杨树MYB转录因子家族基因应答盐胁迫表达特性分析
李晰妍 赵 凯 张雪梅 周博如
(东北林业大学林木遗传育种国家重点实验室,哈尔滨 150040)
杨树MYB转录因子家族基因应答盐胁迫表达特性分析
李晰妍 赵 凯 张雪梅 周博如*
(东北林业大学林木遗传育种国家重点实验室,哈尔滨 150040)
MYB转录因子家族是植物中数量最多的转录因子家族之一,在植物次生代谢调节、信号转导和抗逆等生物过程起重要作用。根据MYB转录因子结构域组成差异可分4个亚家族:即1R-MYB(MYB-relaed)、R2R3-MYB、3R-MYB和4R-MYB。其中,R2R3-MYB亚家族数量最多,可进一步分为22个亚组;利用生物信息学分析杨树MYB转录因子蛋白序列的保守结构域、系统发生、基因组定位、氨基酸组成和理化性质等;参照拟南芥MYB转录因子功能,预测杨树MYB转录因子功能;基于84K杨转录组测序和RT-qPCR分析,从301个杨树MYB转录因子基因中筛选出69个应答盐胁迫基因(P≤0.05)。其中,上调表达基因32个,下调表达基因37个。该研究可为进一步研究杨树MYB家族基因功能提供参考依据。
杨树;MYB转录因子;生物信息学;RT-qPCR
转录因子(Transcription factor)也称为反式作用因子,能够与真核生物基因上游启动子序列中顺式作用元件特异性结合,通过转录激活或抑制调控下游基因的表达,对其下游基因的表达充当开关的角色。不同转录因子一般具有不同的功能区域,植物转录因子按照其结构域的差异可分为WRKY、bZIP、EIL、MYB、NAC、GATA、AP2/ERF、SBP等转录因子家族[1]。
MYB转录因子家族作为植物中最大的转录因子家族之一[2]。MYB转录因子蛋白C端含有转录激活域可与DNA或其他转录因子相互作用,N端具有由51-52个高度保守的氨基酸组成的MYB-DNA结合域。MYB-DNA结合域包含3个α螺旋,第二个和第三个螺旋组成helix-turn-helix(HTH)结构,形成一个疏水核心,对DNA序列的识别具有重要影响。MYB转录因子的MYB-DNA结合域通常由1~4个重复组成,依据其特点可分为4个亚族,即1R-MYB(MYB-related),R2R3-MYB,3R-MYB和4R-MYB[3~5]。其中1R-MYB亚族类仅含有单一的MYB结构域,在不同植物中功能有所差异。如同属于MYB家族的拟南芥LHY,CCA1基因与拟南芥生物钟与激素合成调节有关[6],蜡梅EU11603基因与植物抗逆相关,参与植物的抗逆代谢途径中ABA传导[7];马铃薯过表达StMYB1R-1基因可加快气孔关闭速度,增强植株抗干旱能力[8]。R2R3-MYB亚族蛋白质是MYB转录因子家族中数量最多的一类,在调控次生代谢[9~10]、响应赤霉素(GA)信号调节[11]、器官形成过程中发挥作用[12~13]。玉米ZmC1基因调控查耳酮合成及类黄酮生物合成途径[14],彩艳龙头RCP1基因在成花过程中调控类胡萝卜素生物合成[15],金鱼草MIXTA基因影响金鱼草花瓣形态建成和花青素合成[16]。