猪圆环病毒3型研究进展
张永宁,梅 琳,张 舟,唐丽杰,吴绍强
(1.中国检验检疫科学研究院动物检疫研究所,北京 100176;2.东北农业大学动物医学学院,哈尔滨 150030)
猪圆环病毒3型研究进展
张永宁1,梅 琳1,张 舟1,唐丽杰2,吴绍强1
(1.中国检验检疫科学研究院动物检疫研究所,北京 100176;2.东北农业大学动物医学学院,哈尔滨 150030)
2016年,美国堪萨斯州立大学科研人员利用宏基因组测序技术,从患有皮炎肾病综合征(PDNS)和繁殖障碍母猪及其流产胎儿体内首次鉴定出一种新型猪圆环病毒,命名为猪圆环病毒3型(Porcine circovirus 3,PCV3)。流行病学调查发现PCV3感染多见于具有PDNS和繁殖障碍症状猪群,目前已于美国多个州猪群内普遍流行。同时,美国旧金山血液系统研究所科研人员从3头不明病因导致的心脏和多器官炎症仔猪体内也检测出PCV3。随后,我国湖北、广东等多个省市猪群中也检测出PCV3。近来,波兰和韩国相继报道存在PCV3感染猪群。作为一种新发现病原体,PCV3对猪致病性及在猪圆环病毒相关疾病(PCVAD)中作用以及现有商品化PCV2疫苗对其免疫效力等有待进一步研究。文章综述PCV3最新进展,以期为后续研究提供参考。
宏基因组测序;猪圆环病毒3型;猪皮炎肾病综合征;繁殖障碍;猪圆环病毒相关疾病
猪圆环病毒(Porcine circovirus,PCV)为一种共价闭合、单股环状DNA病毒,是迄今发现最小动物病毒[1]。2016年前,全球范围内仅发现PCV1和PCV2两个基因型[2-3]。其中,PCV1被认为是猪肾细胞PK-15污染物,但不产生细胞病变效应[4],对猪无致病性[5]。PCV2是引起断奶仔猪多系统衰竭综合征(PMWS)主要病原[6],与猪皮炎和肾病综合征(PDNS)[7]、猪呼吸道疾病综合征(PRDC)[8]、繁殖障碍[9]、增生性坏死性肺炎(PNP)[10]和肠炎[11-12]等疾病密切相关,均为猪圆环病毒相关疾病(PCVAD)[13-14]。临床上,PCV2常与猪繁殖与呼吸综合征病毒(PRRSV)[15]、猪细小病毒(PPV)[16]和猪肺炎支原体(M.hyo)[17]等病原体混合/继发感染,造成养猪业巨大经济损失。
2003年全球范围内PCV2发生由PCV2a向PCV2b基因型转变,病毒致病力增强,对养猪业危害加重[18]。2012年PCV2在世界范围内再次发生基因型转变,由PCV2b向致病力更强PCV2d转变[19-20],现有疫苗免疫效果降低。2015年6月,美国北卡罗来纳州某商品化猪场暴发PDNS疫情,与历史平均值相比,该猪场母猪死亡率升高10.2%、受孕率下降0.6%。患病母猪厌食、皮肤呈现多灶性丘疹、斑点和浅表性皮炎,所产胎儿(包括弱胎、死胎、木乃伊胎)具有相似临床症状。Palinski等借助新一代测序(NGS)技术,从患病母猪及流产胎儿体内首次鉴定出一种新病毒[21],该病毒与圆环病毒科病毒具有类似基因组结构和遗传相似性,但与其他圆环病毒衣壳蛋白氨基酸序列同源性低于70%,根据国际病毒分类委员会(ICTV)圆环病毒分类标准[22],将其归类为一种新型圆环病毒,命名为PCV3[21]。作为新动物病原体,PCV3对猪致病性和在PCVAD中作用以及现有商品化PCV2疫苗对其免疫效果等有待进一步研究。
文章综述PCV3在病原学、流行病学、临床症状与病理变化、诊断与防控对策等方面国内外最新进展,以期为后续科学研究提供参考。
1 病原学
1.1 分类
PCV3在分类上属于圆环病毒科(Circoviridae)圆环病毒属(Circovirus)成员[21]。
