糖原合酶激酶-3β在急性坏死性胰腺炎肾损伤中的表达及作用机制
赵凯亮 陈辰 石乔 赵亮 梅方超 王萍 王卫星
糖原合酶激酶-3β在急性坏死性胰腺炎肾损伤中的表达及作用机制
赵凯亮 陈辰 石乔 赵亮 梅方超 王萍 王卫星
目的观察急性坏死性胰腺炎(ANP)大鼠肾脏的组织形态及超微结构变化,检测ANP大鼠肾脏组织糖原合酶激酶-3β(GSK-3β)及磷酸化GSK-3β(p-GSK-3β)的蛋白表达。方法60只雄性SD大鼠随机分为对照组及ANP 3、6、12、24 h组,每组12只。采用胆胰管逆行注射5%牛磺胆酸钠溶液制备大鼠ANP模型。在相应时间点处死大鼠后取血及胰腺和左肾组织,检测血淀粉酶、脂肪酶、肌酐和尿素氮水平,常规行胰腺和肾脏组织病理学检查,透射电镜下观察大鼠肾超微结构改变,采用蛋白质印迹法检测肾脏组织GSK-3β、p-GSK-3β蛋白表达。结果ANP大鼠血淀粉酶、脂肪酶、肌酐、尿素氮水平以及胰腺及肾组织的病理评分均较对照组显著升高,且随着时间延长逐渐升高。ANP大鼠肾小管上皮细胞表面微绒毛肿胀、排列紊乱,胞核固缩、碎裂,核内见染色质浓缩、核膜分离,胞质内线粒体凝集、肿胀,空泡样变。对照组及ANP 3、6、12、24 h组大鼠肾组织GSK-3β蛋白表达量分别为0.702±0.044、0.876±0.017、0.872±0.034、0.855±0.035、0.852±0.032;p-GSK-3β表达量为0.626±0.029、0.790±0.029、0.616±0.021、0.448±0.028、0.439±0.017。ANP各时点组肾组织GSK-3β蛋白表达均较对照组显著增高,差异有统计学意义(P值均<0.05),但ANP各时点组间的差异均无统计学意义。p-GSK-3β表达在造模后3 h升高,以后逐渐下降,其中ANP 3 h组显著高于对照组及ANP其他时点组,ANP 12、24 h组显著低于对照组及ANP 3、6 h组,差异均有统计学意义(P值均<0.05)。结论ANP大鼠肾脏GSK-3β表达在造模3 h即升高,并维持在较高水平,p-GSK-3β在造模3 h时一过性升高,以后逐渐下降,并降至显著低于对照组,这一变化在ANP并发肾损伤的过程中可能发挥重要作用。
胰腺炎,急性坏死性; 急性肾功能不全; 糖原合酶激酶-3β
Fundprogram:National Natural Science Foundation of China(81370562);Fundamental Research Funds for the Central Universities(2042015kf0086)
重症急性胰腺炎(SAP)并发肾功能损伤的概率仅次于肺损伤,严重者可进展为急性肾功能衰竭,病死率可高达80%[1-2]。大量研究表明,微循环障碍、体内产生的大量炎性介质、细胞因子及细胞凋亡等因素在SAP并发肾损伤的发病机制中起着重要的作用,但其具体机制仍不十分清楚。糖原合酶激酶-3β(GSK-3β)是存在于真核细胞内的一种多功能蛋白激酶,属于丝氨酸/苏氨酸蛋白激酶家族。GSK-3β不仅通过激活糖原合成酶调节细胞糖代谢从而调控细胞能量代谢过程,而且在炎症相关疾病的细胞坏死和器官衰竭中发挥重要作用[3-4]。但GSK-3β是否参与SAP并发的肾损伤尚未见相关报道。本研究建立急性坏死性胰腺炎(ANP)并发肾损伤的大鼠模型,检测肾组织GSK-3β蛋白表达及其磷酸化状态(p-GSK-3β)的变化趋势,探讨其在ANP相关性肾损伤发病中的可能作用。
材料与方法
一、实验动物与分组
SPF级雄性SD大鼠60只,体重150~200 g,由湖北省疾病预防控制中心提供。按随机数字表法将大鼠分为假手术组及ANP 3、6、12、24 h组,每组12只。实验前大鼠禁食12 h,自由饮水。采用主胰管逆向注射5%牛磺胆酸钠溶液1 ml/kg体重(Sigma公司)的方法制备ANP模型。对照组胰胆管内注入等容积生理盐水。对照组术后12 h、ANP组按各时间点分批处死大鼠。下腔静脉穿刺采血,离心分离血清,置-20℃保存备用;取部分胰腺组织和左侧肾脏组织置4%多聚甲醛液固定,部分左侧肾脏组织置2%戊二醛液固定,其余肾脏组织立即经液氮冻存后置-80℃冰箱保存。