3R-MYB基因在动物MYB家族数量较大,在植物中数量较少,1941年从禽急性成髓细胞白血病病毒AMV和E26分离出第一个MYB基因v-MYB即是3R-MYB基因[17]。植物中的也有类似基因,如烟草NtMYB-A1,NtMYB-A2,NtMYB-B基因在细胞分裂G2期与M期发挥重要作用[18];水稻OsMYB3R-2基因应答冷、干旱和盐胁迫,过表达OsMYB3R-2基因导致植株有丝分裂指数增加[19~20]。4R-MYB亚家族基因数量最少,拟南芥中虽然有该类基因,但研究较少[21]。
杨树分布广、适应性强、生长速度快,是我国重要的造林和用材树种,也是研究林木生理和基因工程的模式植物。84K杨(Populusalba×Populusglandulosa)来自银白杨和腺毛杨杂交,遗传转化率较高,是杨树基因工程的重要受体树种之一。为探明杨树MYB转录因子的结构特征和应答盐胁迫情况,通过植物转录因子数据库(http://planttfdb.cbi.pku.edu.cn/index.php)筛选出杨树301个MYB转录因子,利用生物信息学分析其结构特点;通过转录组测序分析84K杨中MYB转录因子基因应答盐胁迫情况,利用RT-qPCR对候选基因应答盐胁迫情况进行验证,为筛选杨树耐盐关键基因和改良杨树抗逆性提供参考依据。
1 材料与方法
从植物转录因子数据库(http://planttfdb.cbi.pku.edu.cn/index.php)获得301个杨树MYB转录因子的cDNA及其编码氨基酸序列,以及14个功能已知的拟南芥MYB转录因子氨基酸序列;利用BioEdit软件分别进行序列比对,利用Weblogo 3.0对比对结果进行基序分析(a;b),利用MEGA 5.0构建进化树(NJ),设置Bootstrap为1 000次重复;利用ProtParam在线分析,分析301个杨树MYB转录因子家族蛋白氨基酸数目、分子量、理论等电点、脂肪族氨基酸指数及蛋白质疏水性等理化性质;利用TargetP1.1 Server在线序列分析及Cello v2.5在线分析预测MYB转录因子亚细胞定位情况。
将培养1个月的84K杨(Populusalba×Populusglandulosa)组培苗洗掉培养基,置于水中,在25℃室温,14 h·d-1光照条件下培养1个月后,然后将生长状态相似的植株分为8组,分别用水和0.15 mol·L-1NaCl处理24 h,每个处理重复4次。采集叶片样本,保存于-80℃冰箱,委托金唯智(GENEWIZ)公司进行RNA提取和转录组测序。