1.2 形态与结构
与PCV1和PCV2一样,PCV3病毒粒子呈二十面体结构,无囊膜,直径为17~20 nm。基因组为单股DNA,全长2 000 bp,包含3个主要开放阅读框(ORF)。其中,ORF1编码由297个氨基酸(aa)组成复制酶蛋白Rep,ORF2编码由214个aa组成衣壳蛋白Cap,两者复制方向相反(见图1)。在rep和cap基因间隔区5'端存在茎-环结构(见图1),具有与PCV1和PCV2完全一致、保守九核苷酸基序(TAGTATTAC)[23-24]。
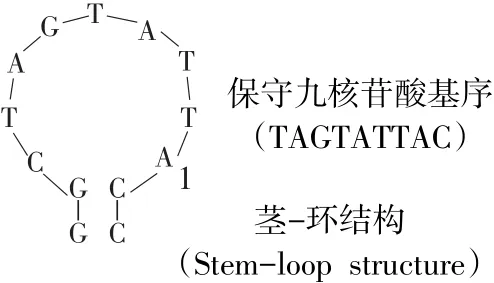
图1 PCV3基因组结构Fig.1 PCV3 genomic structure
按照惯例,将茎-环结构中位于九核苷酸基序内第8位核苷酸残基“A”定义为基因组起始位置“1”[25]。但Rep蛋白编码基因序列中无标准起始密码子ATG,rep基因5'端存在密码子GTC,该替代性起始密码子也见于猪圆环病毒PorkNW2/USA/2009(GenBank登录号ADU77001.1)及禽类圆环病毒(如鹅圆环病毒、鸽圆环病毒和喙羽病病毒)[26-27]。与其他圆环病毒Cap蛋白类似[28],PCV3 Cap蛋白N端富含精氨酸,为病毒核定位信号区域,与病毒在细胞核内定位有关。ORF3编码是一个由231个aa组成的功能未知蛋白质,该蛋白与PorkNW2/USA/2009编码蛋白质具有94%同源性,与鼠疱疹病毒M169具有39%同源性,ORF3编码方向与rep基因相同[29]。与rep基因类似,ORF3起始密码子尚不清楚,其5'端密码子为TCG。位于ORF3第55位aa处甲硫氨酸可能为另一个翻译起始位点,产生一个由177个aa组成蛋白质[21]。
1.3 抗原性
Cap蛋白为PCV主要结构蛋白,诱导动物机体产生特异性免疫应答[30-32]。PCV2与PCV3 Cap蛋白aa同源性仅为30%,两者间存在交叉免疫保护可能性较低[21,33]。湛洋等对PCV3 Cap蛋白三维结构分析发现,PCV3和PCV2 Cap蛋白具有类似由两个β-片层结构组成jelly roll折叠,但PCV3 Cap蛋白其中一个片层仅由3条反平行β-折叠组成,而PCV2 Cap蛋白两个片层均由4条反平行β-折叠组成[33]。借助B细胞抗原表位在线预测软件分析发现,PCV3 Cap蛋白抗原表位主要分布于8个区域(见表1)。
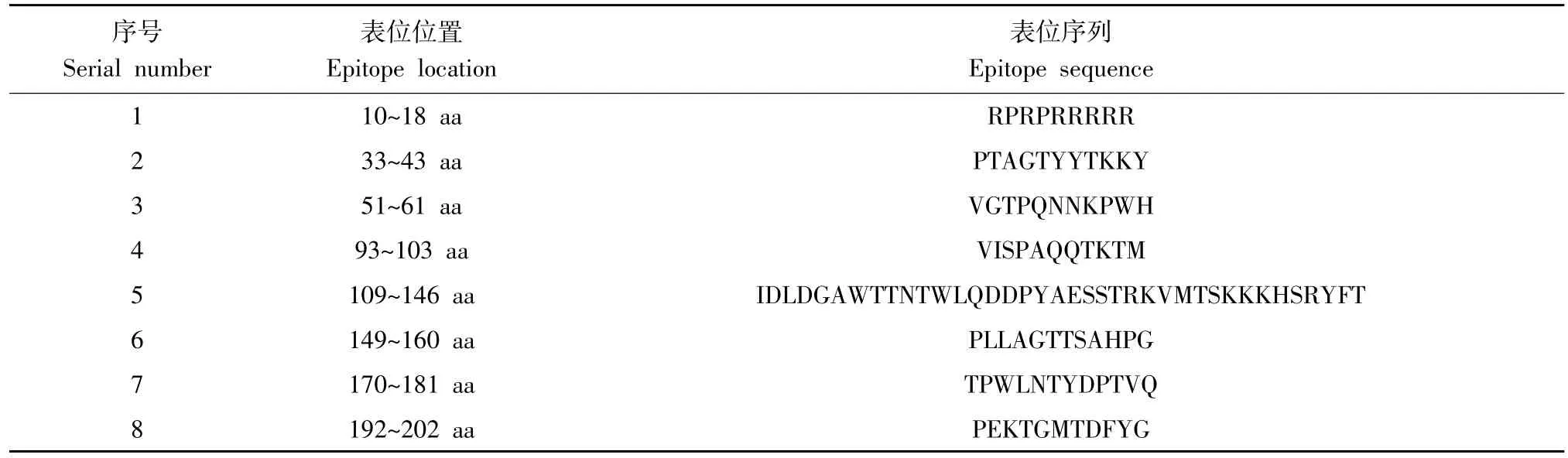
表1 PCV3 Cap蛋白B细胞抗原表位预测Table 1 B-cell epitope prediction for the PCV3 Cap protein
1.4 培养特性
目前,猪肾细胞(PK-15、SK)和猪睾丸细胞(ST)常用于PCV2分离与培养[34],推测PCV3可在此类细胞中增殖[21]。
1.5 理化特性
PCV对不良环境抵抗力较强,可于酸性环境中(pH=3)存活较长时间,无血凝性[35]。Allan和O'Dea等对PCV热稳定性分析发现,PCV1于56和70℃加热15 min后感染性无明显变化[35-36]。PCV2于75℃处理15 min仍有感染性,但80℃处理15 min后完全失去感染性[36],耐热性良好。Welch等研究发现,常规巴氏杀菌、干热处理和湿热处理均难以彻底灭活血液制品中PCV2[37]。由于PCV无囊膜,因此对氯仿、碘酒、酒精等有机溶剂不敏感,但对苯酚、季胺类化合物、氢氧化钠和氧化剂等较敏感[35,38]。鉴于PCV3与PCV2属于同一病毒科,推测两者理化特性相似,可采用相同消毒剂和消毒方法处理PCV3污染圈舍、环境、运输工具等。
