黄芪生长和生理学特性的影响[J]. 西北农业学报,2007,16(1): 46-50.
[17] 郑红丽,邢杰,胡俊.两种丛枝菌根真菌对小麦和大豆生长的影响[J]. 内蒙古农业大学学报,2002, 23(1): 22-23.
[18] 李敏,辛华,郭绍霞,等.AM真菌对盐渍土中番茄、辣椒生长和矿质养分吸收的影响[J].莱阳农学院学报,2005, 22(1): 38-41.
[19] 李义强,王凤龙,龚道新.我国无公害烟叶生产的问题、优势及对策[J]. 中国烟草科学,2009, 30(3): 54-57.
[20] 王瑞敏.电感耦合等离子体质谱法(ICP—MS)测定植物中微量铅和镉[J]. 农业科技与装备,2011(1): 32—34.
[21] 李玲.植物生理学模块实验指导 [M]. 北京:科学出版社,2009:95-98.
[22] ANASTASIS C, GEORGE A M, LOANNIS P, et al. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways [J]. Journal of Experimental Botany, 2013, 64(7): 1953-1966.
[23] ELSTNER E H, HEUPLE A. Inhibition of nitrite formation from hydroxylammonium chloride: a simple assay for superoxide dismutase [J]. Analytical biochemistry, 1976, 70: 616-620.
[24] HWANG S Y, LIN H W, CHERN R H, et al. Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato[J]. Plant Growth Regulation, 1999, 27(3):167-172.
[25] XU P L, GUO Y K, BAI J G, et al. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light[J].Physiologia Plantarum, 2008, 132(4):467-478.
[26] NAKANO Y, ASADA K. Hydrogen peroxide is sca-venged by ascorbate-specific peroxidase in spinach chloroplasts [J]. Plant & Cell Physiology, 1981, 22(5):867-880.
[27] AEBI H. Catalaseinvitro[J]. Methods in Enzymology, 1984, 105: 121-126.
[28] YADAV S K, SINGLA-PAREEK S L, RAY M, et al.Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione [J]. Biochemical and Biophysical Research Communications, 2005, 337: 61-67.
[29] HOSSAIN M A, HOSSAIN M Z, FUJITA M. Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene [J]. Australian Journal of Crop Science, 2009, 3(2): 53-64.
[30] PATRA M, BHOWMIK N, BANDOPADHYAY B, et al. Comparison of mercury,lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance[J]. Environ Exp Bot, 2004, 52(2): 199-223.
[31] GONZáLEZ-CHáVEZ M C, CARRILLO-GONZáLEZ R, WRIGHT S F, et al. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements[J]. Environmental Po-llution, 2004, 130(3): 317-323.
[32] 肖家欣,安静,杨安娜,等.五种丛枝菌根真菌对白三叶耐铜污染的影响[J].中国草地学报, 2011, 33(6): 57-63.
[33] 袁祖丽,吴中红,刘秀敏.镉胁迫对烤烟叶片抗氧化系统的影响[J]. 河南农业科学, 2008, 37(7): 43-46.
[34] YOUNESI O, MORADI A. The effects of arbuscular mycorrhizal fungi inoculation on reactive oxyradical scavenging system of soybean (Glycinemax) nodules under salt stress condition.[J]. Agriculturae Conspectus Scientificus, 2014, 78(4): 321-326.
(责任编辑:常思敏)
DetoxificationeffectofinoculationarbuscularmycorrhizalfungiwithtobaccoonitsPbstress
GAO Shan1, GAO Haimin2, ZHAO Jianan1, YUAN Zuli1
(1.College of Life Sciences, Henan Agricultural University, Zhengzhou 450002, China; 2.Golden Manulfacturing Center, Henan Zhongyan Industrial Co., Ltd, Zhengzhou 450000, China)
To study effects of inoculation arbuscular mycorrhizal fungi with tobacco on detoxification Pb stress, a pot experiment was carried out with tobacco (NicotianatabacumL.) andGlomusintraradice. The result showed that, under Pb stress, after inoculationGlomusintraradicewith tobacco, Pb contentdecreased in leaves, but significant increase in roots. The H2O2content and production rate of superoxide aninwere decreased significantly, and the activity of SOD, CAT, APX, POD and MDA content decline accordingly. As glyoxalase I activity was enhanced and glutathione content increase in the leaves, the potent cytotoxic compound methylglyoxal decreased. The result indicated that inoculation arbuscular mycorrhizal fungi with tobacco could reduce oxidative stress and content of Pb in leaves, and consequently raise the quality of tobacco.
tobacco; Pb; arbuscular mycorrhizal fungi; detoxification
2017-04-03
河南省烟草公司重大专项(620112)
高 杉(1989-),女,河南漯河人,硕士研究生,主要从事烟草重金属方面的研究。
袁祖丽(1963-),女,河南郑州人,教授,博士。
1000-2340(2017)05-0620-06
S 572
A