用琼脂糖凝胶电泳检测转录组测序RNA样品完整性,用Takara的PrimeScriptTMRT reagent kit with gDNA Eraser试剂盒进行cDNA合成。根据69个MYB候选基因序列,利用primer5.0设计定量PCR引物(表1)。其中FN356200(EF1),JM986590(ACT)为内参基因[22]。按照TaKaRa公司SYBR®RPremix Ex TaqaTMП(Tli RNaseH Plus)实时定量试剂盒进行实时定量RT-qPCR分析,所用程序为:95.0℃ 30 s,(95.0℃ 5 s、60.0℃ 34 s、95.0℃ 15 s)35个循环,60.0℃ 60 s,95.0℃ 15 s,利用-△△Ct法进行基因表达分析。
2 结果与分析
2.1 杨树MYB转录因子分类
根据MYB转录因子保守结构域差异将301个杨树MYB转录因子蛋白分为4个亚族:1R-MYB(MYB-relaed)、 R2R3-MYB、3R-MYB和4R-MYB。
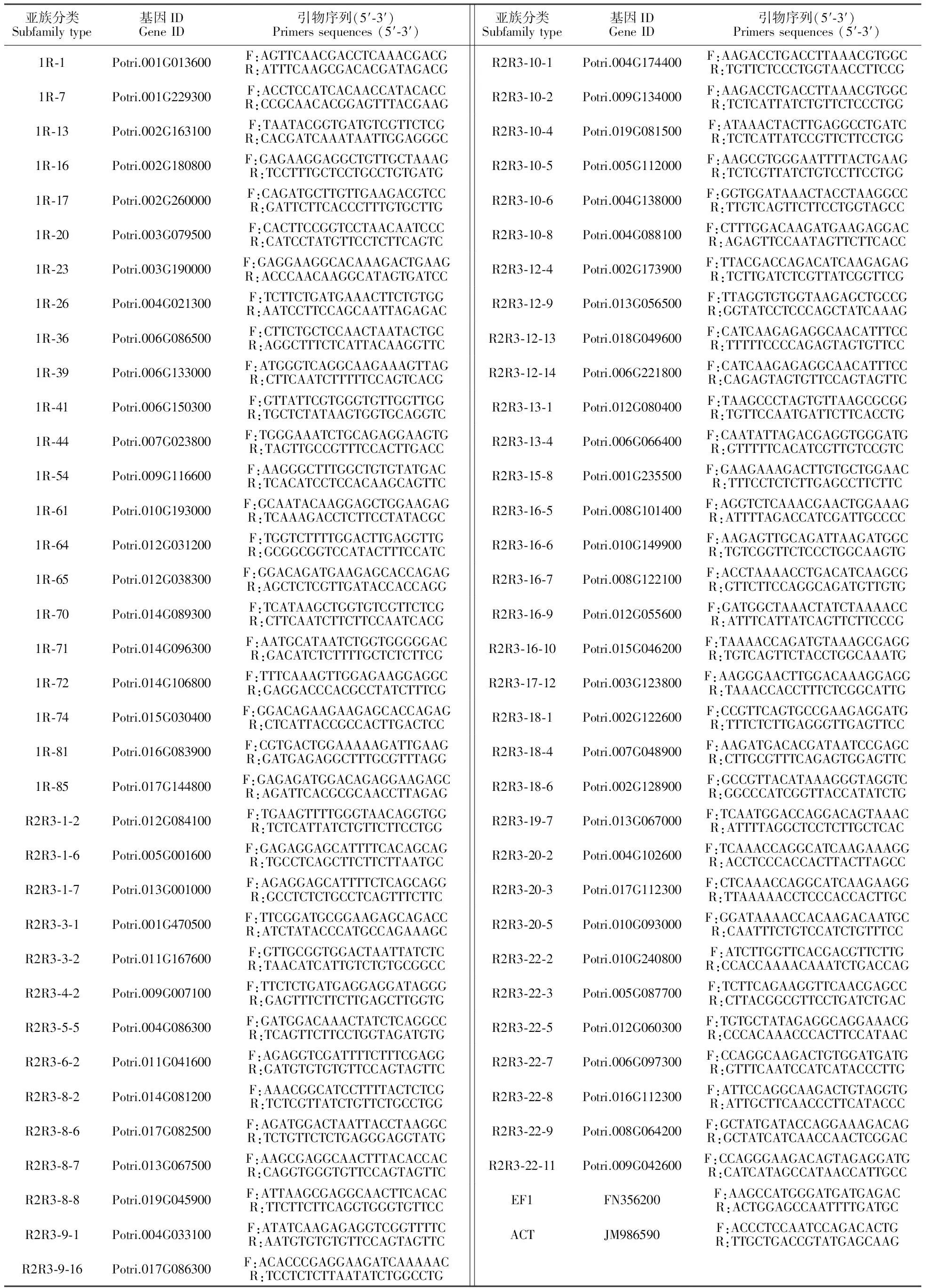
表1 RT-qPCR引物
其中,1R-MYB(MYB-relaed)亚族有89个成员、R2R3-MYB亚族有206个成员、3R-MYB亚族有5个成员、4R-MYB亚族仅有1个成员。将206个R2R3-MYB亚家族转录因子的保守结构域(DNA binding domain)与14个功能已知的拟南芥MYB转录因子家族保守结构域(DNA binding domain)进行比对,将R2R3-MYB亚族分为22个组,命名为R2R3-1~R2R3-22(图1)。其中,有7个转录因子与拟南芥AtMYB61聚在一起,命名为R2R3-1亚组,该亚组基因多与气孔调节和光合作用有关[23];有12个转录因子与拟南芥AtMYB4聚在一起,命名为R2R3-10亚组,该亚组基因多参与调节植物耐紫外辐射过程[24];有3个转录因子与拟南芥AtMYB11聚在一起,命名为R2R3-11亚组,该亚组基因可能参与与细胞发育和形态发生相关蛋白质的功能分化[25];有9个转录因子与拟南芥AtMYB75、AtMYB90和AtMYB113聚在一起,命名为R2R3-14亚组,该亚组基因可能参与调节花青素的合成[26~27];有9个转录因子与拟南芥AtMYB59聚在一起,命名为2R3R-15亚组,该亚组基因可能参与调控植物细胞周期及根的发育过程[28]。
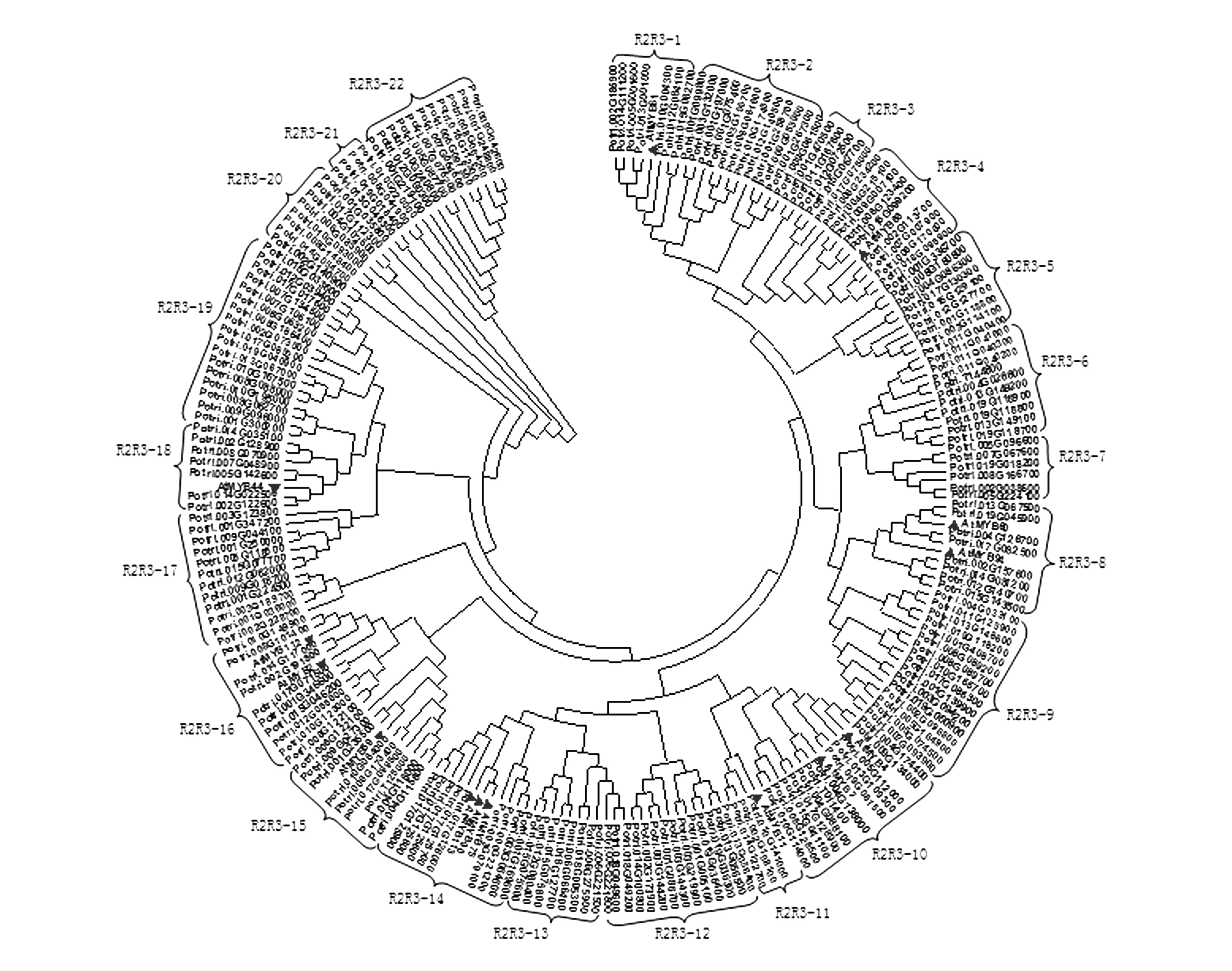
图1 杨树R2R3-MYB亚族保守结构域系统发生树 ▼表示14个功能已知的拟南芥MYB转录因子保守结构域。Fig.1 Phylogenetic tree of Poplar R2R3-MYB subfamily DNA binding domains based on NJ method ▼represents DNA binding domains of the known functions Arabidopsis thaliana MYB transcription factors.
2.2 杨树MYB转录因子保守结构域预测