2 流行病学
2.1 传染源
处于病毒血症期感染猪为主要传染源,包括发病猪和隐性感染猪。病毒随患病猪鼻、口腔分泌物及粪便排出[39-40]。
2.2 传播途径
PCV2可通过呼吸道、消化道和泌尿生殖道分泌物,以直接或间接接触方式传播[40-42]。怀孕母猪感染PCV2后,可经胎盘垂直传染仔猪[43-44]。Palinski等从患有PDNS症状母猪及其流产胎儿体内均发现PCV3全基因组序列[21],间接证实PCV3垂直传播方式。PCV2感染公猪精液中存在间歇性排毒现象[45],将掺入PCV2的健康猪精液人工授精接种健康母猪,可导致母猪发生繁殖障碍,产弱胎、死胎和木乃伊胎[46]。Ku等通过PCR技术从猪精液中检出PCV3核酸,揭示PCV3也可以通过精液传播[47]。
2.3 易感动物
不同年龄、性别和品种均可感染PCV3,但以妊娠母猪和仔猪更易感[48-49]。目前,尚不清楚野猪是否对PCV3易感,也未发现其他物种感染PCV3报道。
2.4 发生与分布

图2 PCV3在美国和中国的流行与分布Fig.2 Prevalenceand distribution of PCV3 in the USA and China
2015年6月,美国北卡罗来纳州某猪场暴发PDNS疫情,与历史平均值相比,母猪死亡率升高10.2%、受孕率下降0.6%。患病母猪出现厌食症状,皮肤呈现多灶性丘疹、斑点和浅表性皮炎,所产胎儿(包括弱胎、死胎、木乃伊胎)具有类似临床症状。2016年,Palinski等借助NGS技术从患病母猪及其流产胎儿体内首次鉴定出PCV3,美国多个州猪群证实存在PCV3感染[21,48],如北卡罗来纳州和爱荷华州等(见图2A)。我国湖北、广东某些发生繁殖障碍母猪以及急性死亡仔猪体内也检测出PCV3[50]。截至目前,我国已有13个省猪场存在PCV3感染与流行[47,51],且主要分布于中东部地区(见图2B),其他省市是否存在PCV3感染目前尚不清楚。Stadejek等对波兰境内14个商品化猪场母猪、仔猪和育成猪血清作PCV3检测,首次证实欧洲地区存在PCV3感染[49]。Kwon等对采自韩国6个省73个猪场的猪唾液样品作PCV3核酸检测,首次发现韩国猪群也存在PCV3感染与流行[52]。
3 临床症状与病理变化
PCV3感染可导致母猪出现厌食症状,皮肤出现多灶性丘疹、斑点和浅表性皮炎,生产性能下降;妊娠母猪发生繁殖障碍,产弱胎、死胎、木乃伊胎和弱仔猪,病情严重甚至造成急性死亡[21,47];不同胎龄胎儿均可发生流产。组织学检查可见,真皮和皮下组织呈以纤维蛋白样变、透壁性嗜中性粒细胞浸润、出血和纤维蛋白渗出为特征的坏死性血管炎;炎性细胞浸润通常蔓延至周围真皮和皮下组织,以“袖套”形式围绕于血管和皮肤附件周围;偶见轻度角化过度表皮增生症。肾脏呈现弥散性膜增生性肾小球肾炎,表现为肾小球硬化和波曼氏囊(Bowman's capsule)增厚、皮质小管衰退和不同程度的间质纤维化;肾小管发生扩张,衰退上皮细胞堆积于管腔,皮质间质和肾小球出现大簇淋巴细胞和巨噬细胞扩散性浸润。肺脏出现不同程度支气管间质性肺炎,偶尔继发化脓性支气管肺炎;中小型气道和小血管周围出现由淋巴细胞和浆细胞组成聚集物,围绕于细支气管和血管周围形成“袖套”样结构;相邻肺泡隔膜发生淋巴细胞和浆细胞浸润;肺泡腔内充满中等数量泡沫巨噬细胞、少量多核巨细胞和小簇嗜中性粒细胞,形成腔内水肿。淋巴结呈现弥漫性肉芽肿淋巴结炎,表现为中度皮质淋巴细胞消耗,甚至出现组织细胞和多核巨细胞替代[21]。