二、方法
1.胰腺、肾脏组织病理学检查:取4%多聚甲醛固定的胰腺、肾脏组织,石蜡包埋、切片,HE染色,光镜下观察。分别参照Schmidt等[5]、Paller等[6]标准对胰腺及肾脏组织进行病理评分。
2.肾脏组织超微结构观察:取1 mm×1 mm×1 mm 2%戊二醛磷酸钠固定的肾脏组织块制成超薄切片,置日立H-300型透射电镜下观察肾脏超微结构变化并拍照。
3.血淀粉酶、脂肪酶、肌酐和尿素氮水平检测:委托武汉大学人民医院检验中心进行检测。
4.肾脏组织GSK-3β及p-GSK-3β蛋白表达检测:取冻存的肾脏组织,应用蛋白裂解液制备匀浆,置冰上孵育30 min,4℃ 13 000 r/min离心30 min,取上清液。应用BCA法测定蛋白浓度,取40 μg蛋白行蛋白质印迹法检测GSK-3β及p-GSK-3β蛋白表达。兔抗大鼠GSK-3β、p-GSK-3β及内参β-actin多克隆抗体均购自Cell Signalling Technology公司,工作浓度分别为1∶1 000、1∶1 000、1∶2 000,辣根过氧化酶标记二抗工作浓度为1∶3 000。最后ECL化学发光,X片曝光、显影、定影。采用Image-ProPlus蛋白灰度分析软件扫描检测蛋白质条带灰度值,以目的条带与内参条带灰度值比表示蛋白相对表达量,并计算p-GSK-3β/GSK-3β比值。实验重复3次,取均值。
三、统计学处理
结 果
一、血淀粉酶、脂肪酶、肌酐、尿素氮水平变化
造模后3 h大鼠血清淀粉酶、脂肪酶活性及肌酐、尿素氮水平即升高,并随病程进展而逐渐增加,分别于24 h、24 h、24 h及12 h达峰值,ANP各时间点的水平均显著高于对照组,差异有统计学意义(P值均<0.01)。ANP 12 h组与24 h组间比较差异无统计学意义,其余各组间差异均有统计学意义(P值均<0.05,表1)。
二、各组大鼠胰腺及肾脏组织病理学改变
对照组胰腺组织无明显病理改变。ANP组各时点胰腺组织呈现不同程度充血、水肿、出血、坏死、炎性细胞浸润和胰周脂肪坏死,随时间进展,胰腺坏死程度加重、范围明显扩大,12 h和24 h时可见大片状坏死、腺体结构破坏严重(图1上)。ANP各时点组病理评分均较对照组显著增加,差异有统计学意义(P值均<0.05),且ANP各时点组间的差异也均有统计学意义(P值均<0.05,表2)。
对照组大鼠肾脏组织无明显病理改变。ANP 3 h组见肾小管上皮细胞水肿,间质血管轻度充血,无出血改变;6 h组见部分肾小球瘀血,肾小管管壁明显充血、水肿,少量肾小管上皮细胞坏死,向管腔脱落,间质可见少量红细胞及炎细胞浸润;12 h组见肾小球淤血性改变,细胞界限模糊,肾小管上皮细胞坏死脱落,管腔内可见大量管型形成,肾小管管腔变窄或闭塞,大量炎症细胞浸润;24 h组见大片肾小球缺血、坏死,肾小管大量管型形成,大量上皮细胞坏死(图1下)。ANP各时点组病理评分均较对照组显著增加,差异有统计学意义(P值均<0.05)。除12与24 h组之外,其他ANP各时点组间的差异也均有统计学意义(P值均<0.05,表2)。
三、肾脏组织超微结构改变
对照组大鼠肾脏组织肾小管上皮细胞微绒毛结构排列整齐,胞核内染色质分布较均匀,无核膜分离现象,线粒体大小及排列均匀一致,内质网排列整齐,无扩张;ANP 12 h组大鼠肾脏的肾小管上皮细胞表面微绒毛肿胀、减少,排列紊乱,微绒毛末端呈杆状膨大,细胞核形不规则,部分足突融合,胞核内可见染色质核膜分离现象,核内染色质浓缩、边积呈环状,有的核固缩、碎裂、呈不规则块状,胞质内线粒体凝集和肿胀,嵴排列紊乱、断裂,甚至可见空泡样变(图2)。