不同杨树MYB转录因子亚家族成员中含有的保守结构域数目不同,1R-MYB亚族成员有1个保守结构域,R2R3-MYB亚族成员有2个保守结构域,3R-MYB亚族成员有3个保守结构域,4R-MYB亚族成员有4个保守结构域。将301个杨树MYB转录因子的第一结构域和第二结构域进行比对,可以发现其保守性均较高,但不同氨基酸的保守性存在差异。图2MYB转录因子结构域中,代表氨基酸的字母越大表示该氨基酸的保守程度越高,从图2a中可以发现MYB第一结构域的第4位,第32位的色氨酸(W)残基极保守,但第4位的色氨酸(W)残基极少数被苯丙氨酸(F)和甲硫氨酸(M)所取代;第32位的色氨酸(W)残基极少数被苯丙氨酸(F)和亮氨酸(L)所取代。从图2b可以发现MYB第二结构域的第32位色氨酸(W)残基始终极保守。总体而言,第一和第二结构域内其他位点的色氨酸(W)也较为保守,只有少数被苯丙氨酸(F),异亮氨酸(I),酪氨酸(Y)和丙氨酸(A)所取代,除此之外,保守结合域内部还有很多保守的氨基酸残基,比如赖氨酸(K),精氨酸(R),甘氨酸(G),天冬氨酸(D),谷氨酸(E),天冬酰胺(N),异亮氨酸(I),酪氨酸(Y)以及亮氨酸(L),共同维持着MYB转录因子家族蛋白结构域的基本稳定。
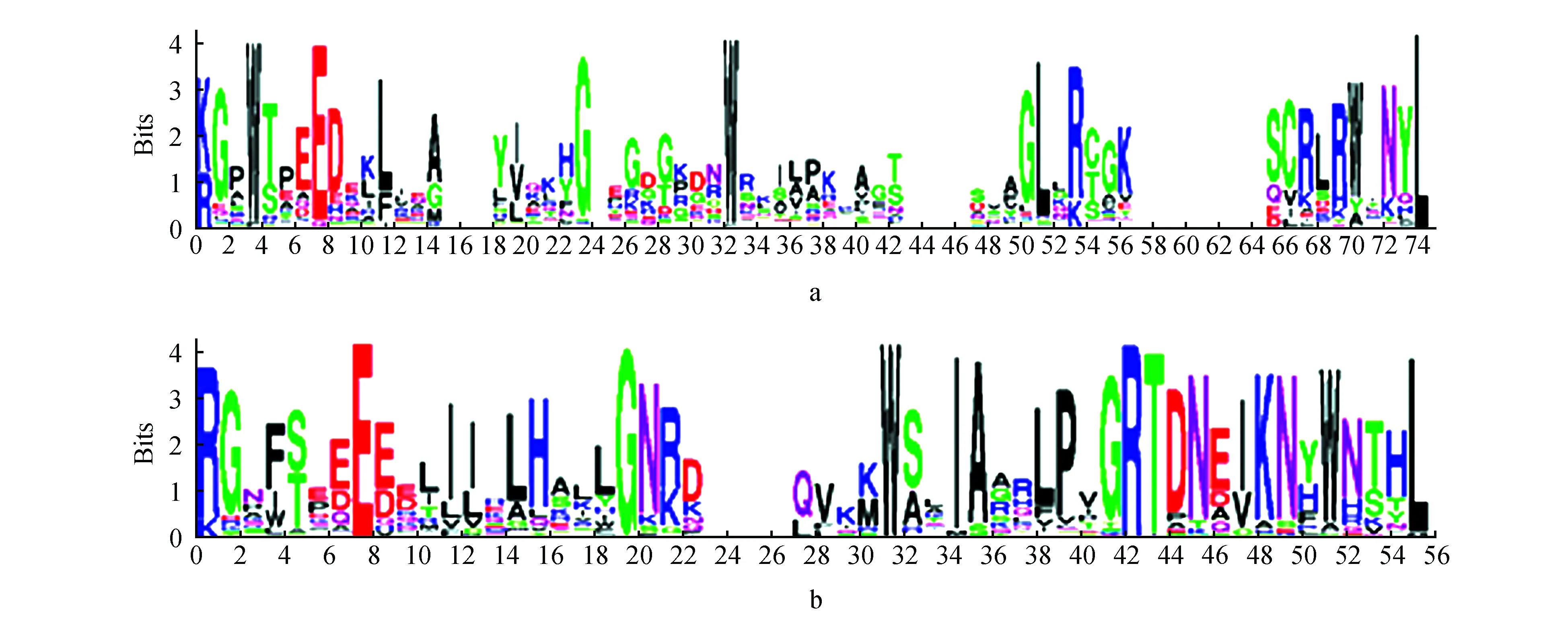
图2 杨树MYB转录因子结构域基序分析 a. 1R-MYB,R2R3-MYB,3R-MYB和4R-MYB亚族第一结构域基序;b. R2R3-MYB,3R-MYB和4R-MYB亚族第二结构域基序Fig.2 Sequence alignment of Poplar MYB transcription factors motif in the first domain and the second domaina. The first domain motif of 1R-MYB,R2R3-MYB,3R-MYB and 4R-MYB subfamilies; b. The second domain motif of R2R3-MYB,3R-MYB and 4R-MYB subfamilies
2.3 杨树MYB转录因子理化性质分析
1R-MYB亚族转录因子平均长度为392 aa,平均分子量为43 558.7 kDa,理论等电点多在碱性范围,细胞核定位占整个亚族的88%。2R3R-MYB亚族转录因子平均长度为346 aa,平均分子量为38 861 kDa,其中最小的是2R3R-14亚组,平均长度只有234.89 aa,平均分子量为26 830.79 kDa;最大的为2R3R-21亚组,平均长度为1 389.25 aa,平均分子量为153 708.10 kDa,除第3、5、21亚组转录因子的等电点都为酸性外,其他2R3R-MYB亚组转录因子的等电点均为碱性。第1、3、17、19、20亚组多数为细胞外定位,过氧化酶体和高尔基体定位,其他亚组多数为细胞核定位,细胞核定位占整个亚族的79.6%。3R-MYB亚族、4R-MYB亚族多为碱性氨基酸,多数定位在细胞核外,过氧化酶体和高尔基体。脂肪族氨基酸指数是衡量蛋白质稳定性的指标,该指数越高蛋白质稳定性越强,MYB转录因子蛋白的稳定性均较强。蛋白质的疏水性负值越大亲水性越强,不同MYB转录因子氨基酸疏水性值虽存在差距,但都属亲水蛋白。