患有体重减轻、呼吸、神经系统疾病以及心脏和全身多发性炎症仔猪可能与PCV3感染有关[48]。但患病仔猪组织同时检出猪星状病毒4型、轮状病毒A、猪巨细胞病毒和猪血凝性脑脊髓炎病毒,目前尚未证实上述临床症状与病理变化是否与PCV3感染直接相关。
4 诊断
4.1 临床诊断
除PCV2感染可以导致PDNS症状外[53],无PCV2存在情况下,将PRRSV以及含有细环病毒(Torque teno virus,TTV)组织匀浆液接种健康猪,也可产生PDNS症状[54]。鉴于PCV3是从患有PDNS症状母猪及其流产胎儿体内检出唯一病原体,PCV3感染猪临床症状与病理变化与PCV2高度相似[21],因此,须结合临床与实验室诊断确诊是否为PCV3感染。
4.2 实验室诊断
4.2.1 病毒分离与鉴定
利用无PCV1和PCV2污染PK-15、SK或ST细胞从患病猪血清、皮肤、肾脏、肺和淋巴结等组织样品中分离PCV3[21],借助DNA测序、中和试验、免疫组化和荧光定量PCR等技术鉴定病毒[21]。
4.2.2 荧光定量PCR
表2 PCV3荧光定量PCR引物和探针Table 2 Primers and probesused for real-time PCR detection of PCV3
美国堪萨斯州立大学、河北出入境检验检疫局和中国检验检疫科学研究院针对PCV3cap基因设计引物和探针,建立PCV3TaqMan探针荧光定量PCR检测方法(见表2)。
4.2.3 DNA测序
Palinski等、Ku等针对PCV3基因组不同区域设计扩增片段相互重叠引物,借助PCR与测序技术获得PCV3全基因组序列[21,47]。
4.2.4 免疫组化
利用PCV3特异性单克隆抗体染色患病猪组织切片[21],观察PCV3组织嗜性及其组织内分布情况。
4.2.5 ELISA
Palinski等利用原核表达PCV3 Cap蛋白包被ELISA板,建立检测PCV3抗体间接ELISA[21]。
5 防控对策
5.1 疫病风险评估
目前,PCV3感染尚未列入世界动物卫生组织(OIE)通报性疫病名录及《中华人民共和国进境动物检疫疫病名录》,该病毒随生猪及其产品国际贸易传播风险尚待评估。相关部门有必要根据该病毒最新研究进展开展风险评估工作,并基于风险评估结果作贸易决定。
5.2 检测技术储备
鉴于PCV3具有传播途径多、传播速度快、流行范围广等特点,应尽快研发适用于出入境口岸PCV3快速筛查与准确鉴定需求的检疫技术。
5.3 疫情处置
我国已出现PCV3感染病例[47,51],主要集中于中东部地区。全国范围内急需开展PCV3流行病学调查,“早发现、早报告、早隔离、早诊断、早扑灭”,及时控制和扑灭疫情。
5.4 疫苗研制
鉴于PCV2与PCV3存在交叉免疫保护可能性较低[21,33],应加快PCV3毒株分离步伐,同时利用感染性克隆技术构建含有PCV3 cap基因PCV1或PCV2嵌合病毒,为疫苗生产奠定基础。
6 结 语
目前针对PCV3研究尚处于起步阶段,需尽快开展相关检测技术研究,建立PCV3快速、灵敏、特异病原学和血清学检测方法,分离与鉴定PCV3毒株,为开展疫苗研制及动物回归试验工作奠定基础,保护我国生猪产业健康发展。
[1] Tischer I,Gelderblom H,Vettermann W,et al.A very small porcine virus with circular single-stranded DNA[J].Nature,1982,295(5844):64-66.