四、各组大鼠肾脏组织GSK-3β、p-GSK-3β蛋白表达的变化
对照组及ANP组3、6、12、24 h组大鼠肾组织GSK-3β蛋白相对表达量分别为0.702±0.044、0.876±0.017、0.872±0.034、0.855±0.035、0.852±0.032;p-GSK-3β表达量为0.626±0.029、0.790±0.029、0.616±0.021、0.448±0.028、0.439±0.017(图3);p-GSK-3β/GSK-3β比值为0.933±0.041、0.868±0.031、0.712±0.054、0.528±0.051、0.516±0.015。ANP各时点组肾组织GSK-3β蛋白表达均较对照组显著增高,差异有统计学意义(P值均<0.05),但ANP各时点组间的差异均无统计学意义。p-GSK-3β表达在造模后3 h升高,以后逐渐下降,其中ANP 3 h组显著高于对照组及ANP其他时点组,ANP 12、24 h组显著低于对照组及ANP 3、6 h组,差异均有统计学意义(P值均<0.05),而ANP 6 h组与对照组,ANP 12 h组与24 h组间的差异均无统计学意义。ANP 3 h组的p-GSK-3β/GSK-3β比值与对照组的差异无统计学意义,而ANP 6、12、24 h组均较对照组显著降低,ANP 12、24 h组又较6 h组显著降低,差异均有统计学意义(P值均<0.05)。
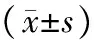
表1 对照组及ANP组大鼠血清学指标的变化
注:与对照组比较,aP<0.05;与ANP 3 h组比较,bP<0.05;与ANP 6 h组比较,cP<0.05

图1 对照组(1A)及ANP 3、6、12、24 h组(1B、1C、1D、1E)大鼠胰腺(上)及肾脏(下)组织病理学改变(HE ×200)
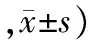
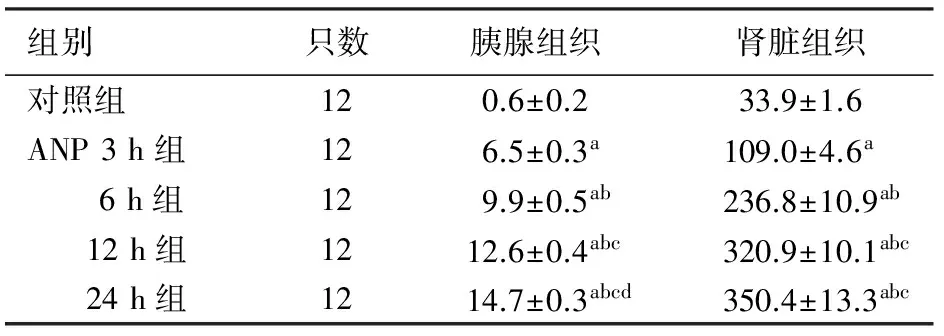
组别只数胰腺组织肾脏组织对照组120.6±0.233.9±1.6ANP3h组126.5±0.3a109.0±4.6a 6h组129.9±0.5ab236.8±10.9ab 12h组1212.6±0.4abc320.9±10.1abc 24h组1214.7±0.3abcd350.4±13.3abc
注:与对照组比较,aP<0.05;与ANP 3 h组比较,bP<0.05;与ANP 6 h组比较,cP<0.05;与ANP 12 h组比较,dP<0.05
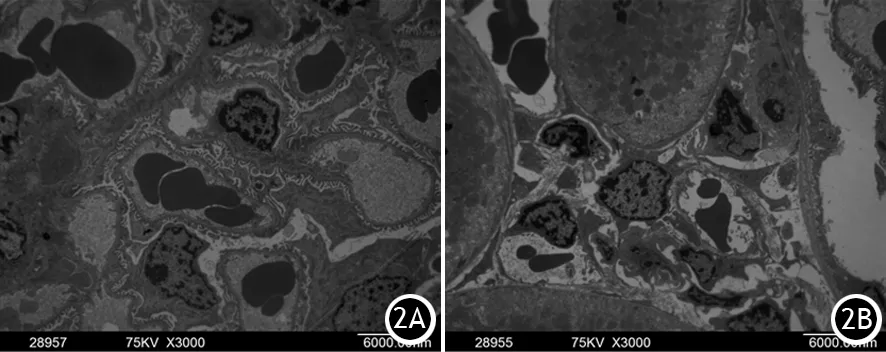
图2 对照组(2A)及ANP组(2B)大鼠肾脏组织的超微结构变化

图3 对照组(1)及ANP 3、6、12、24 h组(2、3、4、5)大鼠肾组织GSK-3β及p-GSK-3β蛋白表达
讨 论
研究表明,GSK-3β在炎症相关疾病的细胞坏死和器官衰竭中起着极其重要的作用[4]。Hoeflich等[7]报道在GSK-3β基因敲除小鼠肝再生模型中,其炎症因子表达情况与NF-κB基因敲除小鼠表达相似,但血清TNF-α、IL-6水平明显低于对照组。Cuzzocrea等[8]报道,抑制AP小鼠GSK-3β表达可降低血清淀粉酶和脂肪酶活性,下调炎症细胞因子表达,并减轻胰腺的病理损伤,对AP时多器官损伤和功能障碍起到保护作用,并有效降低死亡率。
已有研究表明,GSK-3β在静止的细胞中处于活性状态,在受到外界刺激后,其活性可增强,但是GSK-3β位于N末端的丝氨酸残基(GSK-3β Ser9)被磷酸化后,其活性可被抑制。GSK-3β的磷酸化可被各种因素,如缺氧、特异性抑制剂TDZD-8、非特异性抑制剂丙戊酸钠等调控而抑制活性。GSK-3β也可被上游信号如丝裂原活化蛋白激酶(MAPK)、AP-1、蛋白激酶C (PKC)、蛋白激酶A(PKA)、蛋白激酶B(PKB/AKT)等转导通路调控。目前研究显示,由胰岛素或胰岛素样生长因子诱导的PI3K/Akt信号转导通路为GSK-3β的主要调节因素,可导致GSK-3β的丝氨酸残基磷酸化而失去活性[9-10]。