2.4 杨树MYB基因应答盐胁迫分析
根据84K杨转录组测序数据,分析杨树MYB基因应答盐胁迫情况,结果有69个基因表达发生了变化,并且P≤0.05,这些基因定义为盐胁迫应答基因(图3),其中包括37个下调表达基因和32个上调表达基因,分别占家族基因总数的12.33%和10.67%。在37个下调表达基因中,14个属1R-MYB亚族,23个属2R3R-MYB亚族,下调表达超过15倍的基因有7个:分别是Potri.002G260000,Potri.004G102600,Potri.005G112000,Potri.007G023800,Potri.009G116600,Potri.013G001000,Potri.018G049600;在32个上调表达基因中,8个属1R-MYB亚族,24个属2R3R-MYB亚族,上调表达超过15倍的基因有4个:分别是Potri.008G122100,Potri.009G007100,Potri.010G149900,Potri.015G046200。

图3 杨树MYB应答盐胁迫表达情况Fig.3 Expression of Poplar MYB genes in response to salt stress

图4 69个杨树MYB基因的RT-qPCR分析Fig.4 Expression analysis of 69 Poplar MYB genes by RT-qPCR
2.5 杨树应答盐胁迫MYB基因的RT-qPCR验证
用RT-qPCR验证69个应答盐胁迫的MYB基因的表达情况(图4),结果表明RT-qPCR结果与转录组测序分析结果总体趋势基本一致。如在转录组测序分析和RT-qPCR结果中,Potri.007G023800、Potri.018G049600下调表达量均超过15倍;Potri.008G122100、Potri.009G007100、Potri.010G149900、Potri.012G055600、Potri.015G046200上调表达量均超过15倍。但两种方法检测到的基因相对表达水平有所差异,如Potri.012G080400在转录组测序分析中下调表达1.11倍,RT-qPCR分析中下调表达11.14倍;而Potri.008G101400在转录组测序分析中上调表达3.77倍,在RT-qPCR分析中上调表达47.78倍。
我们发现下调表达超过15倍的Potri.018G049600基因与拟南芥AtMYB7基因聚于2R3R-10亚组。有实验证明拟南芥AtMYB7基因在盐胁迫下呈明显下调表达[29];上调表达倍数超过15倍的Potri.008G122100,Potri.010G149900,Potri.012G055600,Potri.015G046200基因与拟南芥AtMYB2,AtMYB112基因同属于2R3R-16亚组,拟南芥AtMYB2和AtMYB112基因在高盐刺激下表达量上升,过量表达的转基因植物耐盐能力明显提高[30~31]。