[2] Ellis J.Porcine circovirus:A historical perspective[J].Vet Pathol,2014,51(2):315-327.
[3] Denner J,Mankertz A.Porcine circoviruses and xenotransplantation[J].Viruses,2017,9(4):83.
[4] Tischer I,Rasch R,Tochtermann G.Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines[J].Zentralbl Bakteriol Orig A,1974,226(2):153-167.
[5] Tischer I,Mields W,Wolff D,et al.Studies on epidemiology and pathogenicity of porcine circovirus[J].Arch Virol,1986,91(3-4):271-276.
[6] Baekbo P,Kristensen CS,Larsen L E.Porcine circovirusdiseases:A review of PMWS[J].Transbound Emerg Dis,2012,59(S1):60-67.
[7] Allan G M,McNeilly E,Kennedy S,et al.PCV-2-associated PDNS in Northern Ireland in 1990.Porcine dermatitis and nephropathy syndrome[J].Vet Rec,2000,146(24):711-712.
[8] Kim J,Chung H K,Chae C.Association of porcine circovirus 2 with porcine respiratory disease complex[J].Vet J,2003,166(3):251-256.
[9] Karuppannan A K,Ramesh A,Reddy Y K,et al.Emergence of porcine circovirus 2 associated reproductive failure in Southern India[J].Transbound Emerg Dis,2016,63(3):314-320.
[10] Szeredi L,Szentirmai C.Proliferative and necrotising pneumonia and severe vascular lesions in pigs naturally infected with porcine circovirus type 2[J].Acta Vet Hung,2008,56(1):101-109.
[11] Opriessnig T,Madson D M,Roof M,et al.Experimental reproduction of porcine circovirus type 2(PCV2)-associated enteritis in pigs infected with PCV2 alone or concurrently with Lawsonia intracellularis or Salmonella typhimurium[J].J Comp Pathol,2011,145(2-3):261-270.
[12] Kim J,Ha Y,Jung K,et al.Enteritis associated with porcine circovirus 2 in pigs[J].Can J Vet Res,2004,68(3):218-221.
[13] Ge X,Wang F,Guo X,et al.Porcine circovirus type 2 and its associated diseases in China[J].Virus Res,2012,164(1-2):100-106.
[14] Chae C.A review of porcine circovirus 2-associated syndromes and diseases[J].Vet J,2005,169(3):326-336.
[15] Harms P A,Sorden S D,Halbur P G,et al.Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus[J].Vet Pathol,2001,38(5):528-539.
[16] Allan G M,Kennedy S,McNeilly F,et al.Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus[J].J Comp Pathol,1999,121(1):1-11.
[17] Opriessnig T,Thacker E L,Yu S,et al.Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2[J].Vet Pathol,2004,41(6):624-640.
[18] Beach N M,Meng X J.Efficacy and future prospects of commercially available and experimental vaccines against porcine circovirus type 2(PCV2)[J].Virus Res,2012,164(1-2):33-42.
[19] Xiao C T,Halbur P G,Opriessnig T.Global molecular genetic analysis of porcine circovirus type 2(PCV2)sequenc-es confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d[J].J Gen Virol,2015,96(7):1830-1841.