本研究结果显示,ANP3h组大鼠肾组织GSK-3β蛋白表达较对照组显著增高,且未随着病程进展进一步升高,而是维持在较高水平;而p-GSK-3β ser9在造模后一过性表达增高,随着病程进展逐渐下降,推测可能与ANP时同时启动机体保护机制有关。ANP时PI3K/AKT信号转导通路被激活,导致GSK-3β的丝氨酸残基磷酸化,p-GSK-3β表达增高,但是随着病情进展,导致GSK-3β磷酸化的保护机制可能受到抑制,从而使p-GSK-3β表达降低。本研究结果提示,GSK-3β不仅参与ANP时全身炎症进展,也参与ANP并发的急性肾损伤的发病过程,而p-GSK-3β表达变化可能在ANP并发的肾损伤病情进展中发挥更重要的作用。但其更为确切的分子机制还需做更深入的研究。
[1] Zhou J, Li Y, Tang Y. Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis[J]. Nephrology,2015,20(7):485-491. DOI:10.1111/nep.12439.
[2] Zhang XP, Wang L, Zhou YF. The pathogenic mechanism of severe acute pancreatitis complicated with renal injury: A review of current knowledge[J]. Dig Dis Sci, 2008, 53(2): 297-306. DOI:10.1007/s10620-007-9866-5.
[3] Takada Y, Fang X, Jamaluddin MS, et al. Genetic deletion of glycogen synthase kinase-3 beta abrogates activation of I kappa B alpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor[J]. J Biol Chem, 2004, 279(38): 39541-39554. DOI:10.1074/jbc.M403449200.
[4] Dugo L, Collin M, Allen DA, et al. GSK-3 beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat[J]. Crit Care Med, 2005, 33(9): 1903-1912.
[5] Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy[J]. Ann Surg, 1992, 215(1): 44-56.
[6] Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat[J]. J Clin Invest, 1984, 74(4): 1156-1164. DOI:10.1172/JCI111524.
[7] Hoeflich KP, Luo J, Rubie EA, et al. Requirement for glycogen synthase kinase-3 beta in cell survival and NF-kappa B activation[J]. Nature, 2000, 406(6791): 86-90. DOI: 10.1038/ 35017574.