Potri.009G007100基因与拟南芥AtMYB68基因属于2R3R-4亚组,拟南芥AtMYB68基因响应环境胁迫[32];Potri.013G067500、Potri.014G081200、Potri.017G082500、Potri.019G045900基因与拟南芥AtMYB60、AtMYB94基因聚于2R3R-8亚组,拟南芥AtMYB60、AtMYB94基因参与盐胁迫和干旱胁迫的生理调节过程[33~34];Potri.002G122600、Potri.002G128900、Potri.007G048900与拟南芥AtMYB44基因属于2R3R-18亚组,过表达AtMYB44通过SOS2负反馈环增强植株的耐盐能力[35]。
4 结论
根据MYB转录因子结构域数量差异将301个MYB转录因子基因分为4个亚族:1R-MYB(MYB-relaed)、R2R3-MYB、3R-MYB和4R-MYB。通过84K杨转录组测序筛选出69个应答盐胁迫的MYB转录因子基因,其中下调表达基因37个,上调表达基因32个。RT-qPCR分析结果与转录组测序结果基本一致。本研究对明确MYB转录因子基因的应答盐胁迫表达特点和探明杨树耐盐分子机制具有参考价值。
1.Kasuga M,Liu Q,Miura S,et al.Improving plant drought,salt,and freezing tolerance by gene transfer of a single stress-inducible transcription factor[J].Nature Biotechnology,1999,17(3):287-291.
2.Frampton J.MYB transcription factors:their role in growth,differentiation and disease:proteins and cell regulation[M].Netherlands:Springer,2004.
3.Rabinowicz P D,Braun E L,Wolfe A D,et al.Maize R2R3 Myb genes:sequence analysis reveals amplification in the higher plants[J].Genetics,1999,153(1):427-444.
4.Chen Y H,Yang X Y,He K,et al.The MYB transcription factor superfamily ofArabidopsis:expression analysis and phylogenetic comparison with the Rice MYB Family[J].Plant Molecular Biology,2006,60(1):107-124.