[20] Guo L,Fu Y,Wang Y,et al.A porcine circovirus type 2(PCV2)mutant with 234 amino acids in capsid protein showed more virulence in vivo,compared with classical PCV2a/b strain[J].PLoS One,2012,7(7):41463-41472.
[21] Palinski R,Pineyro P,Shang P,et al.A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure[J].J Virol,2017,91(1):1879-1891.
[22] Biagini P,Bendinelli M,Hino S,et al.Circoviridae[M]//King A M Q,Adams M J,Carstens E B,et al.Virus Taxonomy:Classification and Nomenclature of Viruses.Ninth report of the International Committee on Taxonomy of Viruses.New York:Elsevier Academic Press,2012:343-349.
[23] Li L,Kapoor A,Slikas B,et al.Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces[J].J Virol,2010,84(4):1674-1682.
[24] Steinfeldt T,Finsterbusch T,Mankertz A.Demonstration of nicking/joining activity at the origin of DNA replication associated with the rep and rep'proteins of porcine circovirus type 1[J].JVirol,2006,80(13):6225-6234.
[25]Todd D,Weston J H,Soike D,et al.Genome sequence determinations and analyses of novel circoviruses from goose and pigeon[J].Virology,2001,286:354-362.
[26] Phenix K V,Weston J H,Ypelaar I,et al.Nucleotide sequence analysis of a novel circovirus of canaries and its relationship to other members of the genus Circovirus of the family Circoviridae[J].J Gen Virol,2001,82(11):2805-2809.
[27]Bassami M R,Ypelaar I,Berryman D,et al.Genetic diversity of beak and feather disease virus detected in psittacine species in Australia[J].Virology,2001,279:392-400.
[28] Liu Q,Tikoo S K,Babiuk L A.Nuclear localization of the ORF2 protein encoded by porcine circovirus type 2[J].Virology,2001,285:91-99.
[29] Zhang W,Li L,Deng X,et al.What is for dinner?Viral metagenomics of US store bought beef,pork,and chicken[J].Virology,2014,(468-470):303-310.
[30] Nawagitgul P,Morozov I,Bolin S R,et al.Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein[J].J Gen Virol,2000,81(9):2281-2287.
[31] 郭春艳,葛俊伟,李一经.猪圆环病毒2型ORF2基因的原核表达[J].东北农业大学学报,2009,40(6):79-84.
[32] 宋月,姜艳萍,崔文,等.猪圆环病毒2型Cap蛋白在杆状病毒系统表达及鉴定[J].东北农业大学学报,2012,43(3):36-41.
[33] 湛洋,王东亮,王乃东,等.猪圆环病毒3型检测及其Cap结构序列和抗原性预测分析[J].畜牧兽医学报,2017,48(6):1076-1084.
[34] Misinzo G,Delputte P L,Lefebvre D J,et al.Porcine circovirus 2 infection of epithelial cells is clathrin-,caveolaeand dynamin-independent,actin and Rho-GTPase-mediated,and enhanced by cholesterol depletion[J].Virus Res,2009,139(1):1-9.
[35] Allan G M,Phenix K V,Todd D,et al.Some biological and physico-chemical properties of porcine circovirus[J].Zentralbl Veterinarmed B,1994,41(1):17-26.
[36]O'Dea M A,Hughes A P,Davies L J,et al.Thermal stability of porcine circovirus type 2 in cell culture[J].J Virol Methods,2008,147(1):61-66.
[37] Welch J,Bienek C,Gomperts E,et al.Resistance of porcine circovirus and chicken anemia virus to virus inactivation procedures used for blood products[J].Transfusion,2006,46(11):1951-1958.
[38] Royer R L,Nawagitgul P,Halbur P G,et al.Susceptibility of porcine circovirus type 2 to commercial and laboratory disinfectants[J].J Swine Health Prod,2001,9(6):281-284.
[39] Patterson A R,Madson D M,Halbur P G,et al.Shedding and infection dynamics of porcine circovirus type 2(PCV2)after natural exposure[J].Vet Microbiol,2011,149(1-2):225-229.
[40] Patterson A R,Ramamoorthy S,Madson D M,et al.Shedding and infection dynamics of porcine circovirus type 2(PCV2)after experimental infection[J].Vet Microbiol,2011,149(1-2):91-98.