[8] Cuzzocrea S, Malleo G, Genovese T, et al. Effects of glycogen synthase kinase-3 beta inhibition on the development of cerulein-induced acute pancreatitis in mice[J]. Critical Care Medicine, 2007, 35(12):2811-2821.
[9] Simao F, Matte A, Pagnussat AS, et al. Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK-3 beta and CREB through PI3-K/Akt pathways[J]. Eur J Neurosci, 2012, 36(7): 2899-2905. DOI: 10.1111/j.1460-9568. 2012.08229.x.
[10] Duarte AI, Santos P, Oliveira CR, et al. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3 beta signaling pathways and changes in protein expression [J]. Biochimi Biophy Acta, 2008, 1783(6): 994-1002. DOI:10.1016/j.bbamcr.2008.02.016.
(本文编辑:屠振兴)
Expressionofglycogensynthasekinase-3βinrenaldamageofacutenecrotizingpancreatitisanditsmechanism
ZhaoKailiang,Chenchen,ShiQiao,Zhaoliang,MeiFangchao,Wangping,WangWeixing.
DepartmentofHepatobiliaryandLaparoscopicSurgery,RenminHospitalofWuhanUniversity,Wuhan430060,China
Correspondingauthor:WangWeixing,Email:sate.llite@163.com
ObjectiveTo observe the changes of tissue morphology and ultrastructure of kidney in the rat model of acute necrotizing pancreatitis (ANP), and to investigate the protein expression of glycogen synthase kinase-3β(GSK-3β) and phosphorylated GSK-3βin renal tissue.MethodsSixty SPF male SD rats were randomly divided into 5 groups (n=12 for each group) according to random number method, including control group, ANP 3 h, 6 h, 12 h, 24 h groups. ANP model was established by retrograde infusion of 5% sodium taurocholate solution into the biliopancreatic duct. Rats were sacrificed at corresponding time points to collect pancreatic and left renal tissue. Serum amylase (AMY), lipase (LIPA), creatinine (Cr) and urea nitrogen (BUN) levels were detected. Pancreatic and renal tissues were routinely pathologically examined.
Rephrocytes′ ultrastructure changes were observed by projection electron microscope. GSK-3β protein expression and phosphorylated GSK-3β(p-GSK-3β) in kidney tissue were quantified by Western-blot.ResultsSerum AMY, LIPA, Cr, Bun and pathological scores for pancreatic and renal tissues in ANP groups were obviously higher than those in control group, which increased gradually with the progress of pancreatitis. In ANP rats, it was observed that the microvilli on the surface of the epithelial cells of renal tubules were swelling and irregularly arranged, the nucleus was condensed and broken, the nuclear chromatin was condensed and separated from the nuclear membrane, the mitochondria was condensed, swelling and vacuolated. The expression levels of GSK-3β protein in the renal tissue of the control group and ANP 3 h, 6 h, 12 h, 24 h groups were 0.702±0.044, 0.876±0.017, 0.872±0.034, 0.855±0.035 and 0.852±0.032, respectively. The expression levels of p-GSK-3β were 0.626±0.029, 0.790±0.029, 0.616±0.021, 0.448±0.028 and 0.439±0.017. GSK-3β protein expression was higher in ANP group than in control group, and the difference was statistically significant (allP<0.05). But there was no statistically significant difference at different time points in ANP group. p-GSK-3β protein expression increased at 3 h after modeling, and then gradually decreased. p-GSK-3β protein expression was higher in ANP 3 h group than control group and other ANP groups, which in ANP 12 h, 24 h group was obviously lower than control group and ANP 3 h, 6 h group, and the difference was statistically significant (P<0.05).ConclusionsGSK-3β expression in the kidney of ANP rats began to increase at 3 h after modeling and maintain a high level. p-GSK-3β was transiently increased at 3 h after modeling and then gradually decreased to a level obviously lower than control group. It indicated that these changes may play a crucial role in ANP associated kidney injury.
Pancreatitis, acute necrotizing; Acute kidney injury; Glycogen synthase kinase-3β
10.3760/cma.j.issn.1674-1935.2017.05.004
430060 武汉,武汉大学人民医院肝胆腔镜外科
王卫星,Email: sate.llite@163.com
国家自然科学基金面上项目(81370562);中央高校基本科研业务费专项资金资助(青年教师资助项目,2042015kf0086)
2017-01-04)