5.Ogate K,Morikawa S,Nakamura H,et al.Comparison of the free and DNA-complexed forms of the DNA-binding domain from c-MYB[J].Nature Structural Biology,1995,2(4):309-320.
6.Carré I A,Kim J Y.MYB transcription factors in theArabidopsiscircadian clock[J].Journal of Experimental Botany,2002,53(374):1551-1557.
7.罗莉莉,刘道凤,茅允彬,等.蜡梅转录因子1R-Myb1的cDNA克隆、分子特性与表达载体的构建[C].//中国园艺学会第八届青年学术讨论会论文集.上海:中国园艺学会,2008.
Luo L L,Liu D F,Mao Y B,et al.Chimonanthuspraecox(L.) link gene 1R-Myb1 with plant-specific SANT/MYB domain protein cDNA clones,molecular character and constructing expression vector[C].//China Horticultural Society Youth Seminar.Shanghai:Chinese Society for Horticultural Science,2008.
8.Shin D,Moon S J,Han S,et al.Expression ofStMYB1R-1,a novel potato single MYB-like domain transcription factor,increases drought to lerance[J].Plant Physiology,2011,155(1):421-432.
9.Zhang P F,Chopra S,Peterson T.A segmental gene duplication generated differentially expressed MYB-homologous genes inMaize[J].The Plant Cell,2000,12(12):2311-2322.
10.Nesi N,Jond C,Debeaujon I,et al.TheArabidopsisTT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed[J].The Plant Cell,2001,13(9):2099-2114.
11.Gubler F,Kalla R,Roberts J K,et al.Gibberellin-regulated expression of a MYB gene in barley aleurone cells:evidence for MYB transactivation of a high-pI α-amylase gene promoter[J].The Plant Cell,1995,7(11):1879-1891.
12.Steiner-lange S,Unte U S,Eckstein L,et al.Disruption ofArabidopsisthalianaMYB26 results in male sterility due to non-dehiscent anthers[J].The Plant Journal,2003,34(4):519-528.
13.Penfield S,Meissner R C,Shoue D A,et al.MYB61 is required for mucilage deposition and extrusion in theArabidopsisseed coat[J].The Plant Cell,2001,13(12):2777-2791.
14.Groteword E,Drummond B J,Bowen B,et al.The MYB-homologousPgene controls phlobaphene pigmentation inMaizefloral organs by directly activating a flavonoid biosynthetic gene subset[J].Cell,1994,76(3):543-553.
15.Sagawa J M,Stanley L E,Lafountain A M,et al.An R2R3-MYB transcription factor regulates carotenoid pigmentation inMimuluslewisiiflowers[J].New Phytologist,2016,209(3):1049-1057.
16.张旸,吴佳岩,吴雅妮,等.MYB转录因子基因MIXTA及其同源基因功能的研究进展[J].中国农业科学,2016,49(7):1230-1241.
Zhang Y,Wu J Y,Wu Y N,et al.Progresses and perspective of the function of MYB Transcription factorMIXTAand its orthologous gene[J].Scientia Agricultura Sinica,2016,49(7):1230-1241.
17.Klempnauer K H,Gonda T J,Bishop J M.Nucleotide sequence of the retroviral leukemia genev-MYBand its cellular progenitorc-MYB:the architecture of a transduced oncogene[J].Cell,1982,31(2):453-463.
18.Ito M,Araki S,Matsunaga S,et al.G2/M-phase-specific transcription during the plant cell cycle is mediated by c-MYB-like transcription factors[J].The Plant Cell,2001,13(8):1891-1905.
19.Ma Q B,Dai X Y,Xu Y Y,et al.Enhanced tolerance to chilling stress inOsMYB3R-2 transgenic Rice is mediated by alteration in cell cycle and ectopic expression of stress genes[J].Plant Physiology,2009,150(1):244-256.
20.Dai X Y,Xu Y Y,Ma Q B,et al.Overexpression of an R1R2R3 MYB gene,OsMYB3R-2,increases tolerance to freezing,Drought,and salt stress in transgenicArabidopsis[J].Plant Physiology,2007,143(4):1739-1751.
21.Cai H S,Tian S,Dong H S.Large scaleinsilicoidentification ofMYBfamily genes from Wheat expressed sequence tags[J].Molecular Biotechnology,2012,52(2):184-192.
22.Wang S J,Yao W J,Wei H R,et al.Expression patterns of ERF genes underlying abiotic stresses in Di-haploidPopulussimonii×P.nigra[J].The Scientific World Journal,2014,2014:745091.