[41] Rose N,Opriessnig T,Grasland B,et al.Epidemiology and transmission of porcine circovirus type 2(PCV2)[J].Virus Res,2012,164(1-2):78-89.
[42] Patterson A R,Opriessnig T.Epidemiology and horizontal transmission of porcine circovirus type 2(PCV2)[J].Anim Health Res Rev,2010,11(2):217-234.
[43] Shen H,Wang C,Madson D M,et al.High prevalence of porcine circovirus viremia in newborn piglets in five clinically normal swine breeding herds in North America[J].Prev Vet Med,2010,97(3-4):228-236.
[44] West K H,Bystrom J M,Wojnarowicz C,et al.Myocarditis and abortion associated with intrauterine infection of sows with porcine circovirus 2[J].J Vet Diagn Invest,1999,11(6):530-532.
[45] Larochelle R,Bielanski A,Muller P,et al.PCR detection and evidence of shedding of porcine circovirus type 2 in boar semen[J].J Clin Microbiol,2000,38(12):4629-4632.
[46] Madson D M,Patterson A R,Ramamoorthy S,et al.Reproductive failure experimentally induced in sows via artificial insemination with semen spiked with porcine circovirus type 2[J].Vet Pathol,2009,46(4):707-716.
[47] Ku X,Chen F,Li P,et al.Identification and genetic characterization of porcine circovirus type 3 in China[J].Transbound Emerg Dis,2017,64(3):703-708.
[48] Phan T G,Giannitti F,Rossow S,et al.Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation[J].Virol J,2016,13(1):184.
[49] Stadejek T,Wozniak A,Milek D,et al.First detection of porcine circovirus type 3 on commercial pig farms in Poland[J].Transbound Emerg Dis,2017,64(5):1350-1353.
[50] Fan S,Ku X,Chen F,et al.Complete genome sequence of
Research progress on porcine circovirus 3/
ZHANG Yongning1,Mei Lin1,ZHANG
Zhou1,TANG Lijie2,WU Shaoqiang2
(1.Institute of Animal Quarantine,Chinese Academy of Inspection and Quarantine,Beijing 100176,China;2.School of Veterinary Medicine,Northeast Agricultural University,Harbin 150030,China)
In 2016,a novel porcine circovirus,designated porcine circovirus 3(PCV3),was first identified in diseased sows with porcine dermatitis and nephropathy syndrome(PDNS)-like clinical signs as well as in the aborted fetuses by researchers from the Kansas State University using metagenomic sequencing technology.Epidemiological survey further demonstrated that PCV3 infection was mainly distributed in the pig herds exhibiting PDNS and reproductive failure symptoms and was widely prevalent in the swine population in several states of the USA.The Blood Systems Research Institute(San Francisco,USA)also discovered PCV3 in three sick piglets with cardiac and multi-systemic inflammation of unknown etiology.PCV3 was also detected in the Chinese pig herds in Hubei and Guangdong Provinces,etc.The presence of PCV3 infection was successively reported in the Polish and Korean pig populations.As a newly emerging pathogen,the pathogenicity of PCV3 for swine,the role of PCV3 played in porcine circovirusassociated diseases(PCVAD),and its potential influence on the immune efficacy of the existing commercial porcine circovirus 2(PCV2)vaccines need to be further studied.In order to provide a reference for follow-up studies,the latest research progress of PCV3 was summarized in this review.
metagenomic sequencing;porcine circovirus 3(PCV3);porcine dermatitis and nephropathy syndrome(PDNS);reproductive failure;porcine circovirus associated diseases(PCVAD)
S852.659
A
1005-9369(2017)09-0089-08
时间 2017-10-19 17:14:28 [URL]http://kns.cnki.net/kcms/detail/23.1391.S.20171019.1714.006.
张永宁,梅琳,张舟,等.猪圆环病毒3型研究进展[J].东北农业大学学报,2017,48(9):89-96.
Zhang Yongning,Mei Lin,Zhang Zhou,et al.Research progress on Porcine Circovirus 3[J].Journal of Northeast Agricultural University,2017,48(9):89-96.(in Chinese with English abstract)
2017-07-20
“十三五”国家重点研发计划项目(2016YFD0501102,2017YFD0501105);中国检验检疫科学研究院“青年英才”计划(CAIQ-YC-20140205)
张永宁(1980-),男,副研究员,博士,研究方向为外来动物疫病检疫。 E-mail:zhangyn@caiq.gov.cn