23.Liang Y K,Dubos C,Dodd I C,et al.AtMYB61,an R2R3-MYB transcription factor controlling stomatal aperture inArabidopsisthaliana[J].Current Biology,2005,15(13):1201-1206.
24.Hemm M R,Herrmann K M,Chapple C.AtMYB4:a transcription factor general in the battle against UV[J].Trends in Plant Science,2001,6(4):135-136.
25.Petroni K,Falasca G,Calvenzani V,et al.The AtMYB11 gene fromArabidopsisis expressed in meristematic cells and modulates growth in planta and organogenesis in vitro[J].Journal of Experimental Botany,2008,59(6):1201-1213.
26.Bhargava A,Mansfield S D,Hall H C,et al.MYB75 functions in regulation of secondary cell wall formation in theArabidopsisinflorescence stem[J].Plant Physiology,2010,154(3):1428-1438.
27.Rajagopalan R,Vaucheret H,Trejo J,et al.A diverse and evolutionarily fluid set of microRNAs inArabidopsisthaliana[J].Genes & Development,2006,20(24):3407-3425.
28.Mu R L,Cao Y R,Liu Y F,et al.An R2R3-type transcription factor geneAtMYB59 regulates root growth and cell cycle progression inArabidopsis[J].Cell Research,2009,19(11):1291-1304.
29.Kim J H,Hyun W Y,Nguyen H N,et al.AtMyb7,a subgroup 4 R2R3 Myb,negatively regulates ABA-induced inhibition of seed germination by blocking the expression of the bZIP transcription factor ABI5[J].Plant,Cell & Environment,2015,38(3):559-571.
30.Urao T,Noji M A,Yamaguchi-shinozaki K,et al.A transcriptional activation domain of ATMYB2,a drought-inducibleArabidopsisMYB-related protein[J].The Plant Journal,1996,10(6):1145-1148.
31.Lotkowska M E,Tohge T,Fernie A R,et al.TheArabidopsistranscription factor MYB112 promotes anthocyanin formation during salinity and under high light stress[J].Plant Physiology,2015,169(3):1862-1880.
32.Feng C P,Andreasson E,Maslak A,et al.ArabidopsisMYB68 in development and responses to environmental cues[J].Plant Science,2004,167(5):1099-1107.
33.Cominelli E,Galbiati M,Vavasseur A,et al.A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance[J].Current Biology Cb,2005,15(13):1196-1200.
34.Lee S B,Kim J,Suh M C.Cuticular Wax biosynthesis is up-regulated by the MYB94 transcription factor inArabidopsis[J].Plant & Cell Physiology,2015,56(1):48-60.
35.Jung C,Seo J S,Han S W,et al.Overexpression ofAtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenicArabidopsis[J].Plant Physiology,2008,146(2):623-635.
National 863 project funding(2013AA102701)
introduction:LI Xi-Yan(1992—),female,graduate,major in forest resistance mechanism.
date:2017-02-16
ExpressionAnalysisofPoplarMYBTranscriptionFactorGeneFamilyinResponsetoSaltStress
LI Xi-Yan ZHAO Kai ZHANG Xue-Mei ZHOU Bo-Ru*
(State Key Laboratory of Tree Genetics and Breeding,Northeast Forestry University,Harbin 150040)
MYB transcription factor family is one of the most important transcription factors in plants, and plays an important role in the process of plant secondary metabolism regulation, signal transduction and stress resistance. According to the difference of domain structure of MYB transcription factor, it can be divided into 4 subfamilies: 1R-MYB(MYB-relaed), R2R3-MYB, 3R-MYB and 4R-MYB. Among them, the R2R3-MYB sub family with the largest number of members can be further divided into 22 subgroups. Using bioinformatics, the characterizations of conserved domain ofPoplarMYB transcription factors, genomic location, amino acid composition and physicochemical properties were analyzed. According to the Arabidopsis MYB transcription factor, the functions ofPoplarMYB transcription factor were predicted. Based on the transcriptome sequencing and RT-qPCR, 69 salt-stress responding genes(P≤0.05) were screened from 301 MYB genes, including 32 up-regulated genes and 37 down regulated genes. The study could provide a reference for further study on the function of MYB family genes inPoplar.
Poplar;MYB transcription factors;bioinformatics;RT-qPCR
国家863课题资助(2013AA102701)
李晰妍(1992—),女,硕士研究生,主要从事林木抗逆机理方面研究。
* 通信作者:E-mail:boruzhou@yahoo.com
2017-02-16
* Corresponding author:E-mail:boruzhou@yahoo.com
S792.11
A
10.7525/j.issn.1673-5102.2017.03.013

